Occurrence of Aflatoxins in Selected Processed Foods from Pakistan
Abstract
:1. Introduction
2. Results and Discussion
2.1. Repeatability and Reproducibility
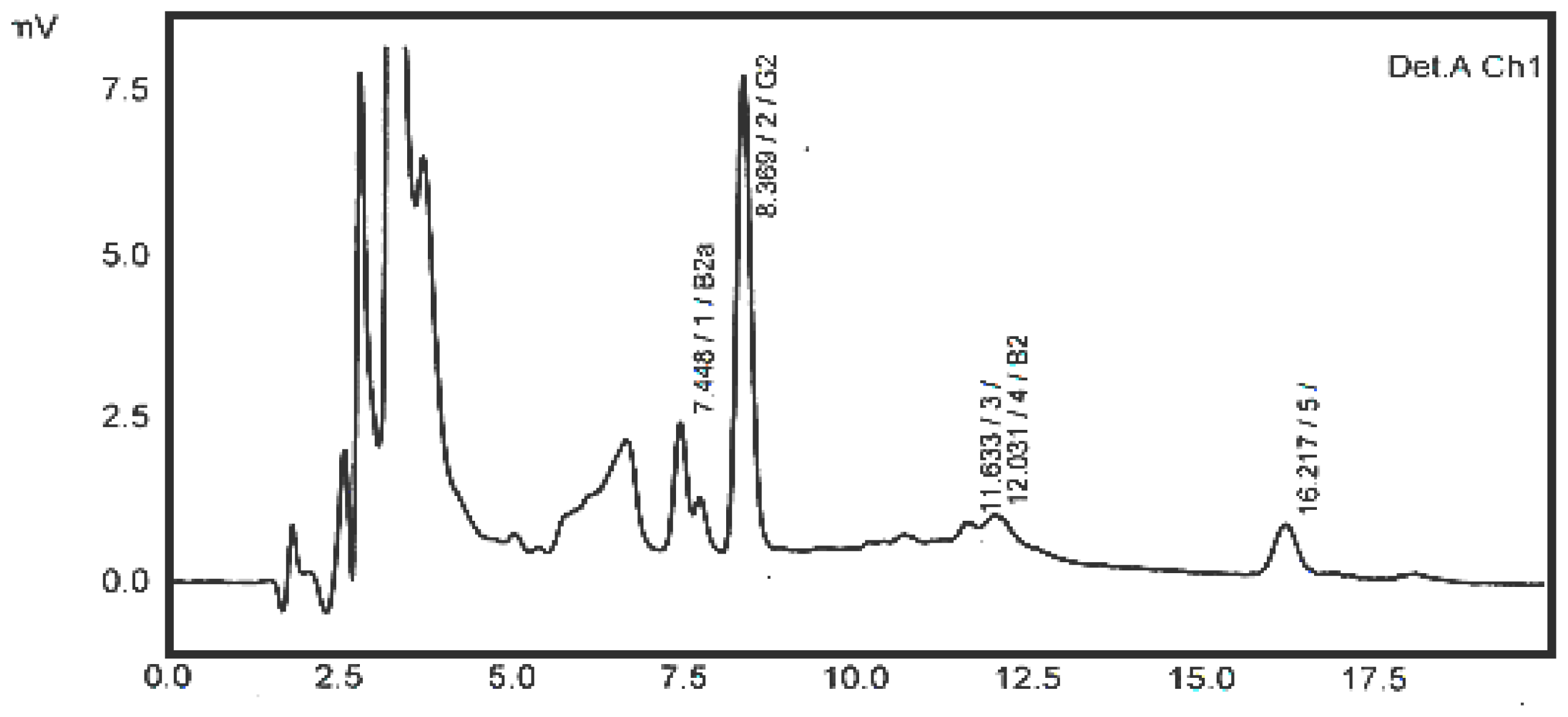
2.2. Sample Analysis
2.3. Cerelac Infant Food Samples
2.4. Noodles
2.5. Baby Powder Milk
2.6. Cream of Rice
2.7. Biscuits
2.8. Wheat Samples
2.9. Chines Fried Rice
2.10. Milk Powder (Tea)
2.11. Gram Flour
2.12. Barian
2.13. Peanuts (Nimko)
3. Experimental Section
3.1. Collection of Samples
3.2. Chemicals and Materials
3.3. Extraction of Aflatoxins
3.4. Clean-Up
3.5. Derivatization
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Ito, Y.; Peterson, S.W.; Wicklow, D.T.; Goto, T. Aspergillus pseudotamarii, a new aflatoxin producing species in Aspergillus section. Flavi. Mycol. Res 2001, 105, 233–239. [Google Scholar]
- Turner, N.W.; Sreenath, S.; Piletsky, S.A. Analytical methods for determination of mycotoxins: A review. Anal. Chim. Act 2009, 632, 168–180. [Google Scholar]
- Strosnider, H.; Azziz-Baumgartner, E.; Banziger, M.; Bhat, R.V.; Breiman, R.; Brune, M.; DeCock, K.; Dilley, A.; Groopman, J.; Hell, K.; et al. Workgroup report: Public health strategies for reducing aflatoxin exposure in developing countries. Environ. Health Perspect 2006, 114, 1989–1903. [Google Scholar]
- Pildain, M.B.; Frisvad, J.C.; Vaamonde, G.; Cabral, D.; Varga, J.; Samson, R.A. Two novel aflatoxin-producing Aspergillus species from Argentinean peanuts. Int. J. Syst. Evol. Microbiol 2008, 58, 725–735. [Google Scholar]
- IARC monographs International Agency for Research on Cancer (IARC), Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organization, International Agency for Research on Cancer: Lyon, France, 2002; Volume 82, pp. 1–556.
- Groopman, J.D.; Kensler, T.W.; Wild, C.P. Protective interventions to prevent aflatoxin-induced carcinogenesis in developing countries. Ann. Rev. Public Health 2008, 29, 187–203. [Google Scholar]
- Park, D.L.; Trucksess, M.W.; Nesheim, S.; Stack, M.E.; Newell, R.F. Solvent-efficient thin-layer chromatographic method for the determination of aflatoxins B1, B2, G1, and G2 in corn and peanut products: Collaborative study. J. Assoc. Off. Anal. Chem 1994, 77, 637–646. [Google Scholar]
