Tandem Aldol-Michael Reactions in Aqueous Diethylamine Medium: A Greener and Efficient Approach to Bis-Pyrimidine Derivatives
Abstract
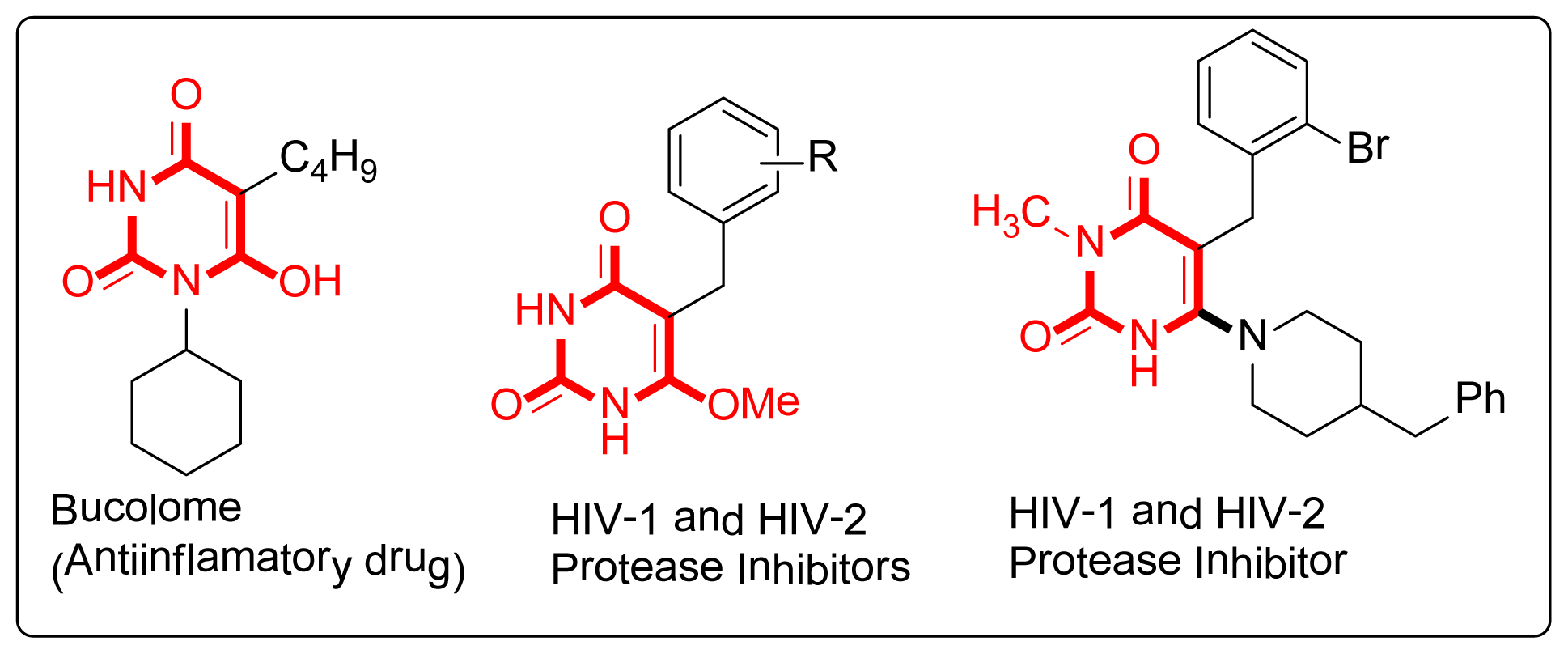
:1. Introduction
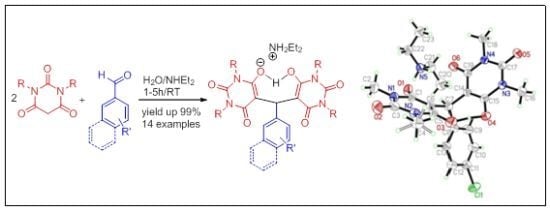
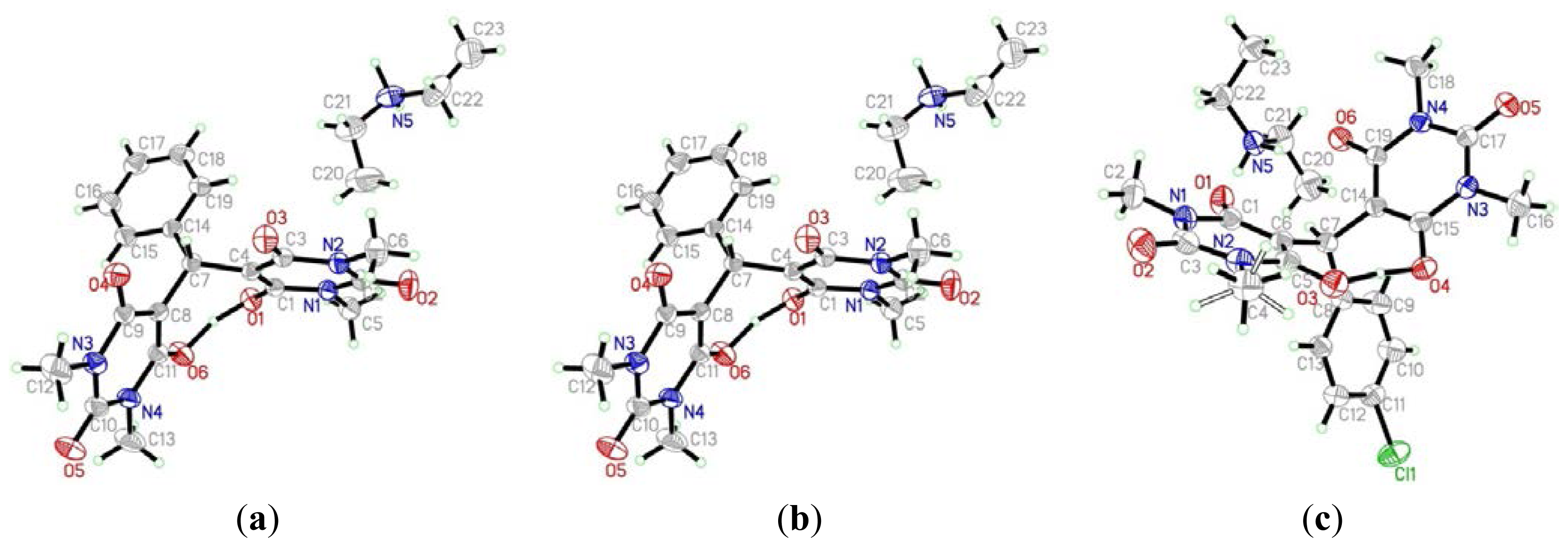
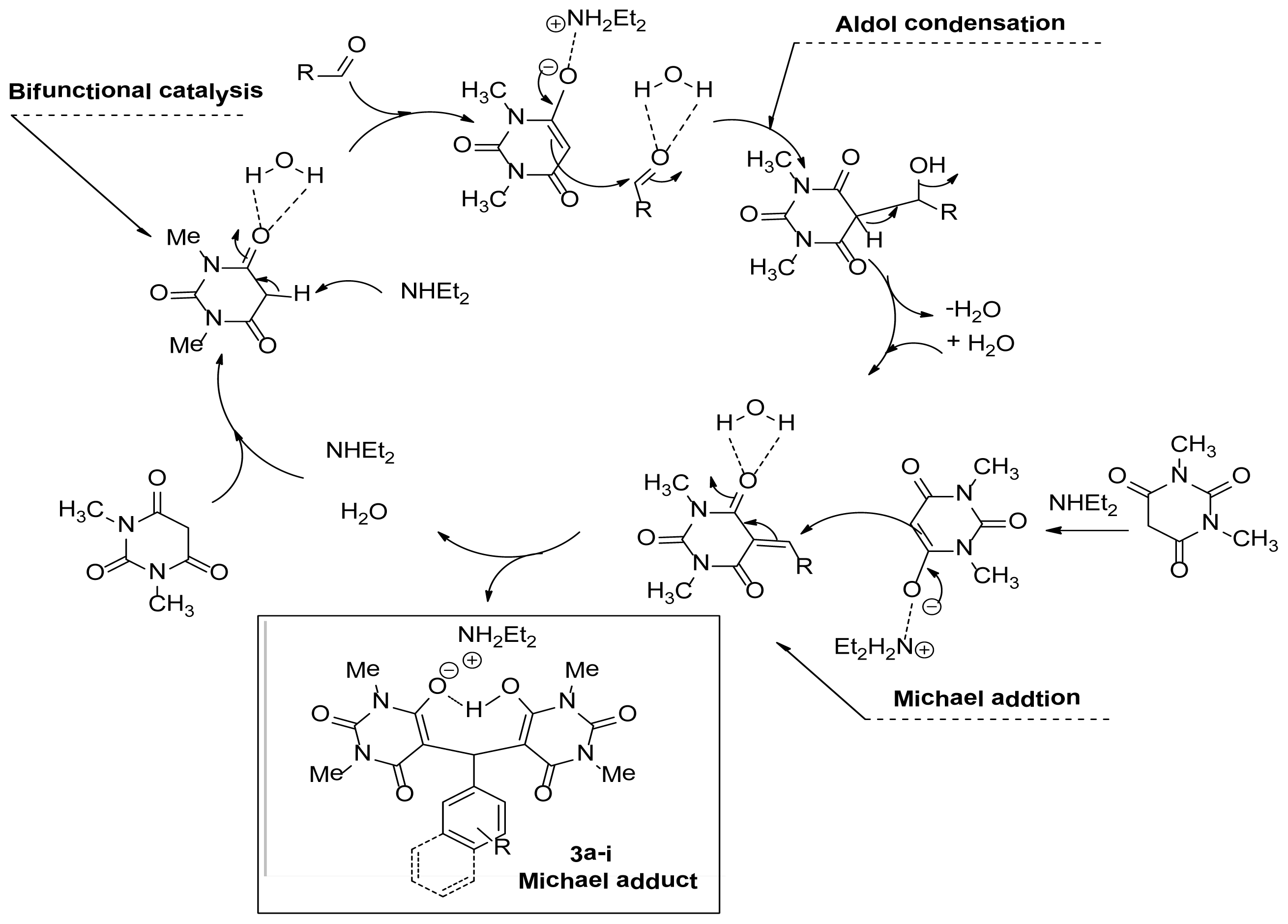
2. Results and Discussion
3. Experimental Section
3.1. General Procedure for Aldol Condensation Michael Addition for the Synthesis of 3a–i and 4a–e (GP1)
3.1.1. 5,5′-(Phenylmethylene)bis(1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione) Diethylaminium Salt 3a
3.1.2. 5,5′-(p-Tolylmethylene)bis(1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione) Diethylaminium Salt 3b
3.1.3. 5,5′-((4-Chlorophenyl)methylene)bis(1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione) Diethylaminium Salt 3c
3.1.4. 5,5′-((4-Bromophenyl)methylene)bis(1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione) Diethylaminium Salt 3d
3.1.5. 5,5′-((3-Bromophenyl)methylene)bis(1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione) Diethylaminium Salt 3e
3.1.6. 5,5′-((4-Methoxyphenyl)methylene)bis(1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione) Diethylaminium Salt 3f
3.1.7. 5,5′-((4-Nitrophenyl)methylene)bis(1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione) Diethylaminium Salt 3g
