Blood Translation Elongation Factor-1δ Is a Novel Marker for Cadmium Exposure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animal and Cadmium Exposure Proceduces
2.2. Cadmium Determination and Organ Functional and Pathological Examination
2.3. Total RNA Isolation and TEF-1δ Expression Detection
2.4. Statistical Analysis
3. Results
3.1. Elevated Weight Coefficient in Cadmium-Exposed Rats
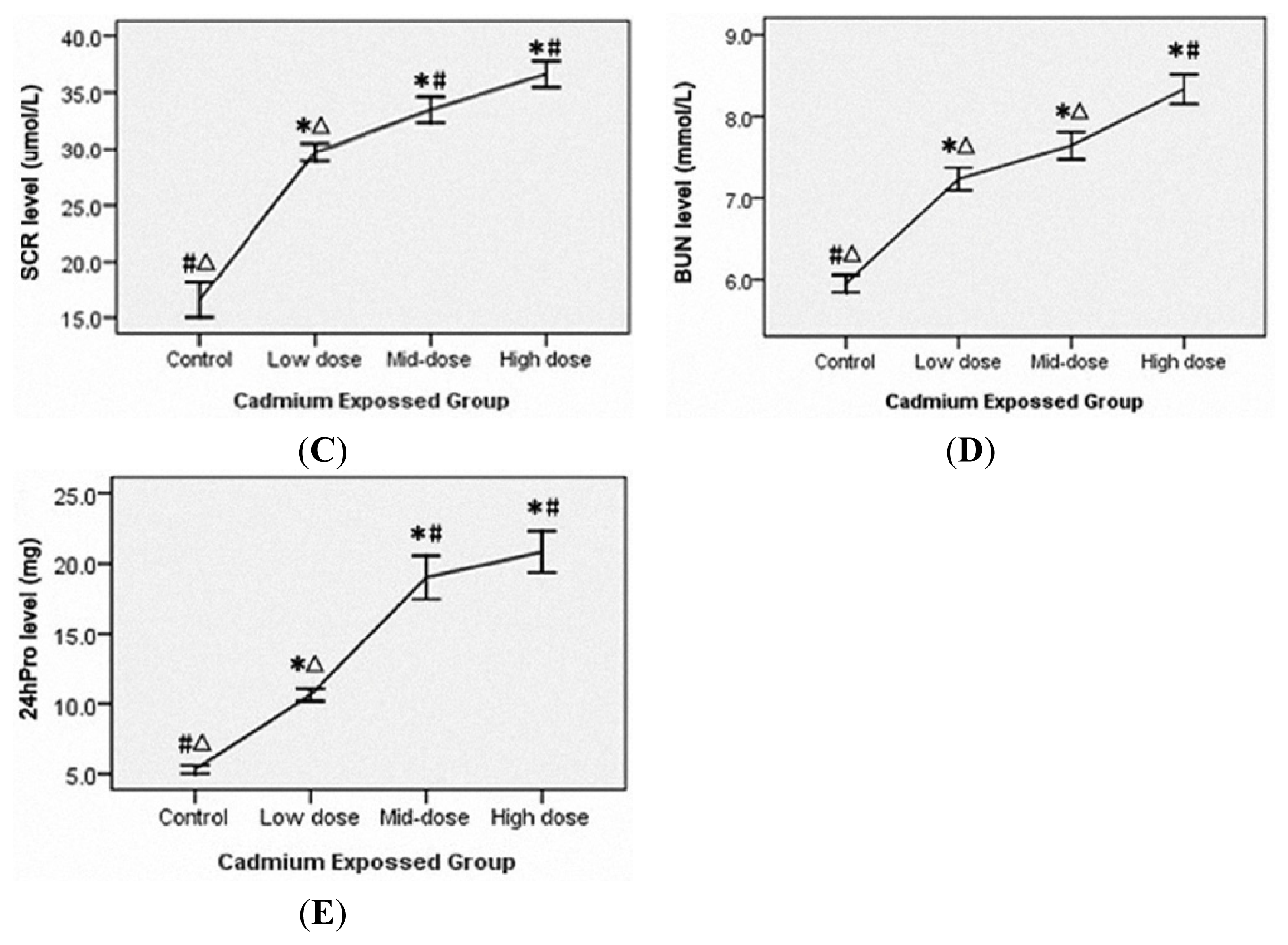
3.2. Accumulation of Cadmium in the Blood, Urine and Multiple Organs
3.3. Liver and Renal Injury in Cadmium-Exposed Rats
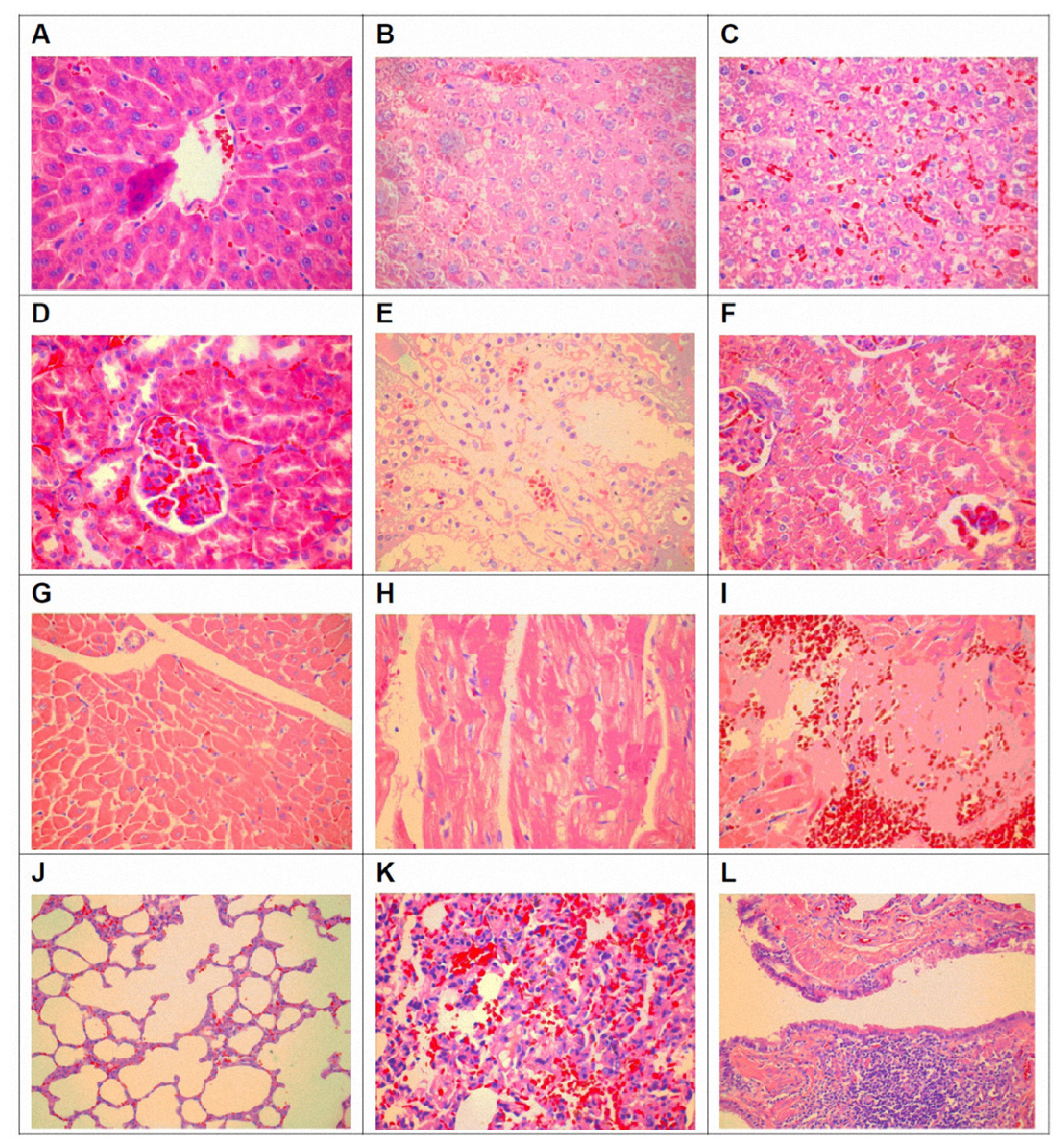
