Degradability Enhancement of Poly(Lactic Acid) by Stearate-Zn3Al LDH Nanolayers
Abstract
:1. Introduction
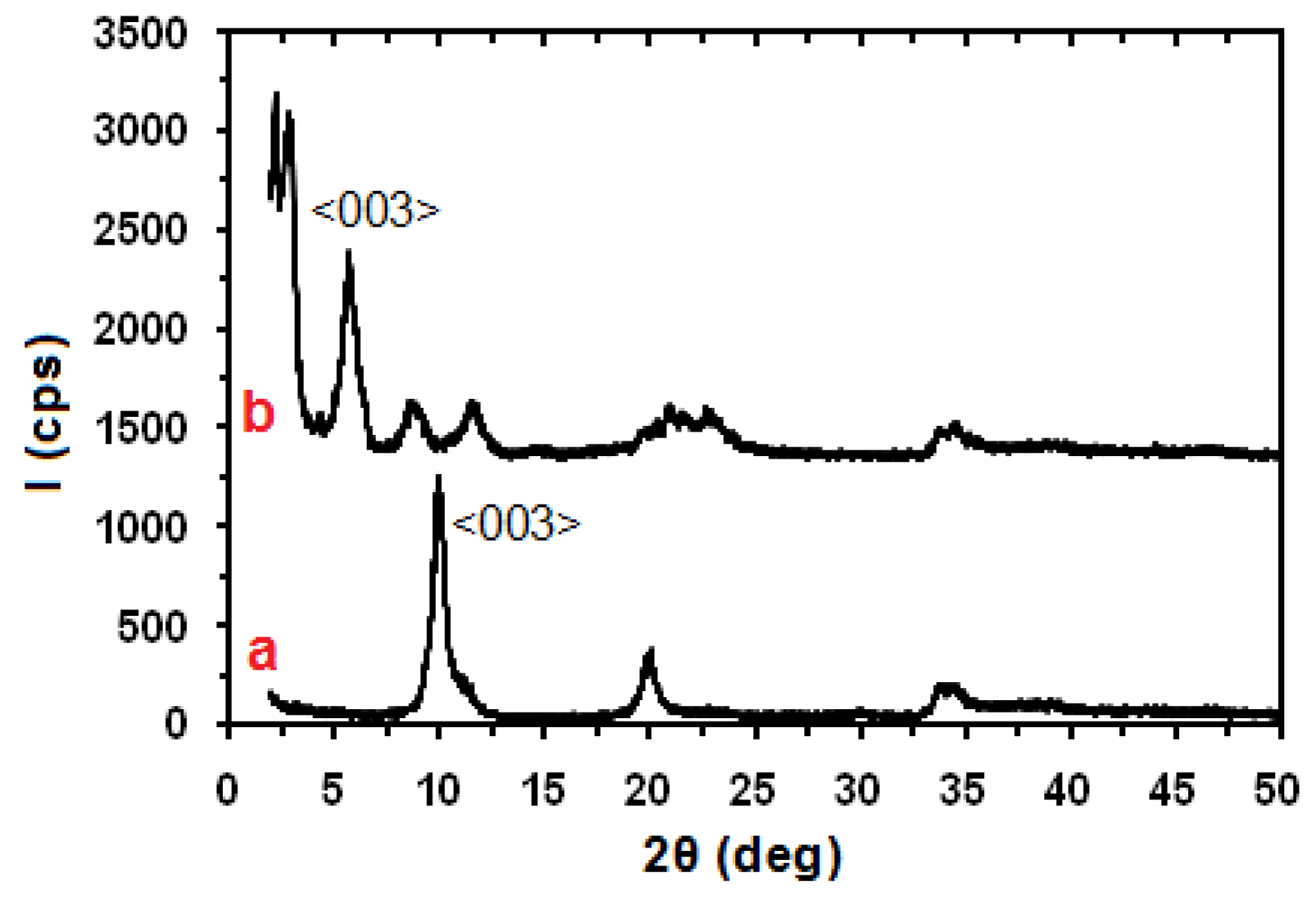
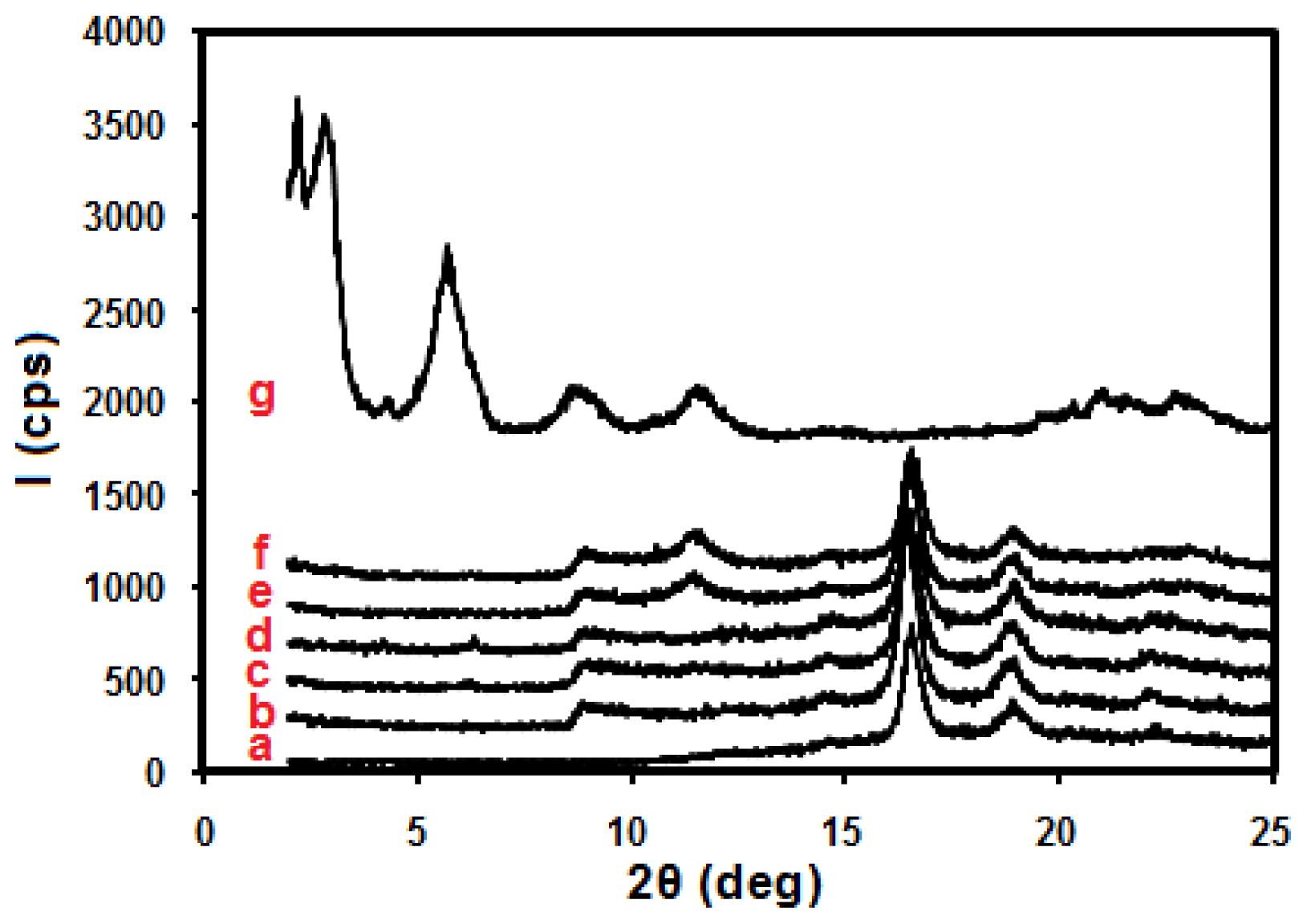
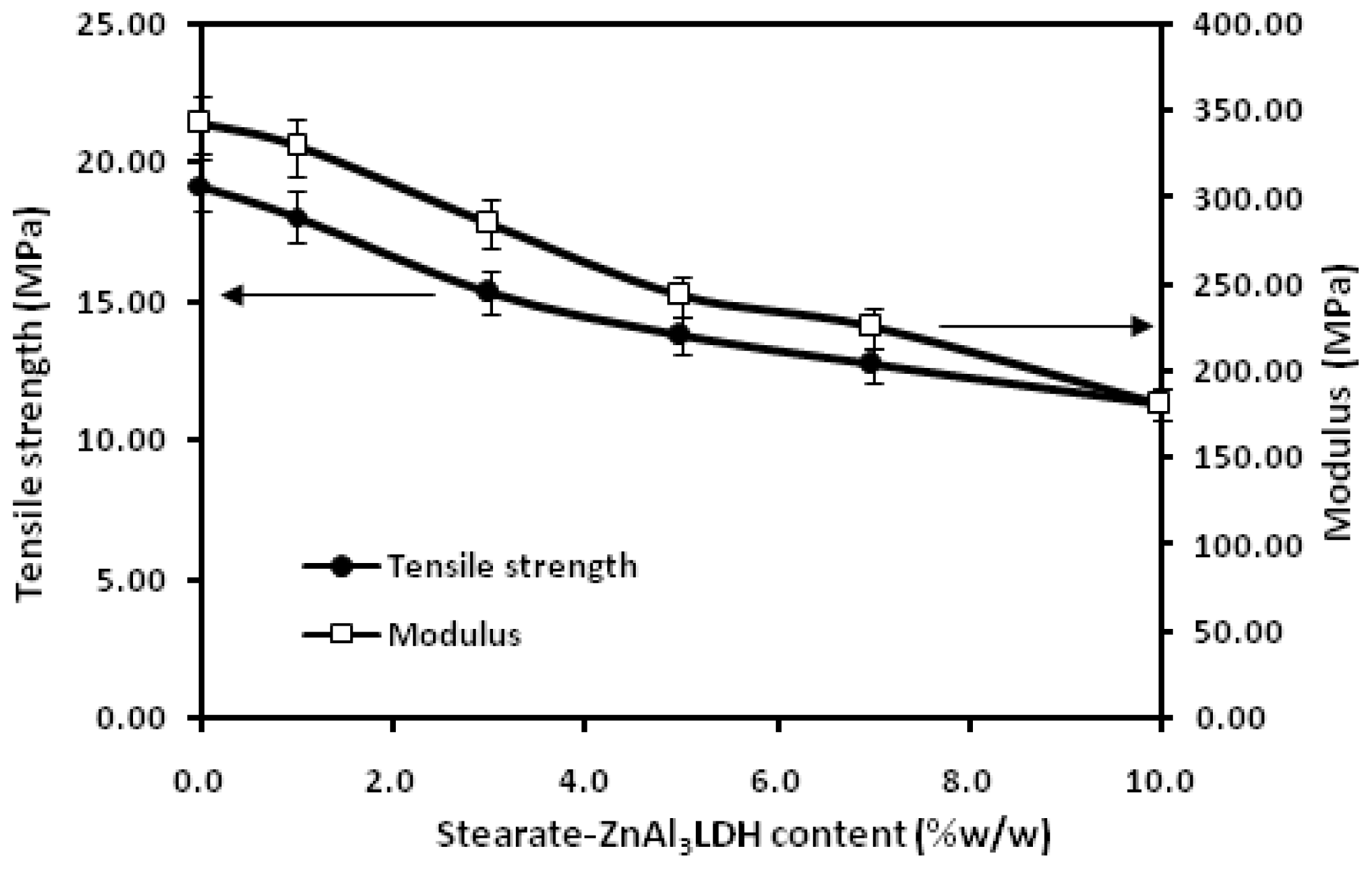
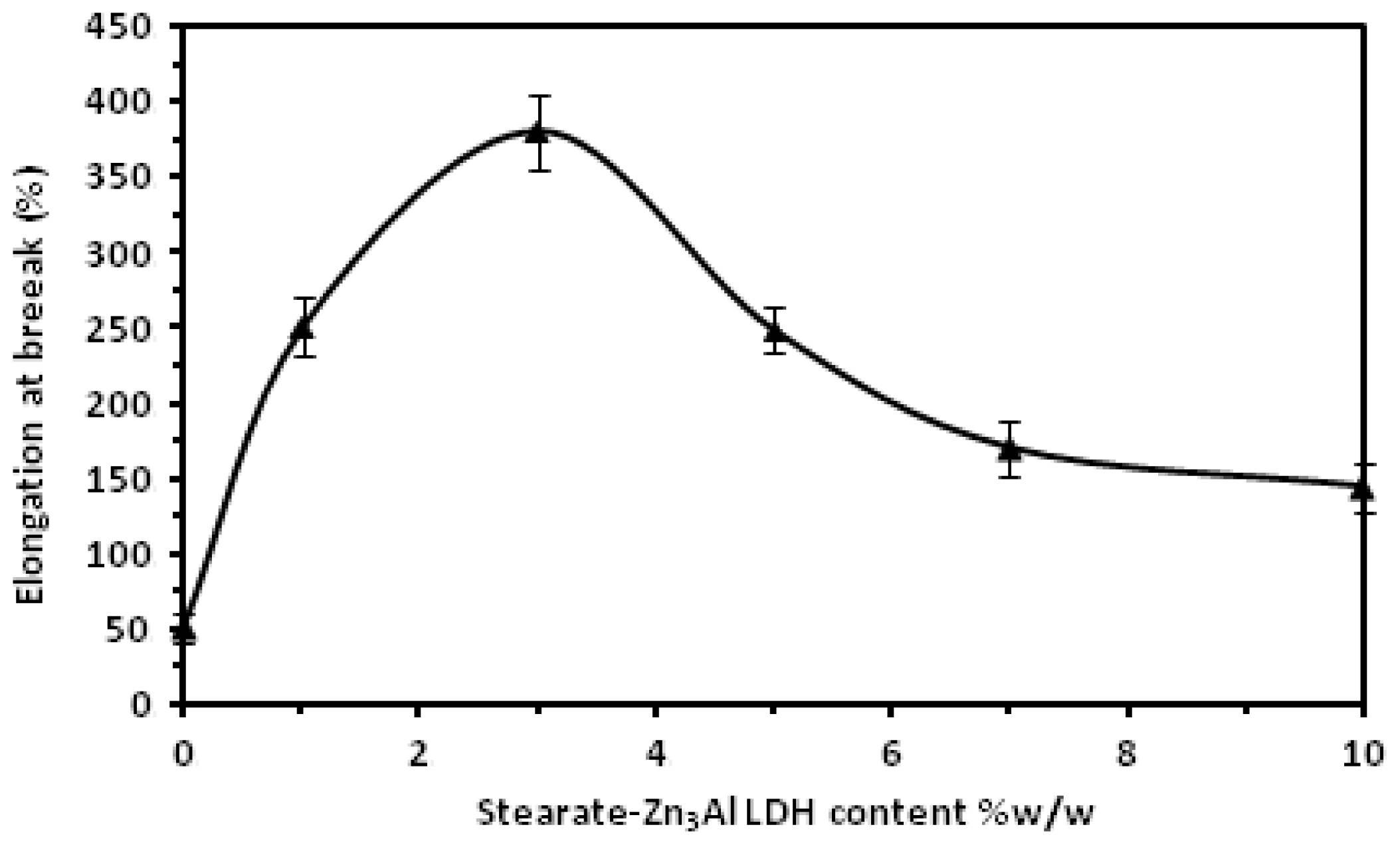
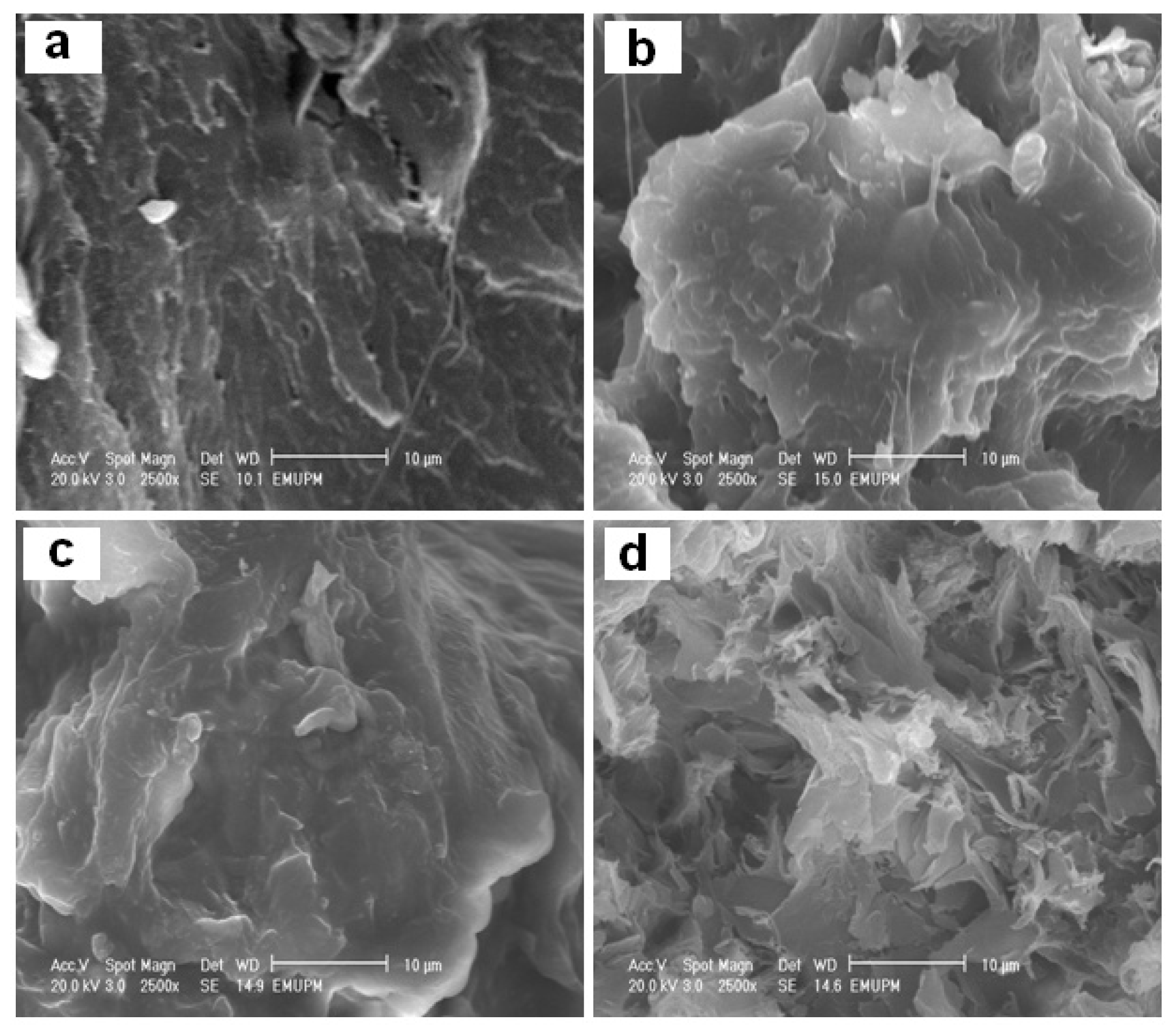
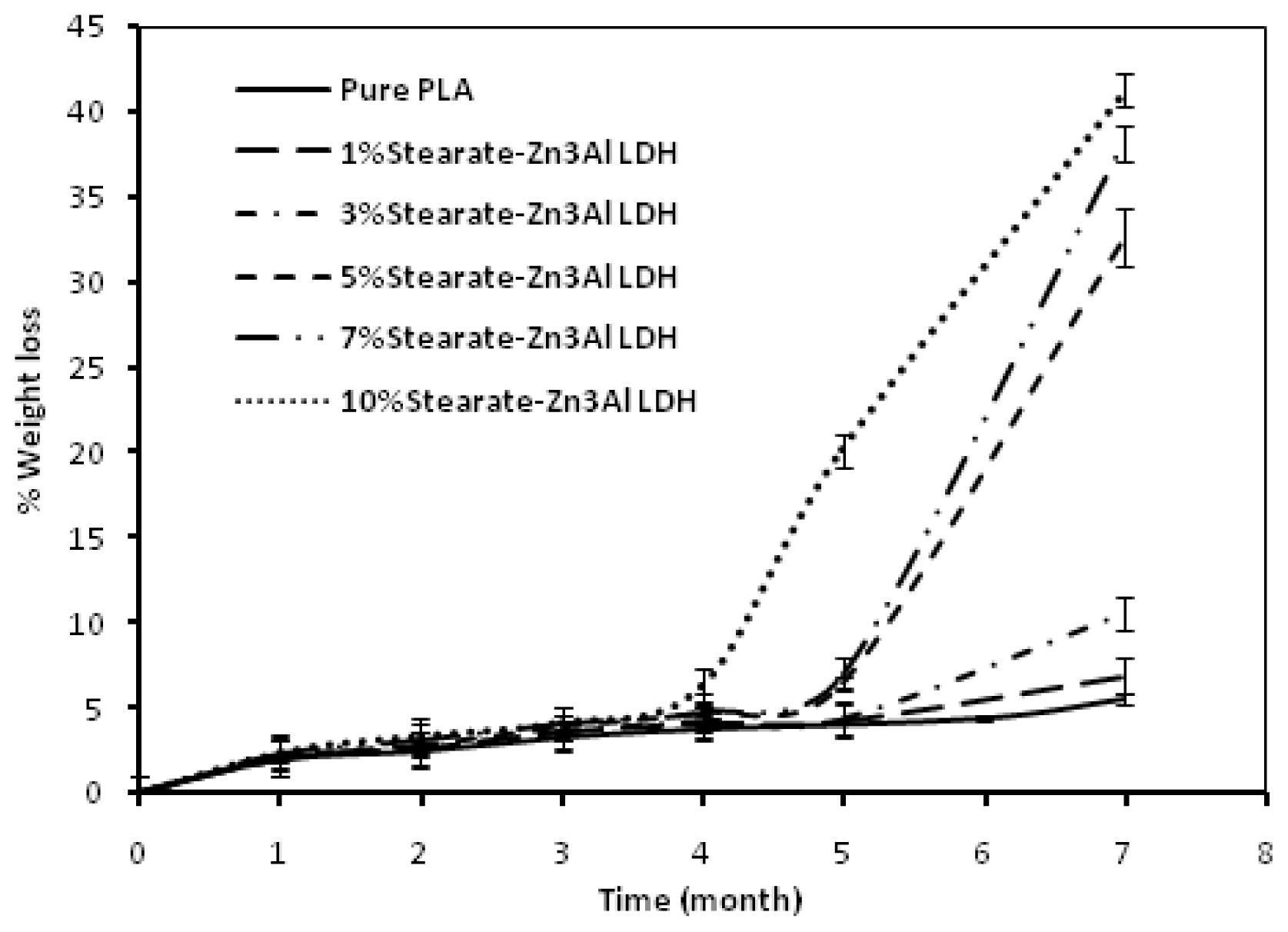
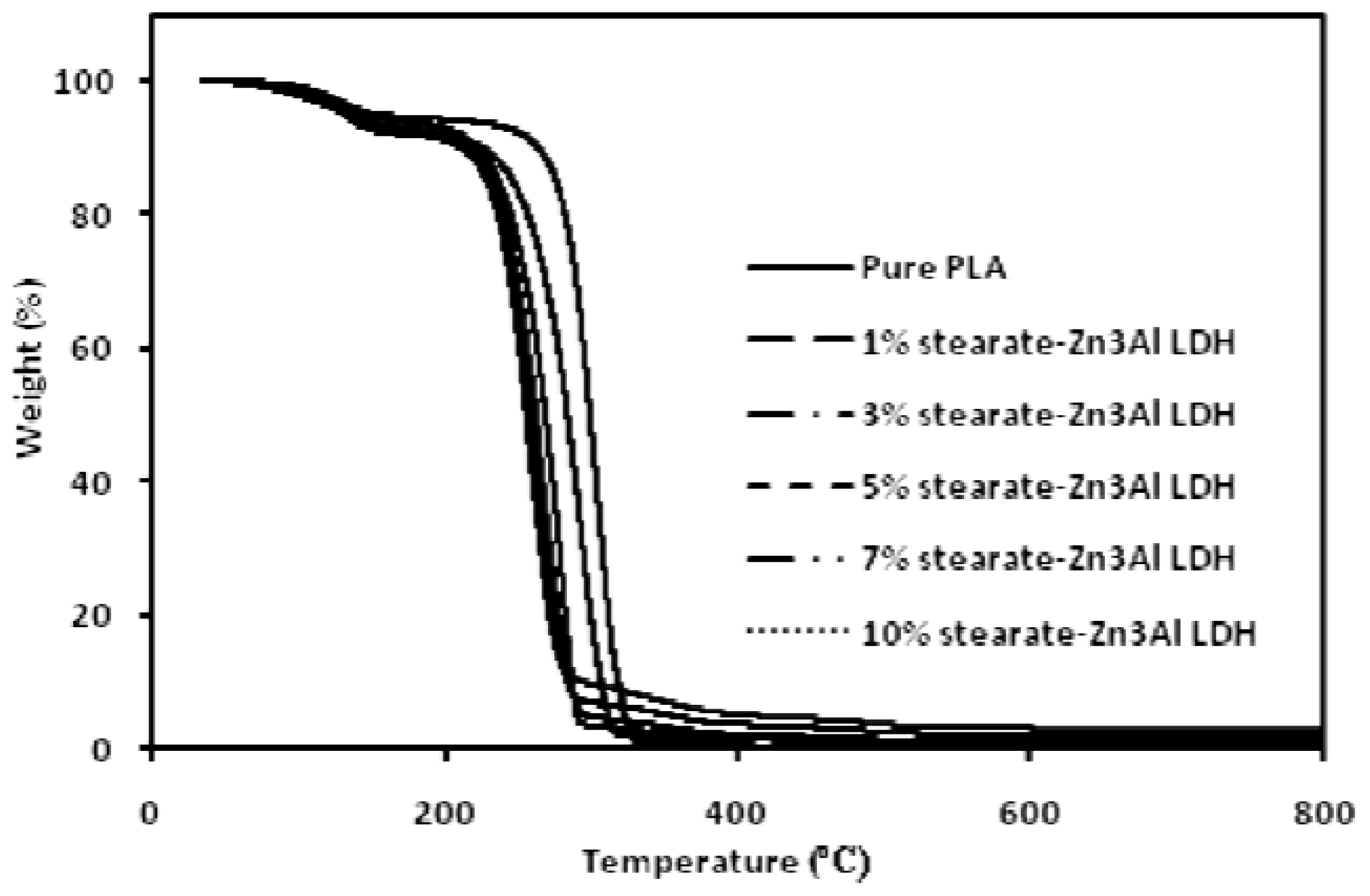
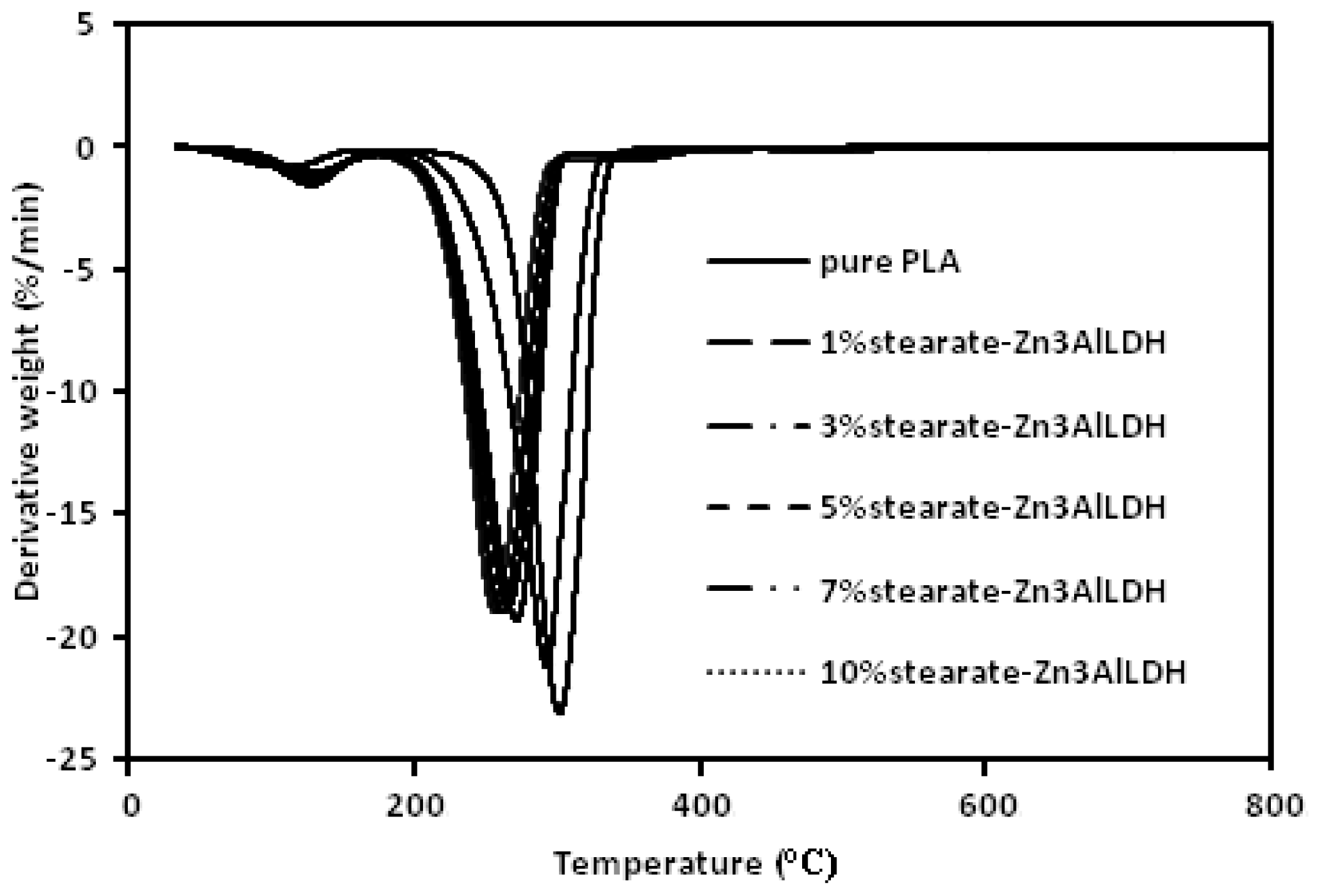
2. Results and Discussion
3. Experimental Section
3.1. Materials
3.2. Synthesis of Zn3Al LDH
3.3. Preparation of Stearate-Zn3Al LDH
3.4. Preparation of PLA/Stearate-Zn3Al LDH Nanocomposites
3.5. Characterization Techniques
4. Conclusions
Acknowledgments
References
- Marten, E.; Müller, R.J.; Deckwer, W.D. Studies on the enzymatic hydrolysis of polyesters I. Low molecular mass model esters and aliphatic polyesters. Polym. Degrad. Stabil 2003, 80, 485–501. [Google Scholar]
- Wu, C.S. Physical properties and biodegradability of maleated-polycaprolactone/starch composite. Polym. Degrad. Stabil 2003, 80, 127–134. [Google Scholar]
- Ray, S.S.; Bousmina, M. Biodegradable polymers and their layered silicate nanocomposites: In greening the 21st century materials world. Prog. Mat. Sci 2005, 50, 962–1079. [Google Scholar]
- Tang, X.; Alavi, S. Recent advances in starch, polyvinyl alcohol based polymer blends, nanocomposites and their biodegradability. Carbohydr. Polym 2011, 85, 7–16. [Google Scholar]