3.1.8. 5,5′-(3-Tolylmethylene)bis(1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione) Diethylaminium Salt 3h
3.1.9. 5,5′-(Naphthalen-2-ylmethylene)bis(1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione) Diethylaminium Salt 3i
3.1.10. 5,5′-(Phenylmethylene)bis(6-hydroxypyrimidine-2,4(1H,3H)-dione) Diethylaminium Salt 4a
3.1.11. 5,5′-(p-Tolylmethylene)bis(6-hydroxypyrimidine-2,4(1H,3H)-dione) Diethylaminium Salt 4b
3.1.12. 5,5′-((4-Chlorophenyl)methylene)bis(6-hydroxypyrimidine-2,4(1H,3H)-dione) Diethylaminium Salt 4c
3.1.13. 5,5′-((4-Methoxyphenyl)methylene)bis(6-hydroxypyrimidine-2,4(1H,3H)-dione) Diethylaminium Salt 4d
3.1.14. 5,5′-(Naphthalen-2-ylmethylene)bis(6-hydroxypyrimidine-2,4(1H,3H)-dione) Diethylaminium Salt 4e
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bojarski, J.T.; Mokrocz, J.L.; Barton, H.J.; Paluchowska, M.H. Recent progress in barbituric acid chemistry. In Adv. Heterocycl. Chem; 1985; Volume 38, pp. 229–297. [Google Scholar]
- Undheim, K.; Bennecke, T.; Katritzky, A.R.; Rees, C.W.; Scriven, E.F.V.; Boulton, A.J. (Hrsg.); Comprehensive Heterocyclic Chemistry; Elsevier Pergamon: Oxford, UK, 1996; Volume 6, Suppl 93. [Google Scholar]
- Von Angerer, S. Product class 12: Pyrimidines. Sci. Synth 2004, 16, 379–572. [Google Scholar]