- Yazdanpanah, H.; Mohammadi, T.; Abouhossain, G.; Cheraghali, A.M. Effect of roasting on degradation of aflatoxins in contaminated pistachio nuts. Food. Chem. Toxicol 2005, 43, 1135–1139. [Google Scholar]
- Sergent, T.; Ribonnet, L.; Kolosova, A.; Garsou, S.; Schaut, A.; de Saeger, S.; van Peteghem, C.; Larondelle, Y.; Pussemier, L.; Schneider, Y.J. Molecular and cellular effects of food contaminants and secondary plant components and their plausible interactions at the intestinal level. Food Chem. Toxicol 2008, 46, 813–841. [Google Scholar]
- European Commission. Commission regulation (EC) No. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 1, 5–24.
- Bankole, S.A.; Adebanjo, A. Mycotoxins in food in West Africa: Current situation and possibilities of controlling it: Review. Afr. J. Biotechnol 2003, 2, 254–263. [Google Scholar]
- Hu, Y.Y.; Zheng, P.; Zhang, Z.X.; He, Y.Z. Determination of aflatoxins in high-pigment contents samples by matrix solid-phase dispersion and high-performance liquid chromatography. J. Agric. Food Chem 2006, 54, 4126–4130. [Google Scholar]
- Nilu, F.D.; Boyaciog, L.D. Comparative study of three different methods for the determination of aflatoxins in Tahini. J. Agric. Food Chem 2002, 50, 3375–3379. [Google Scholar]
- Horwitz, W. Chapter 49: Natural Toxins. In Official Methods of Analysis of AOAC International, 17th ed; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Sheibani, A.; Ghaziaskar, H. Pressurized fluid extraction for quantitative recovery of aflatoxins B1 and B2 from pistachio. Food Control 2009, 20, 124–128. [Google Scholar]
- Wang, Y.; Chai, T.; Lu, G.; Quan, C.; Duan, H.; Yao, M.; Zucker, B.A.; Schlenker, G. Simultaneous detection of airborne aflatoxin, ochratoxin and zearalenone in a poultry house by immunoaffinity clean-up and high-performance liquid chromatography. Environ. Res 2008, 107, 139–144. [Google Scholar]
- Beltran, E.M.I.; Yosa, V.; Felix, H. UHPLC-MS/MS highly sensitive determination of aflatoxins, the aflatoxin metabolite M1 and ochratoxin A in baby food and milk. Food Chem 2011, 126, 737–744. [Google Scholar]
- Sizoo, E.A.; Egmond, V. Analysis of duplicate 24-hour diet samples for aflatoxin B1, aflatoxin M1 and ochratoxin A. Food Addit. Contam 2005, 22, 163–172. [Google Scholar]
- Douglas, H. Noodle. Online Etymology Dictionary. Retrieved 14-10-2009. Available online: http://www.etymonline.com/index.php accessed on 15 May 2011.