- Umare, S.S.; Chandure, A.S.; Pandey, R.A. Synthesis, characterization and biodegradable studies of 1,3-propanediol based polyesters. Polym. Degrad. Stabil 2007, 92, 464–479. [Google Scholar]
- Chandra, R.; Rustgi, R. Biodegradable polymers. Prog. Polym. Sci 1998, 23, 1273–1335. [Google Scholar]
- Scherer, T.M.; Fuller, R.C.; Lenz, R.W.; Goodwin, S. Hydrolase activity of an extracellular depolymerase from aspergillus fumigatus with bacterial and synthetic polyesters. Polym. Degrad Stabil 1999, 64, 267–275. [Google Scholar]
- Athanasiou, K.A.; Agrawal, C.M.; Barber, F.A.; Burkhart, S.S. Orthopaedic applications for PLA-PGA biodegradable polymers. Arthrosc. J. Arthrosc. Relat. Surg 1998, 14, 726–737. [Google Scholar]
- Muller, R.J. Biodegradability of polymers: Regulations and methods for testing. Biopolym. Online 2005. [Google Scholar] [CrossRef]
- Liu, J.W.; Zhao, Q.; Wan, C.X. Research progresses on degradation mechanism in vivo and medical applications of polylactic acid. Space Med. Med. Eng 2001, 14, 308–312. [Google Scholar]
- Bordes, P.; Pollet, E.; Avérous, L. Nano-biocomposites: Biodegradable polyester/nanoclay systems. Prog. Polym. Sci 2009, 34, 125–155. [Google Scholar]
- Fukushima, K.; Abbate, C.; Tabuani, D.; Gennari, M.; Camino, G. Biodegradation of poly(lactic acid) and its nanocomposites. Polym. Degrad. Stabil 2009, 94, 1646–1655. [Google Scholar]