- Sans, S.R.G.; Chosaz, M.G. Historical aspects and applications of barbituric acid derivatives. Pharmazie 1988, 43, 827–829. [Google Scholar]
- Taylor, J.B. Modern Medical Chemistry; Prentice Hall: New York, NY, USA, 1994. [Google Scholar]
- Guerin, D.J.; Mazeas, D.; Musale, M.S.; Naguib, F.N.M.; Safarjalani, O.N.A.; Kouni, M.H.; Panzica, R.P. Uridine phosphorylase inhibitors: Chemical modification of benzyloxybenzyl barbituric acid and its effects on UrdPase inhibition. Bioorg. Med. Chem. Lett 1999, 9, 1477–1480. [Google Scholar]
- Andrews, G. Medical Pharmacology; The CV Mosby Co: St. Louis, MO, USA, 1976; pp. 243–250. [Google Scholar]
- Foye, W.O. Principles of Medicinal Chemistry; Lea & Febiger: Pennsylvania, PA, USA, 1989; pp. 143–237. [Google Scholar]
- Goodman, L.S.; Gilman, A. The Pharmacological Basis of Therapeutics; Mc Graw-Hill: New Delhi, India, 1991; pp. 358–360. [Google Scholar]
- Fisher, E.; von Mering, J. Ueber eine neue Klasse von Schlafmitteln. Ther. Ggw 1903, 44, 97–105. [Google Scholar]
- Doran, W.J. Barbituric acid hypnotics. Med. Chem 1959, 4, 164–167. [Google Scholar]
- Bobranski, B. Progress in chemistry of barbituric acid. In Wiad. Chem; 1977; Volume 31, pp. 231–278. [Google Scholar]