- Seta, E.; Erkmen, O. The aflatoxin contamination of ground red pepper and pistachio nuts sold in Turkey. Food Chem. Toxicol 2010, 48, 2532–2537. [Google Scholar]
- Sirhan, A.Y.; Tan, G.H.; Wong, R.C.S. Method validation in the determination of aflatoxins in noodle samples using the QuEChERS method and high performance liquid chromatography coupled to a fluorescence detector (HPLC-FLD). Food Control 2011, 22, 1839–1843. [Google Scholar]
- Isadora, B.S. FDA Consumer Magazine. 1996. Available online: http://www.fda.gov/fdac/features/596_baby.html accessed on 15 May 2011.
- Fomon, S.J. Infant feeding in the 20th century: Formula and beikost. J. Nutr 2001, 131, 409S–420S. [Google Scholar]
- Ryan, A. The Resurgence of Breastfeeding in the United States. Pediatrics. 1997, 99, pp. 4–12. Available online: http://pediatrics.aappublications.org accessed on 25 May 2008.
- Bullerman, L.B; Bianchini, A. Stability of mycotoxins during food processing. Int. J. Food Microbiol 2007, 119, 140–146. [Google Scholar]
- Giray, B.; Girgin, G.; Basak-Engin, A.; Aydin, S.; Shahin, G. Aflatoxin levels in wheat samples consumed in some regions of Turkey. Food Control 2007, 18, 23–29. [Google Scholar]
- Zinedine, J.; Molto, C.A.J.; Idrissi, C.L.; Manes, J. Aflatoxins levels in dried fruits and nuts from Rabat-Sale area, Morocco. Food Control 2008, 19, 849–854. [Google Scholar]
- Reiter, E.V.; Vouk, F.; Bohm, J.; Razzazi-Fazeli, E. Aflatoxins in rice—A limited survey of products marketed in Austria. Food Control 2010, 21, 988–991. [Google Scholar]
- Codex Alimentarius Commission. Presented at Joint FAO/WHO Food Standards Program. Thirty sixth session, Rotterdam, the Netherlands, 22–26 March 2004.