- Tuominen, J.; Kylma, J.; Kapanen, A.; Venelampi, O.; Itävaara, M.; Seppälä, J. Biodegradation of lactic acid based polymers under controlled composting conditions and evaluation of the ecotoxicological impact. Biomacromolecules 2002, 3, 445–455. [Google Scholar]
- Gupta, B.; Revagade, N.; Hilborn, J. Poly(lactic acid) fiber: An overview. Prog. Polym. Sci 2007, 32, 455–482. [Google Scholar]
- Lee, S.Y.; Chen, H.; Hanna, M.A. Preparation and characterization of tapioca starch-poly(lactic acid) nanocomposite foams by melt intercalation based on clay type. Ind. Crop. Prod 2008, 28, 95–106. [Google Scholar]
- Li, B.; Dong, F.X.; Wang, X.L.; Yang, J.; Wang, D.Y.; Wang, Y.Z. Organically modified rectorite toughened poly(lactic acid): Nanostructures, crystallization and mechanical properties. Eur. Polym. J 2009a, 45, 2996–3003. [Google Scholar]
- Pavlidou, S.; Papaspyrides, C.D. A review on polymer-layered silicate nanocomposites. Prog. Polym. Sci 2008, 33, 1119–1198. [Google Scholar]
- Carrado, K.A.; Kostapapas, A.; Suib, S.L. Layered double hydroxides (LDHs). Solid State Ion 1988, 26, 77–86. [Google Scholar]
- Hsueh, H.B.; Chen, C.Y. Preparation and properties of LDHs/polyimide nanocomposites. Polymer 2003, 44, 1151–1161. [Google Scholar]
- Li, E.; Xu, Z.P.; Rudolph, V. MgCoAl-LDH derived heterogeneous catalysts for the ethanol transesterification of canola oil to biodiesel. Appl. Catal. Environ 2009b, 88, 42–49. [Google Scholar]