- Senda, S.; Izumi, H.; Fujimura, H. Uracil derivatives and related compounds. VI. Derivatives of 5-alkyl-2,4,6-trioxoperhydropyrimidine as anti-inflammatory agents. Arzneim. Forsch 1967, 17, 1519–1523. [Google Scholar]
- Anderson, G.L.; Shim, J.L.; Brown, A.D. Pyrido[2,3-d]pyrimidines. IV. Synthetic studies leading to various oxopyrido[2,3-d]pyrimidines. J. Org. Chem 1976, 41, 1095–1099. [Google Scholar]
- Grivaky, E.M.; Lee, S.; Siyal, C.W.; Duch, D.S.; Nichol, C.A. Synthesis and antitumor activity of 2,4-diamino-6-(2,5-dimethoxybenzyl)-5-methylpyrido[2,3-d]pyrimidine. J. Med. Chem 1980, 23, 327–329. [Google Scholar]
- Gawande, M.B.; Bonifacio, V.D.B.; Luque, R.; Branco, P.S.; Varma, R.S. Benign by design: Catalyst-free in-water, on-water green chemical methodologies in organic synthesis. Chem. Soc. Rev 2013, 42, 5522–5551. [Google Scholar]
- Ho, T.L. Tandem Organic Reactions; Wiley: New York, NY, USA, 1992. [Google Scholar]
- Nicolaou, K.C.; Yue, E.W.; Oshima, T. New Roads to Molecular Complexity. In The New Chemistry; Hall, N., Ed.; Cambridge University Press: Cambridge, UK, 2001; pp. 168–198. [Google Scholar]
- Tietze, L.F.; Hautner, F. Domino Reaction in Organic Synthesis. An Approach to Efficiency, Elegance, Ecological Benefit, Economic Advantage, and Preservation of Our Resources in Chemical Transformation. In Stimulating Concepts in Chemistry; Vögtle, F., Stoddart, J., Shibasaki, F.M., Eds.; Wiley-VCH: Weinheim, Germany, 2000; pp. 38–64. [Google Scholar]