- Chen, C.Y.; Li, W.J.; Peng, K.Y. Determination of aflatoxin M1 in milk and milk powder using high-flow solid-phase extraction and liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem 2005, 53, 8474–8480. [Google Scholar]
- Ozdemir, M. Determination of aflatoxin M1 levels in goat milk consumed in Kilis province. Ankara Univ. Vet. Fak. Derg 2007, 54, 99–103. [Google Scholar]
- Herzallah, S.H. Determination of aflatoxins in eggs, milk, meat and meat products using HPLC fluorescent and UV detectors. Food Chem 2009, 114, 1141–1146. [Google Scholar]
- Thompson, C.; Henke, S.E. Effect of climate and type of storage container on aflatoxin production in corn and its associated risks to wildlife species. J. Wildl. Dis 2000, 36, 172–179. [Google Scholar]
- Villa, P.; Markaki, P. Aflatoxin B1 and ochratoxin A in breakfast cereals from athens market: Occurrence and risk assessment. Food Control 2009, 20, 455–561. [Google Scholar]
- Ghali, R.K.; Hmaissia-Khlifa, K.; Ghorbel, H.; Maaroufi, K. Incidence of aflatoxins, ochratoxin A and zearalenone in Tunisian foods. Food Control 2009, 19, 921–924. [Google Scholar]