- Coronado, E.; Martí-Gastaldo, C.; Navarro-Moratalla, E.; Ribera, A. Intercalation of [M(ox)3]3− (M = Cr, Rh) complexes into NiIIFeIII-LDH. Appl. Clay Sci 2009, 48, 228–234. [Google Scholar]
- Triantafyllidis, K.S.; Peleka, E.N.; Komvokis, V.G.; Mavros, P.P. Iron-modified hydrotalcite-like materials as highly efficient phosphate sorbents. J. Colloid Interf. Sci 2009, 342, 427–436. [Google Scholar]
- Schwenzer, B.; Neilson, J.R.; Sivula, K.; Woo, C.; Fréchet, J.M.J.; Morse, D.E. Nanostructured p-type cobalt layered double hydroxide/n-type polymer bulk heterojunction yields an inexpensive photovoltaic cell. Thin Solid Films 2009, 517, 5722–5727. [Google Scholar]
- Forano, C.; Hibino, T.; Leroux, F.; Taviot-Guého, C. Layered double hydroxides. Dev. Clay Sci 2006, 1, 1021–1095. [Google Scholar]
- Olfs, H.W.; Torres-Dorante, L.O.; Eckelt, R.; Kosslick, H. Comparison of different synthesis routes for Mg-Al layered double hydroxides (LDH): Characterization of the structural phases and anion exchange properties. Appl. Clay Sci 2009, 43, 459–464. [Google Scholar]
- Villa, M.V.; Sánchez-Martín, M.J.; Sánchez-Camazano, M. Hydrotalcites and organo-hydrotalcites as sorbents for removing pesticides from water. J. Environ. Sci. Health Pestic. Food Contam. Agric. Wastes 1999, 34, 509–525. [Google Scholar]