- Tietze, L.F.; Beifuss, U. Sequential transformations in organic chemistry: A synthetic strategy with a future. Angew. Chem. Int. Ed. Engl 1993, 32, 131–163. [Google Scholar]
- Tietze, L.F. Domino reactions in organic synthesis. Chem. Rev 1996, 96, 115–136. [Google Scholar]
- Thompson, L.A. Recent applications of polymer-supported reagents and scavengers in combinatorial, parallel, or multistep synthesis. Curr. Opin. Chem. Biol 2000, 4, 324–337. [Google Scholar]
- Nefzi, A.; Ostresh, J.M.; Houghten, R.A. The current status of heterocyclic combinatorial libraries. Chem. Rev 1997, 97, 449–472. [Google Scholar]
- Yanovskaya, L.A.; Dombrovsky, V.A.; Khusid, A. Cyclopropanes with Functional Groups. Synthesis and Application; Nauka: Moscow, Russia, 1980. [Google Scholar]
- Tsuji, T.; Nishida, S. The Chemistry of the Cyclopropyl Group; Wiley and Sons: New York, NY, USA, 1987. [Google Scholar]
- Boche, G.; Walbirsky, H.M. Cyclopropane Derived Intermediates; John Wiley and Sons: New York, NY, USA, 1990. [Google Scholar]
- Rappoport, Z. The Chemistry of the Cyclopropyl Group; Wiley and Sons: New York, NY, USA, 1996. [Google Scholar]
- Posner, G.H. Multicomponent one-pot annulations forming 3 to 6 bonds. Chem. Rev 1986, 86, 831–834. [Google Scholar]