3.4. Pathological Changes of Organ Toxicity from Cadmium Exposure
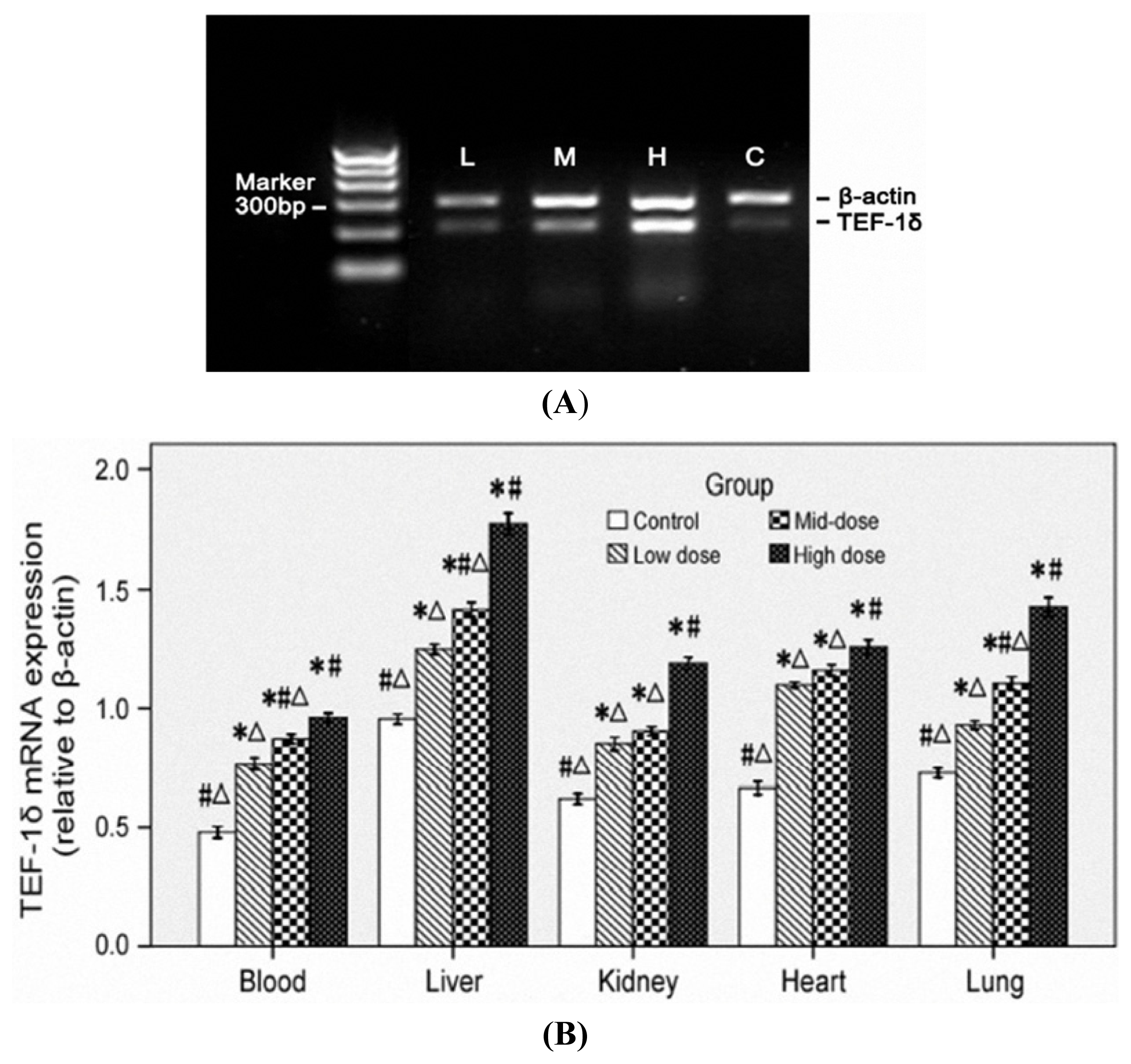
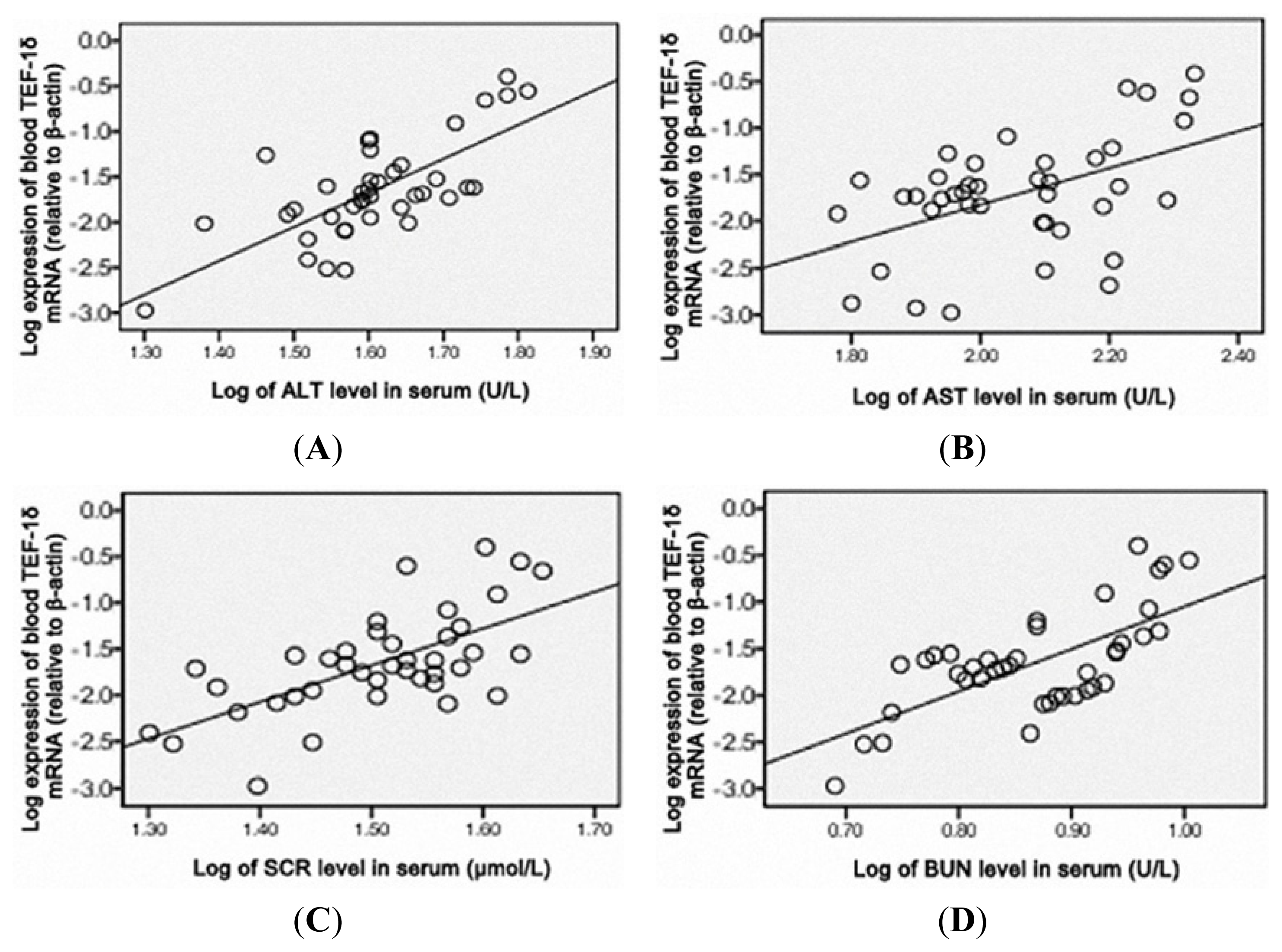
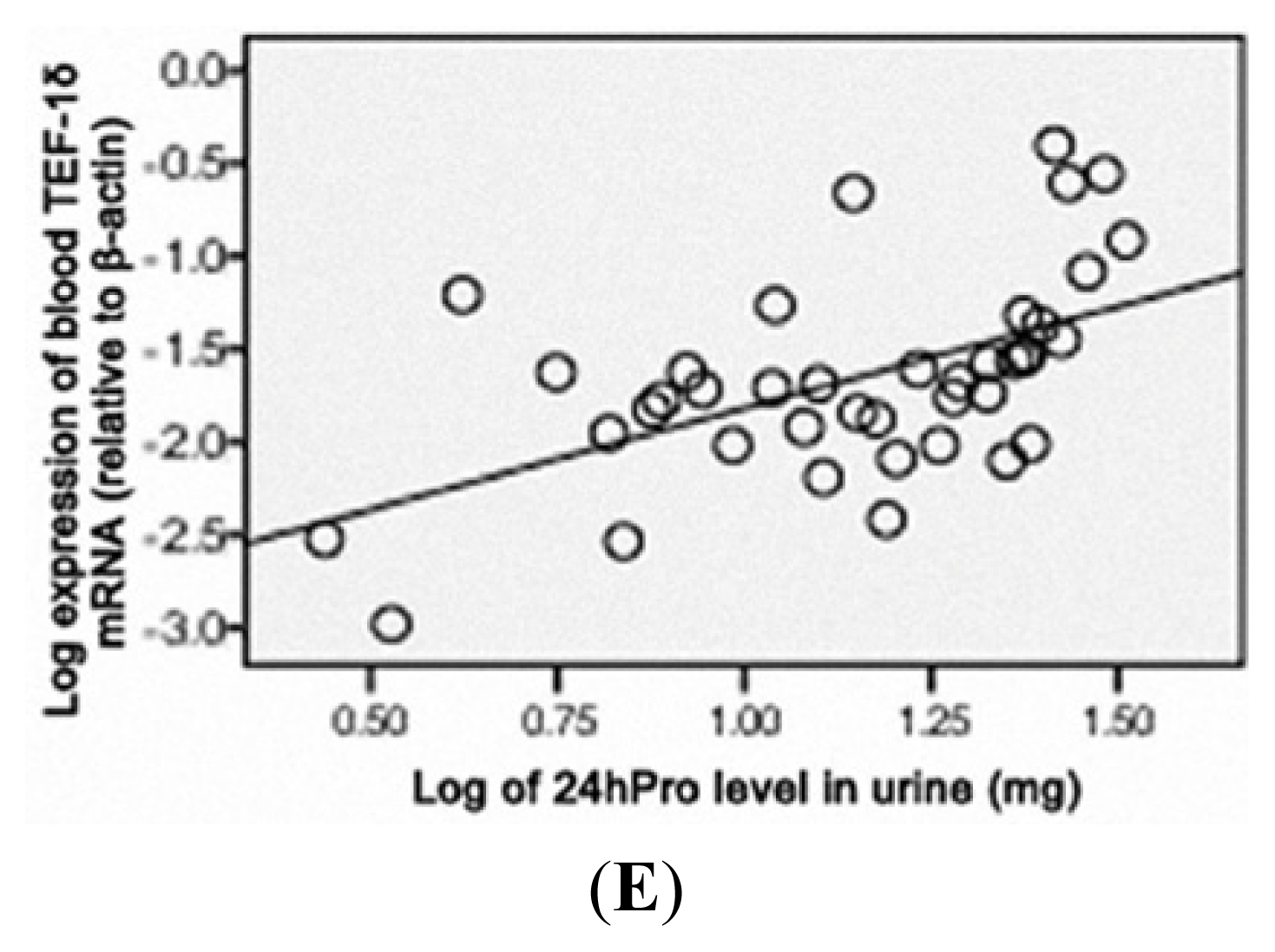
3.5. TEF-1δ Is Elevated in Cadmium-Exposed Rats
4. Discussion
Acknowledgements
- Funding InformationThis work was supported by the National Natural Science Foundation of China (81072322 and 30771781 to Yi-xiong Lei); Natural Science Foundation of Guangdong Province, China (06022672 to Yi-xiong Lei); Science and Technology Key Project of Guangdong Municipality, China (2003Z2-E0191 and 2003Z2-E0192 to Yi-xiong Lei).