- Kameda, T.; Takeuchi, H.; Yoshioka, T. Preparation of organic acid anion-modified magnesium hydroxides by coprecipitation: A novel material for the uptake of heavy metal ions from aqueous solutions. J. Phys. Chem. Solids 2009, 70, 1104–1108. [Google Scholar]
- Costa, F.R.; Leuteritz, A.; Wagenknecht, U.; Jehnichen, D.; Häußler, L.; Heinrich, G. Intercalation of Mg–Al layered double hydroxide by anionic surfactants: Preparation and characterization. Appl. Clay Sci 2008, 38, 153–164. [Google Scholar]
- Sugahara, Y.; Yokoyama, N.; Kuroda, K.; Kato, C. AlN formation from a hydrotalcite-polyacrylonitrile intercalation compound by carbothermal reduction. Ceram. Int 1988, 14, 163–167. [Google Scholar]
- Oriakhi, C.O.; Farr, I.V.; Lerner, M.M. Incorporation of poly(acrylic acid), poly(vinylsulfonate) and poly(styrenesulfonate) within layered double hydroxides. J. Mater. Chem 1996, 6, 103–107. [Google Scholar]
- Hsueh, H.B.; Chen, C.Y. Preparation and properties of LDHs/polyimide nanocomposites. Polymer 2003, 44, 1151–1161. [Google Scholar]
- Yuan, Y.; Zhang, Y.; Shi, W. A novel approach for preparing exfoliated UV-cured polymer/LDH nanocomposites via pre-exfoliated organic LDH. Appl. Clay Sci 2011, 53, 608–614. [Google Scholar]
- Ardanuy, M.; Velasco, J.I.; Realinho, V.; Arencón, D.; Martínez, A.B. Non-isothermal crystallization kinetics and activity of filler in polypropylene/Mg–Al layered double hydroxide nanocomposites. Thermochim. Acta 2008, 479, 45–52. [Google Scholar]