- Bunce, R.A. Recent advances in the use of tandem reactions for organic synthesis. Tetrahedron 1995, 48, 13103–13159. [Google Scholar]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Gruttadauria, M.; Giacalone, F.; Marculesco, A.M.; Meo, P.L.; Riela, S.; Noto, R. Hydrophobically directed Aldol reactions: Polystyrene-supported l-proline asa recyclable catalyst for direct asymmetric Aldol reactions in the presence of water. Eur. J. Org. Chem 2007, 4688–4698. [Google Scholar]
- Breslow, R. Determining the geometries of transition states by use of antihydrophobic additives in water. Acc. Chem. Res 2004, 37, 471–478. [Google Scholar]
- Blackmond, D.G.; Armstrong, A.; Coombe, V.; Wells, A. Water in organocatalytic processes: Debunking the myths. Angew. Chem. Int. Ed. Engl 2007, 46, 3798–3800. [Google Scholar]
- Abaee, M.S.; Cheraghi, S.; Navidipoor, S.; Mojtahedi, M.M.; Forghani, S. An efficient tandem aldol condensation-thia-Michael addition process. Tetrahedron Lett 2012, 53, 4405–4408. [Google Scholar]
- Barakat, A.; Al-Majid, A.A.; Shahidul Islam, M.; Al-Othman, Z.A. Highly enantioselective Friedel–Crafts alkylations of indoles with α,β-unsaturated ketones under Cu(II)-simple oxazoline-imidazoline catalysts. Tetrahedron 2013, 69, 5185–5192. [Google Scholar]