- Leong, Y.H.; Ismail, N.; Latif, A.A.; Ahmad, R. Aflatoxin occurrence in nuts and commercial nutty products in Malaysia. Food Control 2010, 21, 334–338. [Google Scholar]
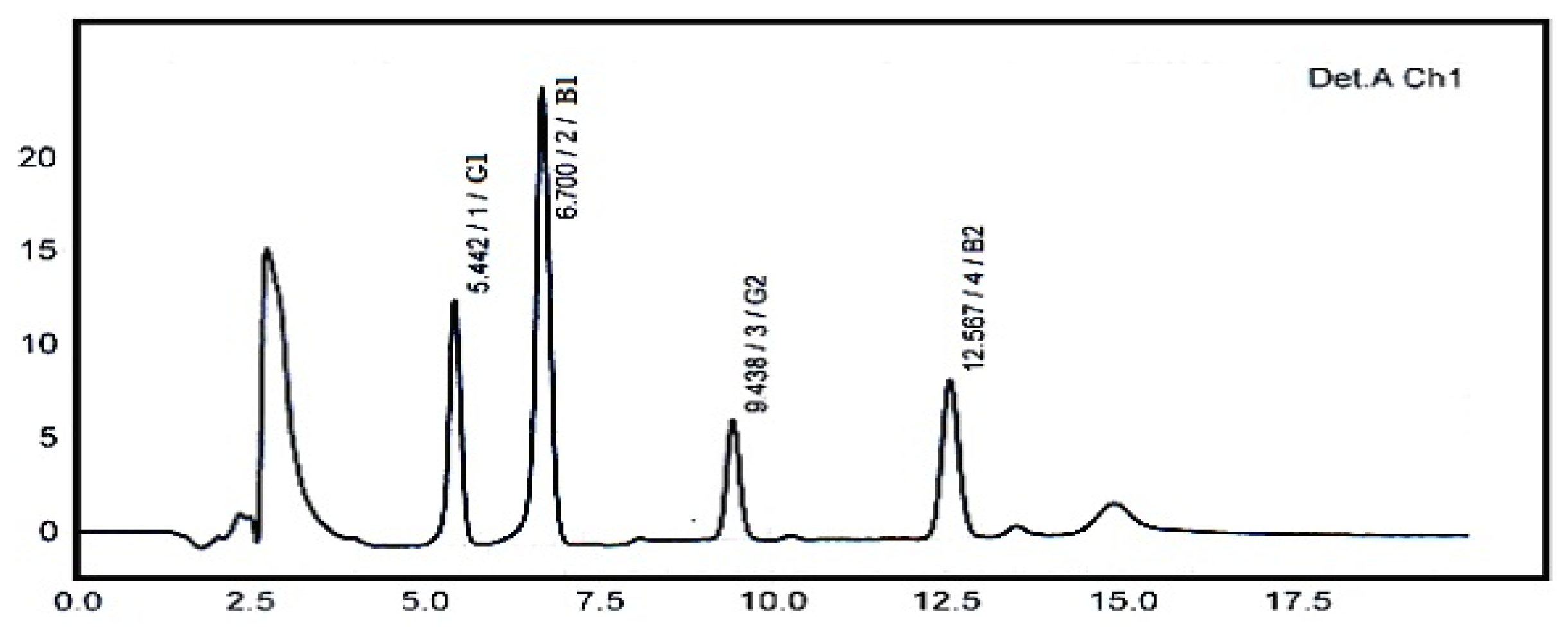
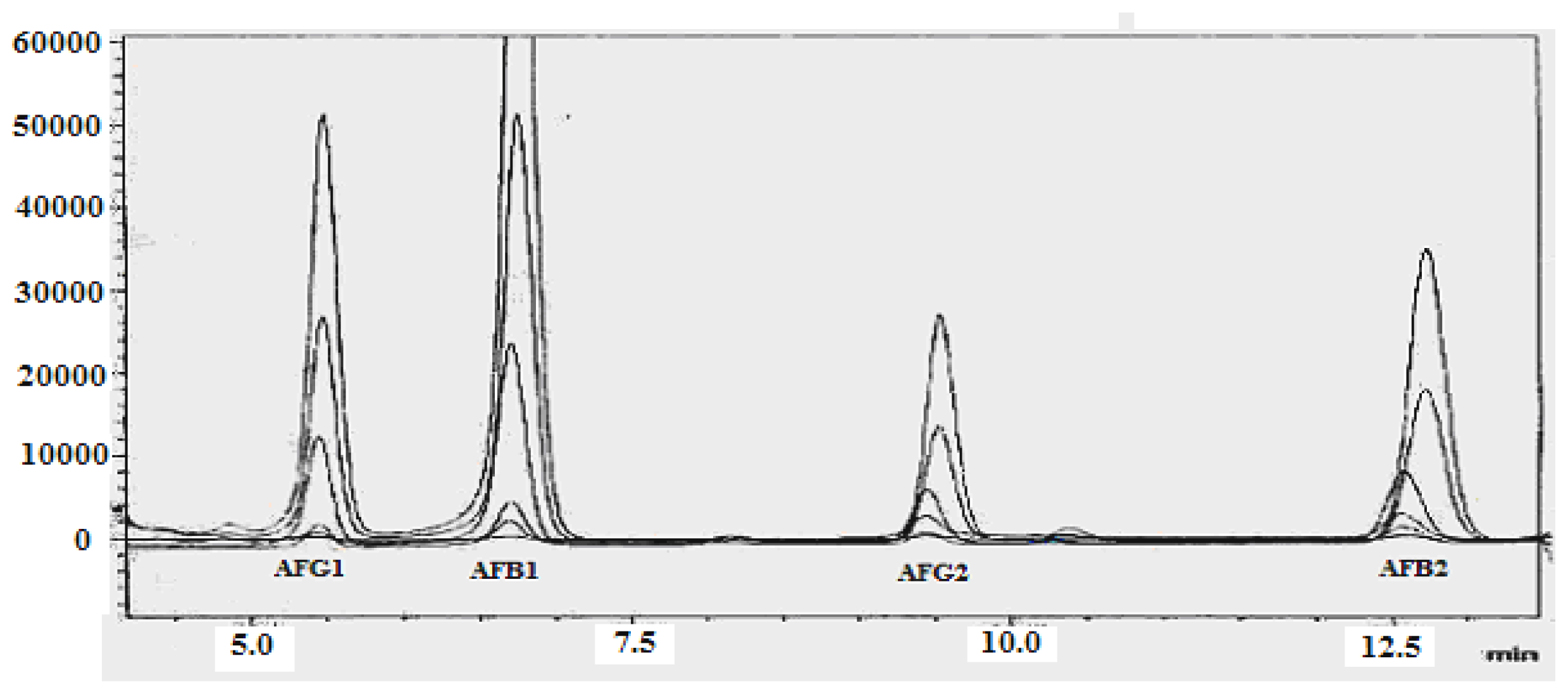
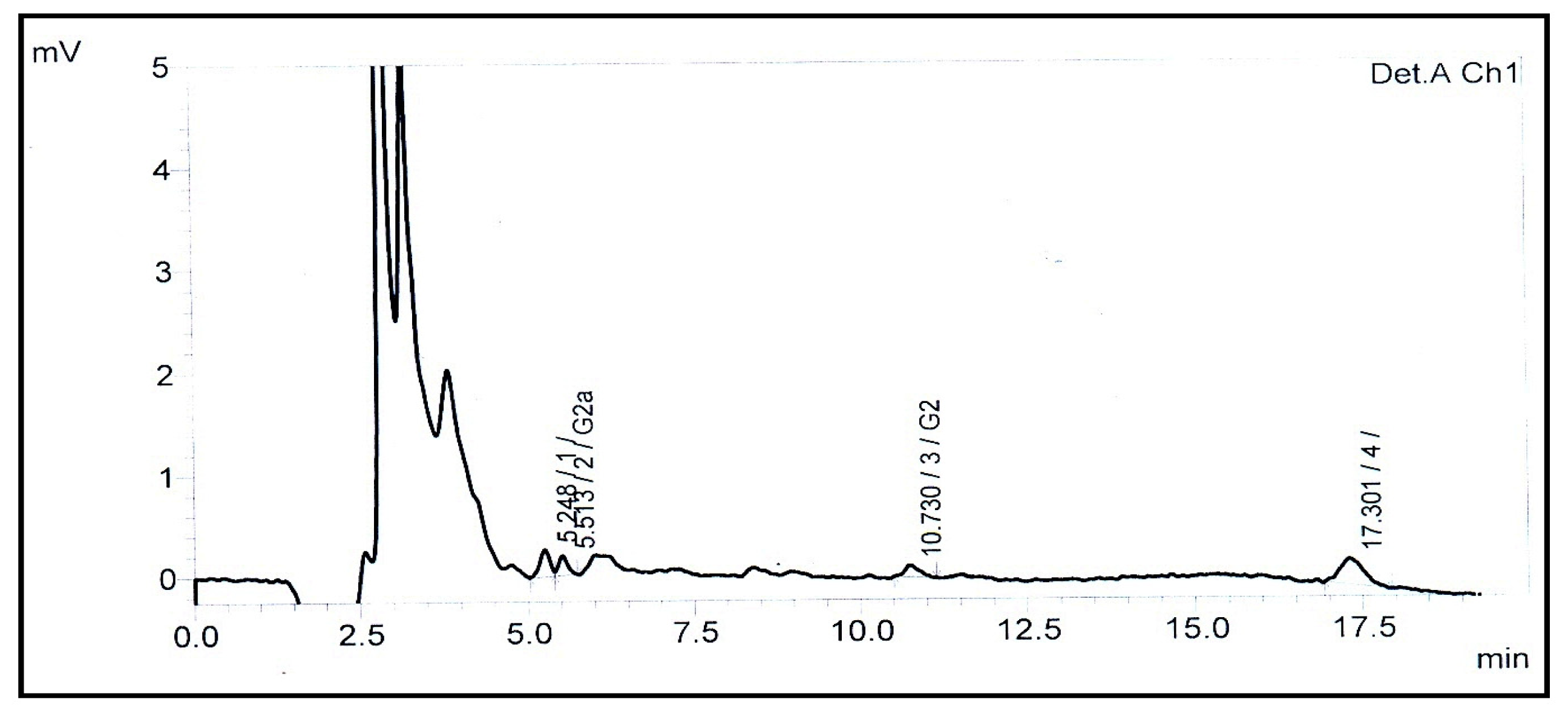
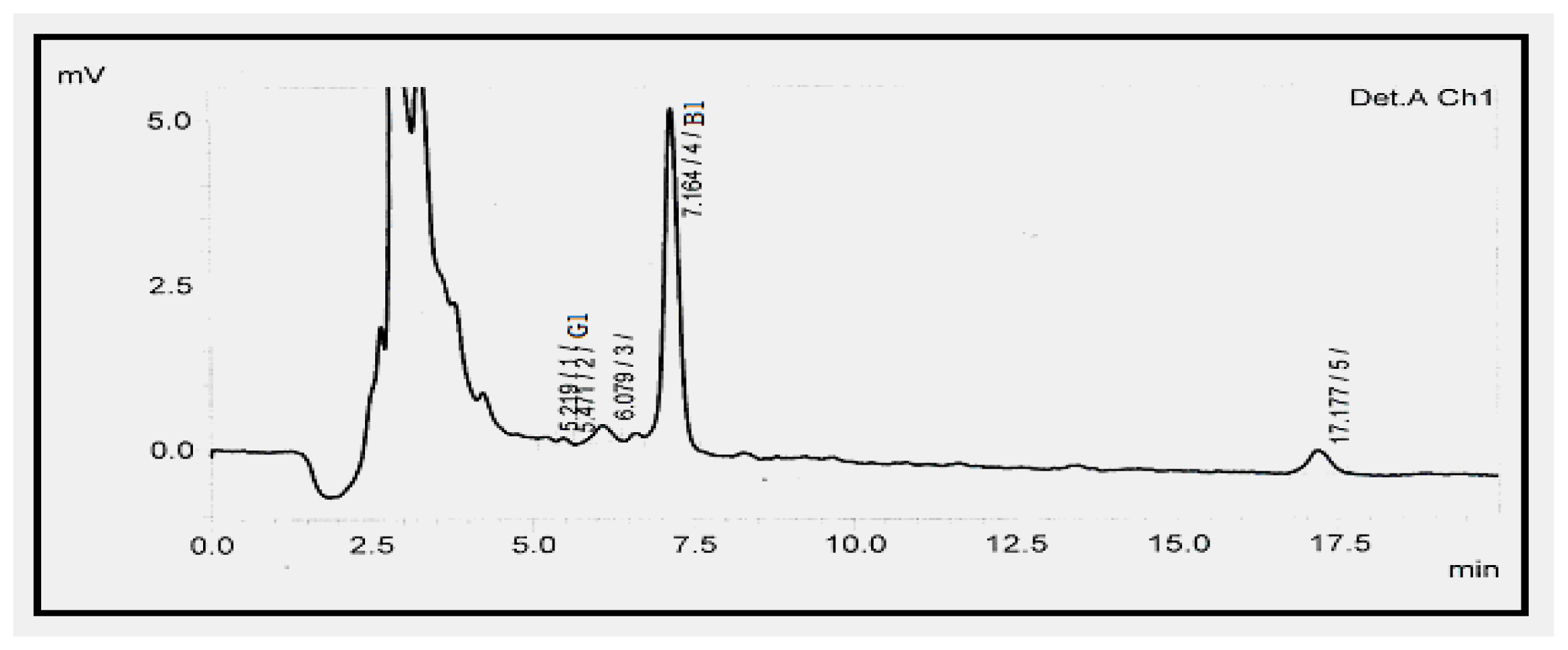
| Aflatoxin | LOD a (ng g−1) | LOQ b (ng g−1) | Calibration Curve | R2 | Recovery (%) c | Mean (μg kg−1) ± RSD (%) d |
|---|---|---|---|---|---|---|
| AFB1 | 0.02 | 0.05 | y = 68,983x + 34,942 | 0.9997 | 97.6 | 125.3 ± 9.12 |
| AFB2 | 0.01 | 0.02 | y = 104,767x – 6,094 | 0.9995 | 91.2 | 15.3 ± 2.01 |
| AFG1 | 0.02 | 0.05 | y = 32,045x + 2,780 | 0.9996 | 97.6 | 15.3 ± 1.44 |
| AFG2 | 0.01 | 0.02 | y = 61,801x − 85.618 | 0.9991 | 91.2 | 6.3 ± 3.42 |
| Sample Typed | Number of Samples | Positive Samples | Samples Having AFB1 > 0.1 μg kg−1 | Samples Having AFB1 < 0.1 μg kg−1 | Total Aflatoxin (mean ± SD) mg kg−1 |