References
- Diao, W.P.; Ni, W.Z.; Ma, H.Y.; Yang, X.E. Cadmium pollution in paddy soil as affected by different rice (Oryza sativa L.) cultivars. Bull. Environ. Contam. Toxicol 2005, 75, 731–738. [Google Scholar]
- Swaddiwudhipong, W.; Mahasakpan, P.; Funkhiew, T.; Limpatanachote, P. Changes in cadmium exposure among persons living in cadmium-contaminated areas in northwestern Thailand: A five-year follow-up. J. Med. Assoc. Thai 2010, 93, 1217–1222. [Google Scholar]
- Thun, M.J.; Elinder, C.G.; Friberg, L. Scientific basis for an occupational standard for cadmium. Am. J. Ind. Med 1991, 20, 629–642. [Google Scholar]
- Boguszewska, A.; Pasternak, K. Cadmium-influence on biochemical processes of the human organism. Ann. Univ. Mariae. Curie. Sklodowska 2004, 59, 519–523. [Google Scholar]
- Koyu, A.; Gokcimen, A.; Ozguner, F.; Bayram, D.S.; Kocak, A. Evaluation of the effects of cadmium on rat liver. Mol. Cell Biochem 2006, 20, 1–5. [Google Scholar]
- Nordberg, G.; Jin, T.; Bernard, A.; Fierens, S.; Buchet, J.P.; Ye, T.; Kong, Q.; Wang, H. Low bone density and renal dysfunction following environmental cadmium exposure in China. Ambio 2002, 31, 478–481. [Google Scholar]
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, environmental exposure, and health outcomes. Environ. Health Perspect 2010, 118, 182–190. [Google Scholar]
- Schöpfer, J.; Drasch, G.; Schrauzer, G.N. Selenium and cadmium levels and ratios in prostates, livers, and kidneys of nonsmokers and smokers. Biol. Trace Elem. Res 2010, 134, 180–187. [Google Scholar]
- Wennberg, M.; Lundh, T.; Bergdahl, I.A.; Hallmans, G.; Jansson, J.H.; Stegmayr, B.; Custodio, H.M.; Skerfving, S. Time trends in burdens of cadmium, lead, and mercury in the population of northern Sweden. Environ. Res 2006, 100, 330–338. [Google Scholar]
- Fay, R.M.; Mumtaz, M.M. Development of a priority list of chemical mixture occurring at 1188 hazardous waste sites, using the HazDat database. Food Chem. Toxicol 1996, 34, 1163–1165. [Google Scholar]
- Benbrahim-Tallaa, L.; Tokar, E.J.; Diwan, B.A.; Dill, A.L.; Coppin, J.F.; Waalkes, M.P. Cadmium malignantly transforms normal human breast epithelial cells into a basal-like phenotype. Environ. Health Perspect 2009, 117, 147–152. [Google Scholar]
- Goyer, R.A.; Liu, J.; Waalkes, M.P. Cadmium and cancer of prostate and testis. Biometals 2004, 17, 555–558. [Google Scholar]
- Kriegel, A.M.; Soliman, A.S.; Zhang, Q.; El-Ghawalby, N.; Ezzat, F.; Soultan, A.; Abdel-Wahab, M.; Fathy, O.; Ebidi, G.; Bassiouni, N.; et al. Serum cadmium levels in pancreatic cancer patients from the East Nile Delta region of Egypt. Environ. Health Perspect 2006, 114, 113–119. [Google Scholar]
- Sorahan, T.; Esmen, N.A. Lung cancer mortality in UK nickel-cadmium battery workers, 1947–2000. Occup. Environ. Med 2004, 61, 108–116. [Google Scholar]
- Waalkes, M.P. Cadmium carcinogenesis. Mutat. Res 2003, 533, 107–120. [Google Scholar]
- Huff, J.; Lunn, R.M.; Waalkes, M.P.; Tomatis, L.; Infante, P.F. Cadmium-induced cancers in animals and in humans. Int. J. Occup. Environ. Health 2007, 13, 202–212. [Google Scholar]
- The International Agency for Research on Cancer (IARC), Beryllium, Cadmium, Mercury and Exposures in the Glass Manufacturing Industry; World Health Organization International Agency for Research on Cancer: Lyon, France, 1993.