- Jursic, B.S. Preparation of unsubstituted dipyridine-dibarbituric acid ylide through dimerization of pyridin-2-ylmethylenepyrimidinetrione as a reactive intermediate. J. Heterocyclic Chem 2003, 40, 167–170. [Google Scholar]
- Jursic, B.S.; Neumann, D.M. Preparation of 5,5′-pyrilidene and 5,5′-quinolidene bis-barbituric acid derivatives. J. Heterocycl. Chem 2003, 40, 465–474. [Google Scholar]
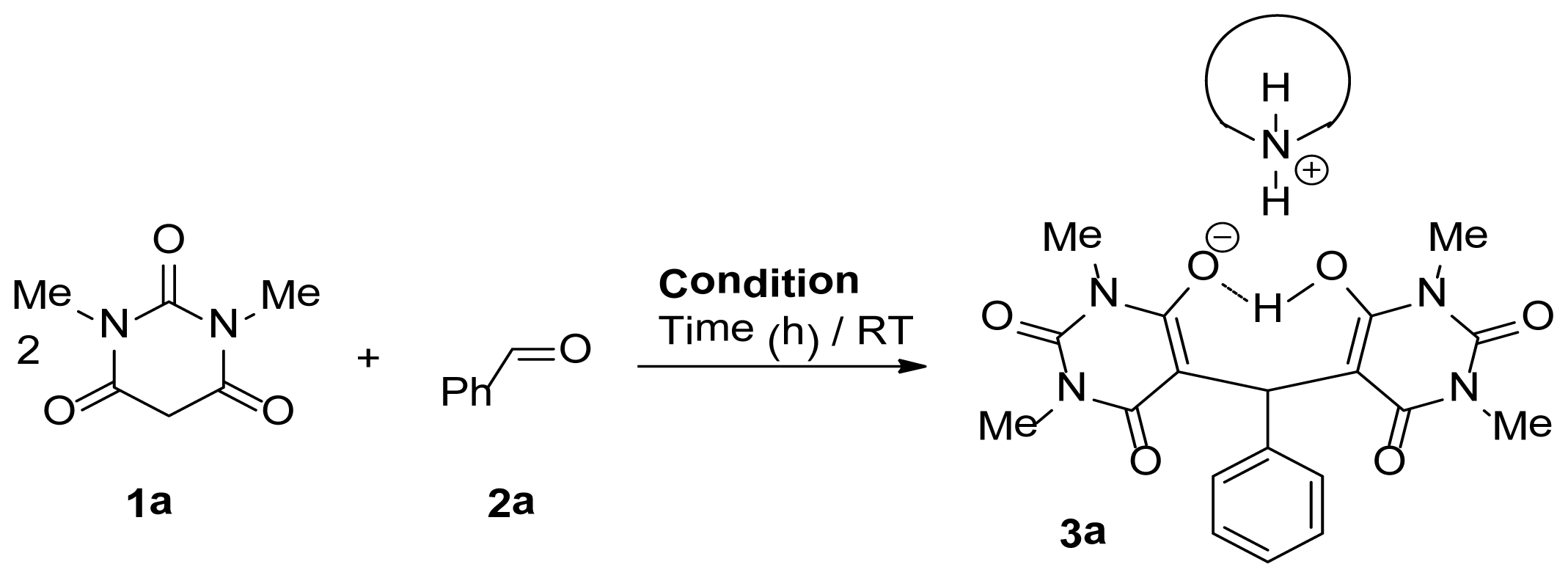 | |||
|---|---|---|---|
| Entry | Condition | Time (h) | Yield (%) b |
| 1 | Et2NH/H2O | 1 | 99 |
| 2 | iPr2NH/H2O | 5 | 85 |
| 3 | (Cyclohexyl)2NH/H2O | 4 | 82 |
| 4 | Morpholine/H2O | 3 | 78 |
| 5 | NaOH/H2O | 8 | 65 |
| 6 | Et2NH | 12 | 10 |
| 7 | H2O | 12 | 0 |
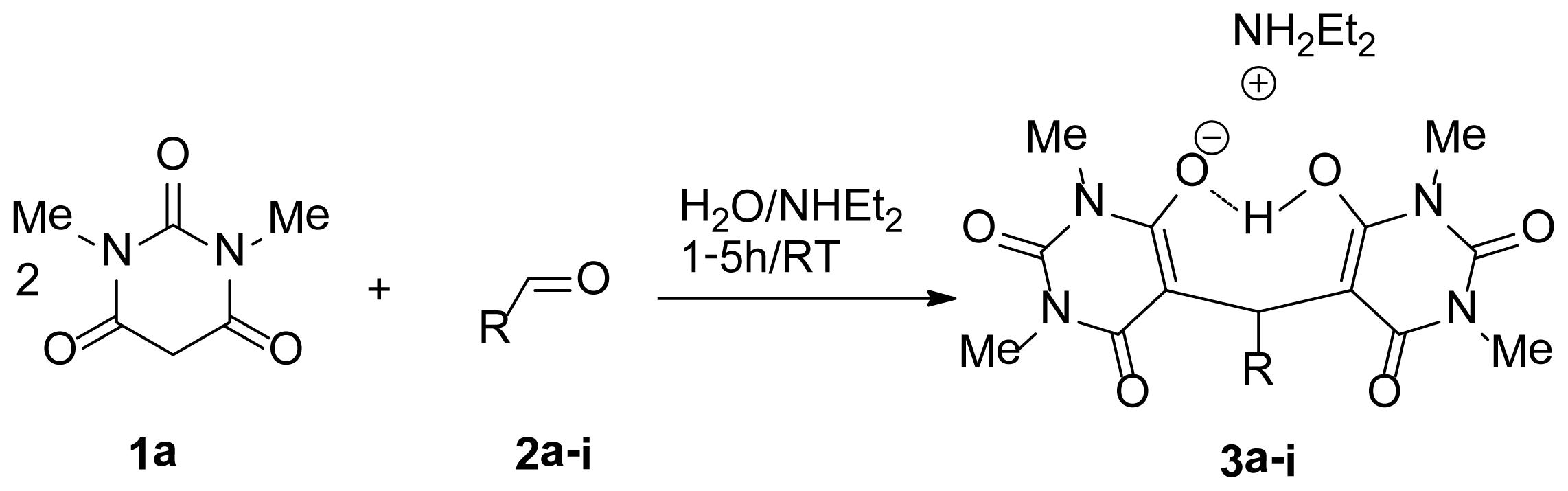 | |||
|---|---|---|---|
| Entry | 3 | R | Yield (%) b |
| 1 | 3a | Ph | 99 |
| 2 | 3b | p-CH3Ph | 97 |
| 3 | 3c | p-ClPh | 95 |
| 4 | 3d | p-BrPh | 92 |
| 5 | 3e | m-BrPh | 92 |
| 6 | 3f | p-CH3OPh | 90 |
| 7 | 3g | p-NO2Ph | 88 |
| 8 | 3h | m-CH3Ph | 92 |
| 9 | 3i | 2-Naphthaldehyde | 94 |
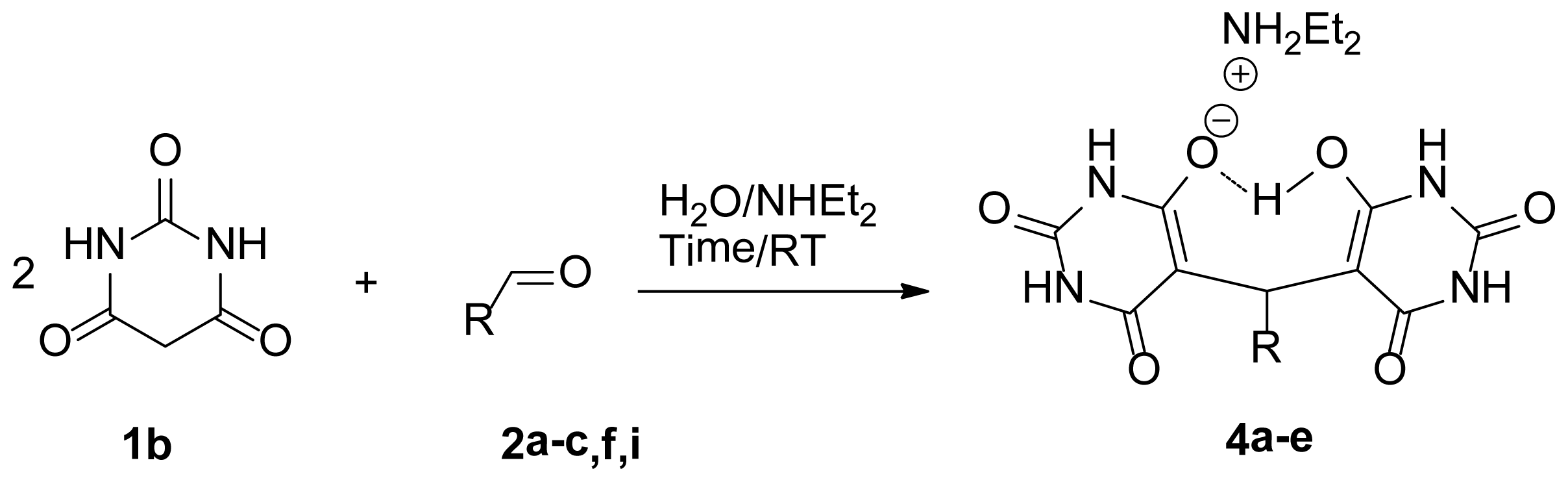 | |||
|---|---|---|---|
| Entry | 4 | R | Yield (%) b |
| 1 | 4a | Ph | 98 |
| 2 | 4b | p-CH3Ph | 95 |
| 3 | 4c | p-ClPh | 95 |
| 4 | 4d | p-CH3OPh | 91 |