|---|---|---|---|---|---|
| N | n (%) | n (%) | n (%) | ||
| Cerelac | 10 | 4 (40) | 2(20) | 2 (20) | 0.052 ± 0.020–0.19 ± 0.06 |
| Powder Milk | 10 | 3 (33) | 2 (20) | 1 (13) | 0.030 ± 0.001–0.36 ± 0.07 |
| Noodles | 10 | 5 (50) | 4(40) | 1 (10) | 0.025 ± 0.001–0.40 ± 0.09 |
| Cream of rice | 5 | 1 (20) | - | 1(20) | 0.025 ± 0.001–0.16 ± 0.05 |
| Biscuits | 10 | 3 (20) | 1 (32) | 2 (68) | (0.041 ± 0.02)–(2.48 ± 0.38) |
| Corn Products | 10 | 7 (70) | 5 (50) | 2 (20) | (0.050 ± 0.020)–(3.74 ± 0.62) |
| Oatmeal | 5 | 1 (20) | - | 1 (20) | (0.02 ± 0.001)–(0.025 ± 0.001) |
| Potato sticks | 5 | 1 (20) | 1 (50) | 1 (50) | (0.026 ± 0.001)–(2.96 ± 1.35) |
| Wheat Porridge | 5 | - | - | - | - |
| Total | 70 | 25 (35) | 15 (21) | 10 (14) | - |
| Sample Type | n | Incidence (%) | Aflatoxin Range (μg kg−1) | ||||
|---|---|---|---|---|---|---|---|
| AFB1 | AFB2 | AFG1 | AFG2 | Total aflatoxin (AFT) | |||
| Chines fried rice | 5 | 1 (20) | ND | ND | 0.025 | 1.3 | (0.03 ± 0.001)–(1.30 ± 0.002) |