- Joseph, P.; Lei, Y.X.; Whong, W.Z.; Ong, T.M. Oncogenic potential of mouse translation elongation factor-1 delta, a novel cadmium-responsive proto-oncogene. J. Biol. Chem 2002, 277, 6131–6136. [Google Scholar]
- Lei, Y.X.; Chen, J.K.; Wu, Z.L. Blocking the translation elongation factor-1δ with its antisense mRNA results in a significant reversal of its oncogenic potential. Teratog. Carcinog. Mutagen 2002, 22, 377–383. [Google Scholar]
- Thomas, R.S.; Bao, W.; Chu, T.M.; Bessarabova, M.; Nikolskaya, T.; Nikolsky, Y.; Andersen, M.E.; Wolfinger, R.D. Use of short-term transcriptional profiles to assess the long-term cancer-related safety of environmental and industrial chemicals. Toxicol. Sci 2009, 112, 311–321. [Google Scholar]
- Vogelstein, B.; Kinzler, R.W. The mulistep nature of cancer. Trends Genet 1993, 9, 138–141. [Google Scholar]
- Lee, T.; Pelletier, J. Eukaryotic initiation factor 4F: A vulnerability of tumor cells. Future Med. Chem 2012, 4, 19–31. [Google Scholar]
- Riis, B.; Rattan, S.I.; Clark, B.F.; Merrick, W.C. Eukaryotic translation elongation factors. Trends Biochem. Sci 1990, 15, 420–424. [Google Scholar]
- Sonenberg, N. Translation factors as effectors of cell growth and tumorigenesis. Curr. Opin. Cell Biol 1993, 5, 955–960. [Google Scholar]
- Chi, K.; Jones, D.V.; Frazier, M.L. Expression of an elongation factor 1 gamma-related sequence in adenocarcinomas of the colon. Gastroenterology 1992, 103, 98–102. [Google Scholar]
- Grant, A.G.; Flomen, R.M.; Tizard, M.L.; Grant, D.A. Differential screening of human pancreatic adenocarcinoma lambda gt11 expression library has identified increased transcription of elongation factor EF-1 alpha in tumor cells. Int. J. Cancer 1992, 50, 740–745. [Google Scholar]
- Khoury, T.; Alrawi, S.; Ramnath, N.; Li, Q.; Grimm, M.; Black, J.; Tan, D. Eukaryotic initiation factor-4E and cyclin D1 expression associated with patient survival in lung cancer. Clin. Lung Cancer 2009, 10, 58–66. [Google Scholar]
- Siebke, C.; James, T.C.; Cummins, R.; O’Grady, T.; Kay, E.; Bond, U. Phage display biopanning identifies the translation initiation and elongation factors (IF1α-3 and eIF-3) as components of Hsp70-peptide complexes in breast tumour cells. Cell Stress Chaperones 2012, 17, 145–156. [Google Scholar]
- Yoshizawa, A.; Fukuoka, J.; Shimizu, S.; Shilo, K.; Franks, T.J.; Hewitt, S.M.; Fujii, T.; Cordon-Cardo, C.; Jen, J.; Travis, W.D. Overexpression of phospho-eIF4E is associated with survival through AKT pathway in non-small cell lung cancer. Clin. Cancer Res 2010, 6, 240–248. [Google Scholar]
- Lei, Y.X.; Wang, M.; Wei, L.; Li, M.; Lin, H.Z. Alterative expression and sequence analysis of human elongation factor-1δ during malignant transformation of human bronchial epithelial cells induced by cadmium chloride. Biomed. Environ. Sci 2010, 23, 151–157. [Google Scholar]
- Ikeda, M.; Ohashi, F.; Fukui, Y.; Sakuragi, S.; Moriguchi, J. Closer correlation of cadmium in urine than that of cadmium in blood with tubular dysfunction markers in urine among general women populations in Japan. Int. Arch. Occup. Environ. Health 2011, 84, 121–129. [Google Scholar]
- Järup, L.; Akesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol 2009, 238, 201–208. [Google Scholar]
- Fowler, B.A. Monitoring of human populations for early markers of cadmium toxicity: A review. Toxicol. Appl. Pharmacol 2009, 238, 294–300. [Google Scholar]
- Menke, A.; Muntner, P.; Silbergeld, E.K.; Platz, E.A.; Guallar, E. Cadmium levels in urine and mortality among U.S. adults. Environ. Health Perspect 2009, 117, 190–196. [Google Scholar]