- Tsai, T.Y.; Lu, S.W.; Li, F.S. Preparation and characterization of epoxy/layered double hydroxides nanocomposites. J. Phys. Chem. Solids 2008, 69, 1386–1390. [Google Scholar]
- Matusinovic, Z.; Rogosic, M.; Sipusic, J. Synthesis and characterization of poly(styrene-co-methyl methacrylate)/layered double hydroxide nanocomposites via in situ polymerization. Polym. Degrad. Stab 2009, 94, 95–101. [Google Scholar]
- Peng, D.; Baojun, Q. Synthesis and characterization of polystyrene/layered double-hydroxide nanocomposites via in situ emulsion and suspension polymerization. J. Appl. Polym. Sci 2006, 101, 3758–3766. [Google Scholar]
- Pradhan, S.; Costa, F.R.; Wagenknecht, U.; Jehnichen, D.; Bhowmick, A.K.; Heinrich, G. Elastomer/LDH nanocomposites: Synthesis and studies on nanoparticle dispersion, mechanical properties and interfacial adhesion. Eur. Polym. J 2008, 44, 3122–3132. [Google Scholar]
- Mai, Y.W.; Yu, Z.Z. Polymer Nanocomposites; Woodhead Publishing Ltd: Cambridge, UK, 2006. [Google Scholar]
- Kornmann, X.; Lindberg, H.; Berglund, L.A. Synthesis of epoxy-clay nanocomposites: Influence of the nature of the clay on structure. Polymer 2001, 42, 1303–1310. [Google Scholar]
- Peng, H.; Han, Y.; Liu, T.; Tjiu, W.C.; He, C. Morphology and thermal degradation behavior of highly exfoliated CoAl-layered double hydroxide/polycaprolactone nanocomposites prepared by simple solution intercalation. Thermochim. Acta 2010, 502, 1–7. [Google Scholar]