| Bread slices | 3 | 1 (33.3) | 0.02–0.06 | BD | 0.06 | ND | (0.1 ± 0.003)–(0.26 ± 0.004) |
| Sohn Halwa | 3 | 1 (33.3) | BD | ND | ND | ND | (0.04 ± 0.001)–(0.16 ± 0.003) |
| Chapati local wheat cleaned with air | 3 | 3 (100.0) | 0.02–0.27 | ND | ND | 0.03–0.94 | (0.05 ± 0.002)–(1.21 ± 0.04) |
| Chapati local wheat cleaned with water | 3 | 0 (0) | ND | ND | ND | ND | ND |
| Chapati (commercial flour) | 3 | 2 (66.7) | BD | 0.025 | 1.36 | 0.03 | (0.07 ± 0.003)–(1.36 ± 0.06) |
| Every Day Milk | 9 | 2 (22) | 0.0474 | ND | 0.141 | 0.084 | (0.05 ± 0.002)–(0.27 ± 0.005) |
| gram flour | 5 | 3 (60) | 0.32–1.02 | 0.12 | 0.58 | 0.34 | (0.8 ± 0.06)–(2.1 ± 0.01) |
| Peanuts (nimko) | 5 | 3 (60) | 0.21–1.24 | 0.13032 | ND | 1.741 | (0.27 ± 0.003)–(2.08 ± 0.01) |
| Lays | 3 | 1 (60) | BD | ND | 0.57 | ND | 0.57 |
| Bread slices | 5 | 3 (20) | 0.021 | ND | 0.06 | ND | 0.85 |
| Soji Halwa | 3 | 0 (0) | ND | ND | ND | ND | ND |
| Barian | 5 | 1 (20) | 0.07 | 0 | 0.25 | 0.15 | (0.03 ± 0.001)–(0.48 ± 0.006) |
| Total | 55 | 22 (40) | |||||