- Nawrot, T.S.; Staessen, J.A.; Roels, H.A.; Munters, E.; Cuypers, A.; Richart, T.; Ruttens, A.; Smeets, K.; Clijsters, H.; Vangronsveld, J. Cadmium exposure in the population: From health risks to strategies of prevention. Biometals 2010, 23, 769–782. [Google Scholar]
- Peters, J.L.; Perlstein, T.S.; Perry, M.J.; McNeely, E.; Weuve, J. Cadmium exposure in association with history of stroke and heart failure. Environ. Res 2010, 110, 199–206. [Google Scholar]
- Asagba, S.O.; Adaikpoh, M.A.; Kadiri, H.; Obi, F.O. Influence of aqueous extract of Hibiscus sabdariffa L. petal on cadmium toxicity in rats. Biol. Trace. Elem. Res 2007, 115, 47–57. [Google Scholar]
- Bernard, A.; Lauwerys, R. Effects of Cadmium Exposure in Humans. In Handbook of Experimental Pharmacology; Foulkes, E.C., Ed.; Springer-Verlag: Berlin, Germany, 1986. [Google Scholar]
- Brzóska, M.M.; Moniuszko-Jakoniuk, J.; Piłat-Marcinkiewicz, B.; Sawicki, B. Liver and kidney function and histology in rats exposed to cadmium and ethanol. Alcohol Alcohol 2003, 38, 2–10. [Google Scholar]
- Nogawa, K.; Kobayashi, E.; Honda, R.; Ishizaki, A.; Kawano, S.; Matsuda, H. Renal dysfunctions of inhabitants in a cadmium-polluted area. Environ. Res 1980, 23, 13–23. [Google Scholar]
- Tanimoto, A.; Hamada, T.; Koide, O. Cell death and regeneration of renaproximal tubular cells in rats with subchronic cadmium intoxication. Toxicol. Pathol 1993, 21, 341–352. [Google Scholar]
- Zhou, Y.; Vaidya, V.S.; Brown, R.P.; Zhang, J.; Rosenzweig, B.A.; Thompson, K.L.; Miller, T.J.; Bonventre, J.V.; Goering, P.L. Comparison of kidney injury molecule-1 and other nephrotoxicity biomarkers in urine and kidney following acute exposure to gentamicin, mercury, and chromium. Toxicol. Sci 2008, 101, 159–170. [Google Scholar]
- Lamberti, A.; Caraglia, M.; Longo, O.; Marra, M.; Abbruzzese, A.; Arcari, P. The translation elongation factor 1A in tumorigenesis, signal transduction and apoptosis. Amino Acids 2004, 26, 443–448. [Google Scholar]
- Caraglia, M.; Budillon, A.; Vitale, G.; Lupoli, G.; Tagliaferro, P.; Abruzzese, A. Modulation of molecular mechanisms involved in protein synthesis machinery as a new tool for control of cell proliferation. Eur. J. Biochem 2000, 267, 3919–3936. [Google Scholar]
- Silvera, D.; Formenti, S.C.; Schneider, R.J. Translational control in cancer. Nat. Rev. Cancer 2010, 10, 254–266. [Google Scholar]
- Anand, N.; Murthy, S.; Amann, G.; Wernick, M.; Porter, L.A.; Cukier, I.H.; Collins, C.; Gray, J.W.; Diebold, J.; Demetrick, D.J.; Lee, J.M. Protein elongation factor EEF1A2 is a putative oncogene in ovarian cancer. Nat. Genet 2002, 31, 301–305. [Google Scholar]
- Edmonds, B.T.; Wyckoff, J.; Yeung, Y.G.; Wang, Y.; Stanley, E.R.; Jones, J.; Segall, J.; Condeelis, J. Elongation factor-1 alpha is an overexpressed actin binding protein in metastatic rat mammary adenocarcinoma. J. Cell Sci 1996, 109, 2705–2714. [Google Scholar]
- Joseph, P.; Lei, Y.X.; Ong, T.M. Up-regulation of expression of translation factors—A novel molecular mechanism for cadmium carcinogenesis. Mol. Cell Biochem 2004, 255, 93–101. [Google Scholar]
- Kawata, K.; Shimazaki, R.; Okabe, S. Comparison of gene expression profiles in HepG2 cells exposed to arsenic, cadmium, nickel, and three model carcinogens for investigating the mechanisms of metal carcinogenesis. Environ. Mol. Mutagen 2009, 50, 46–59. [Google Scholar]
- Waisberg, M.; Joseph, P.; Hale, B.; Beyersmann, D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology 2003, 192, 95–117. [Google Scholar]
- Zhou, Z.H.; Lei, Y.X.; Wang, C.X. Analysis of aberrant methylation in DNA repair genes during malignant transformation of human bronchial epithelial cells induced by cadmium. Toxicol. Sci.