- Kulinski, Z.; Piorkowska, E. Crystallization, structure and properties of plasticized poly(L-lactide). Polymer 2005, 46, 10290–10300. [Google Scholar]
- Landreau, E.; Tighzert, L.; Bliard, C.; Berzin, F.; Lacoste, C. Morphologies and properties of plasticized starch/polyamide compatibilized blends. Eur. Polym. J 2009, 45, 2609–2618. [Google Scholar]
- Sun, S.; Song, Y.; Zheng, Q. Morphology and mechanical properties of thermo-molded bioplastics based on glycerol-plasticized wheat gliadins. J. Cereal Sci 2008, 48, 613–618. [Google Scholar]
- Yang, J.H.; Yu, J.G.; Ma, X.F. Study on the properties of ethylenebisformamide and sorbitol plasticized corn starch (ESPTPS). Carbohydr. Polym 2006, 66, 110–116. [Google Scholar]
- Baiardo, M.; Frisoni, G.; Scandola, M.; Rimelen, M.; Lips, D. Thermal and mechanical properties of plasticized poly(L-lactic acid). J. Appl. Polym. Sci 2003, 90, 1731–1738. [Google Scholar]
- Piorkowska, E.; Kulinski, Z.; Galeski, A.; Masirek, R. Plasticization of semicrystalline poly(L-lactide) with poly(propylene glycol). Polymer 2006, 47, 7178–7188. [Google Scholar]
- Chang, J.H.; An, Y.U.; Cho, D.; Giannelis, E.P. Poly(lactic acid) nanocomposites: Comparison of their properties with montmorillonite and synthetic mica (II). Polymer 2003, 44, 3715–3720. [Google Scholar]