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mushtaq, M.; Sultana, B.; Anwar, F.; Khan, M.Z.; Ashrafuzzaman, M. Occurrence of Aflatoxins in Selected Processed Foods from Pakistan. Int. J. Mol. Sci. 2012, 13, 8324-8337. https://doi.org/10.3390/ijms13078324
Mushtaq M, Sultana B, Anwar F, Khan MZ, Ashrafuzzaman M. Occurrence of Aflatoxins in Selected Processed Foods from Pakistan. International Journal of Molecular Sciences. 2012; 13(7):8324-8337. https://doi.org/10.3390/ijms13078324
Chicago/Turabian StyleMushtaq, Muhammad, Bushra Sultana, Farooq Anwar, Muhammad Zargham Khan, and Muhammad Ashrafuzzaman. 2012. "Occurrence of Aflatoxins in Selected Processed Foods from Pakistan" International Journal of Molecular Sciences 13, no. 7: 8324-8337. https://doi.org/10.3390/ijms13078324
APA StyleMushtaq, M., Sultana, B., Anwar, F., Khan, M. Z., & Ashrafuzzaman, M. (2012). Occurrence of Aflatoxins in Selected Processed Foods from Pakistan. International Journal of Molecular Sciences, 13(7), 8324-8337. https://doi.org/10.3390/ijms13078324