| Primer | Sequence | Length |
|---|---|---|
| RT-PCR | ||
| Forward | 5′-ACC AGC AGA GGA CGA TGA-3′ | 227 bp |
| Reverse | 5′-AAT TGG ATG GAA CGC ACA-3′ | |
| FQ-PCR | ||
| Forward | 5′-GGAGCGGCTACGGCAGTAC-3′ | 90 bp |
| Reverse | 5′-TCCCAAGGCTTGACATCCA-3′ | |
| Probe | 5′-CACTGGTGGCCAAGTCCTCCATCCTAMARA-3′ |
| Groups | Liver (%) | Kidney (%) | Heart (%) | Lung (%) |
|---|---|---|---|---|
| High dose | 5.368 ± 0.203 *,# | 0.921 ± 0.023 *,# | 0.428 ± 0.015 * | 0.625 ± 0.018 *,# |
| Mid-dose | 4.721 ± 0.181 * | 0.898 ± 0.028 * | 0.414 ± 0.012 * | 0.576 ± 0.014 * |
| Low dose | 4.542 ± 0.154 *,Δ | 0.828 ± 0.020 *,Δ | 0.397 ± 0.011 * | 0.529 ± 0.016 *,Δ |
| Control | 3.950 ± 0.127 #,Δ | 0.706 ± 0.015 #,Δ | 0.349 ± 0.008 #,Δ | 0.457 ± 0.014 #,Δ |
| Groups | Blood (μg/L) | Urine (μg/L.Cr) |
|---|---|---|
| High dose | 313.25 ± 28.81 *,# | 38.31 ± 3.78 *,# |
| Mid-dose | 210.72 ± 11.64 *,#,Δ | 17.95 ± 1.50 *,#,Δ |
| Low dose | 128.63 ± 8.71 *,Δ | 6.49 ± 0.53 *,Δ |
| Control | 0.50 ± 0.04 #,Δ | 0.65 ± 0.06 #,Δ |
| Groups | Liver (μg/g) | Kidney (μg/g) | Heart (μg/g) | Lung (μg/g) |
|---|---|---|---|---|
| High dose | 133.79 ± 44.36 *,# | 141.34 ± 30.87 *,# | 5.51 ± 1.66 *,# | 3.52 ± 1.48 *,# |
| Mid-dose | 66.99 ± 30.26 *,#,Δ | 70.02 ± 16.12 *,#,Δ | 2.30 ± 0.87 *,#Δ | 1.94 ± 0.42 *,#,Δ |
| Low dose | 39.15 ± 16.95 *,Δ | 42.29 ± 14.03 *,Δ | 1.02 ± 0.31 *,Δ | 1.36 ± 0.54 *,Δ |
| Control | 0.04 ± 0.00 #,Δ | 0.22 ± 0.02 #,Δ | 0.11 ± 0.02 #,Δ | 0.20 ± 0.02 #,Δ |
| Groups | Blood | Liver | Kidney | Heart | Lung |
|---|---|---|---|---|---|
| High dose | 152.87 ± 121.87 *,# | 3.00 ± 2.70 *,# | 1.27 ± 0.70 *,# | 1.00 ± 0.44 *,# | 0.82 ± 0.27 *,# |
| Mid-dose | 27.80 ± 7.283 *,#,Δ | 0.50 ± 0.31 *,#,Δ | 0.43 ± 0.23 *,#,Δ | 0.40 ± 0.18 *,Δ | 0.40 ± 0.13 *,#,Δ |
| Low dose | 16.50 ± 3.393 *,Δ | 0.14 ± 0.03 *,Δ | 0.13 ± 0.08 *,Δ | 0.24 ± 0.08 *,Δ | 0.24 ± 0.05 *,Δ |
| Control | 6.49 ± 3.357 #,Δ | 0.04 ± 0.02 #,Δ | 0.01 ± 0.01 #,Δ | 0.08 ± 0.04 #,Δ | 0.10 ± 0.06 #,Δ |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lu, Q.; Lei, Y.-X.; He, C.-C.; Lei, Z.-N. Blood Translation Elongation Factor-1δ Is a Novel Marker for Cadmium Exposure. Int. J. Mol. Sci. 2013, 14, 5182-5197. https://doi.org/10.3390/ijms14035182
Lu Q, Lei Y-X, He C-C, Lei Z-N. Blood Translation Elongation Factor-1δ Is a Novel Marker for Cadmium Exposure. International Journal of Molecular Sciences. 2013; 14(3):5182-5197. https://doi.org/10.3390/ijms14035182
Chicago/Turabian StyleLu, Qian, Yi-Xiong Lei, Chao-Cai He, and Zi-Ning Lei. 2013. "Blood Translation Elongation Factor-1δ Is a Novel Marker for Cadmium Exposure" International Journal of Molecular Sciences 14, no. 3: 5182-5197. https://doi.org/10.3390/ijms14035182