| 5 | 4e | 2-Naphthaldehyde | 93 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Al-Majid, A.M.; Barakat, A.; AL-Najjar, H.J.; Mabkhot, Y.N.; Ghabbour, H.A.; Fun, H.-K. Tandem Aldol-Michael Reactions in Aqueous Diethylamine Medium: A Greener and Efficient Approach to Bis-Pyrimidine Derivatives. Int. J. Mol. Sci. 2013, 14, 23762-23773. https://doi.org/10.3390/ijms141223762
Al-Majid AM, Barakat A, AL-Najjar HJ, Mabkhot YN, Ghabbour HA, Fun H-K. Tandem Aldol-Michael Reactions in Aqueous Diethylamine Medium: A Greener and Efficient Approach to Bis-Pyrimidine Derivatives. International Journal of Molecular Sciences. 2013; 14(12):23762-23773. https://doi.org/10.3390/ijms141223762
Chicago/Turabian StyleAl-Majid, Abdullah M., Assem Barakat, Hany J. AL-Najjar, Yahia N. Mabkhot, Hazem A. Ghabbour, and Hoong-Kun Fun. 2013. "Tandem Aldol-Michael Reactions in Aqueous Diethylamine Medium: A Greener and Efficient Approach to Bis-Pyrimidine Derivatives" International Journal of Molecular Sciences 14, no. 12: 23762-23773. https://doi.org/10.3390/ijms141223762
APA StyleAl-Majid, A. M., Barakat, A., AL-Najjar, H. J., Mabkhot, Y. N., Ghabbour, H. A., & Fun, H.-K. (2013). Tandem Aldol-Michael Reactions in Aqueous Diethylamine Medium: A Greener and Efficient Approach to Bis-Pyrimidine Derivatives. International Journal of Molecular Sciences, 14(12), 23762-23773. https://doi.org/10.3390/ijms141223762