- Murariu, M.; da Silva Ferreira, A.; Pluta, M.; Bonnaud, L.; Alexandre, M.; Dubois, P. Polylactide (PLA)-CaSO4 composites toughened with low molecular weight and polymeric ester-like plasticizers and related performances. Eur. Polym. J 2008, 44, 3842–3852. [Google Scholar]
- Ray, S.S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci 2003, 28, 1539–1641. [Google Scholar]
- Ray, S.S.; Yamada, K.; Okamoto, M.; Ueda, K. New polylactide-layered silicate nanocomposites. 2. Concurrent improvements of material properties, biodegradability and melt rheology. Polymer 2003, 44, 857–866. [Google Scholar]

© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Eili, M.; Shameli, K.; Ibrahim, N.A.; Wan Yunus, W.M.Z. Degradability Enhancement of Poly(Lactic Acid) by Stearate-Zn3Al LDH Nanolayers. Int. J. Mol. Sci. 2012, 13, 7938-7951. https://doi.org/10.3390/ijms13077938
Eili M, Shameli K, Ibrahim NA, Wan Yunus WMZ. Degradability Enhancement of Poly(Lactic Acid) by Stearate-Zn3Al LDH Nanolayers. International Journal of Molecular Sciences. 2012; 13(7):7938-7951. https://doi.org/10.3390/ijms13077938
Chicago/Turabian StyleEili, Mahboobeh, Kamyar Shameli, Nor Azowa Ibrahim, and Wan Md Zin Wan Yunus. 2012. "Degradability Enhancement of Poly(Lactic Acid) by Stearate-Zn3Al LDH Nanolayers" International Journal of Molecular Sciences 13, no. 7: 7938-7951. https://doi.org/10.3390/ijms13077938



