Supercritical Fluid Extraction of Eucalyptus globulus Bark—A Promising Approach for Triterpenoid Production
Abstract
:1. Introduction
2. Results and Discussion
2.1. Soxhlet Extraction of Eucalyptus globulus Deciduous Bark
2.2. One-Step Supercritical Fluid Extraction of Eucalyptus globulus Deciduous Bark
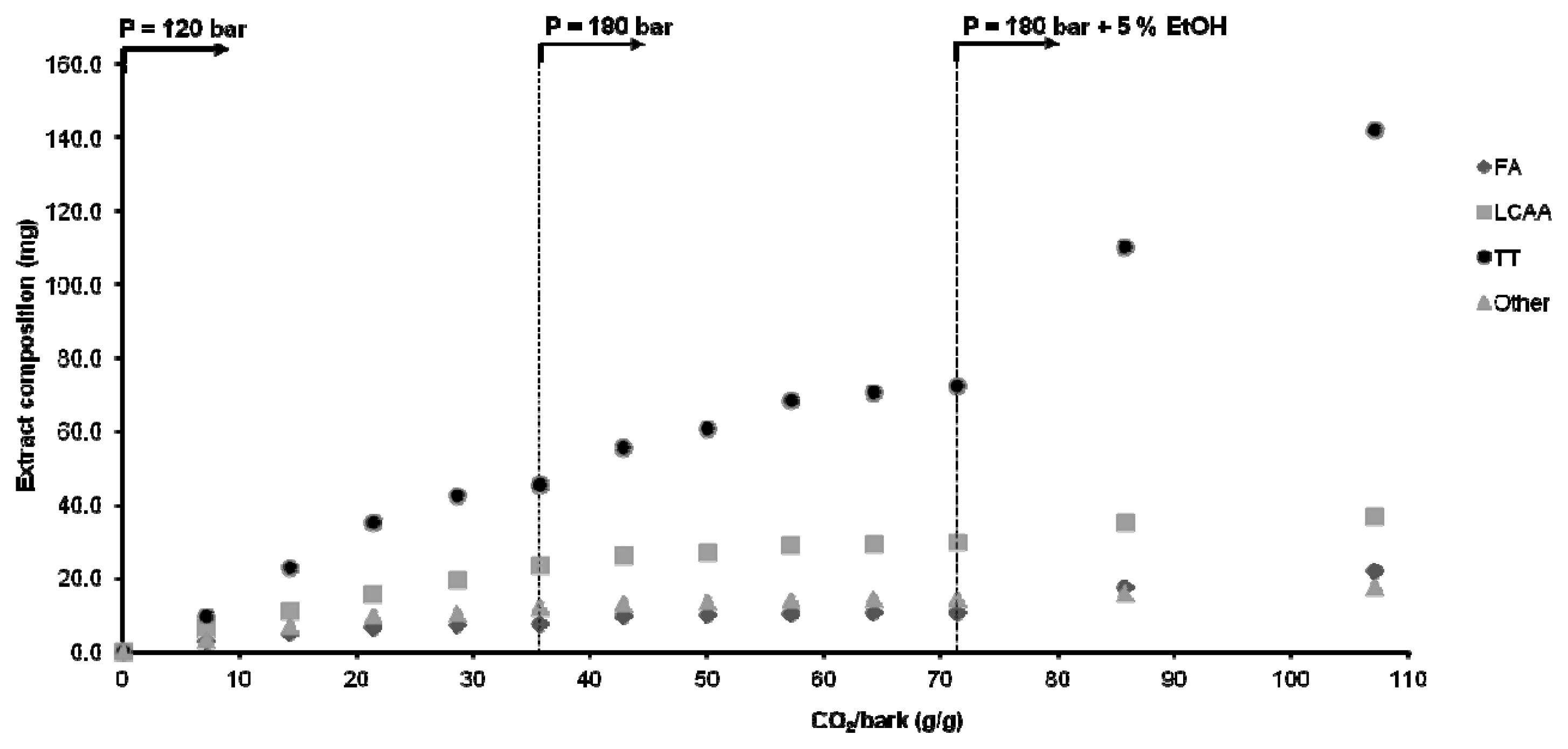
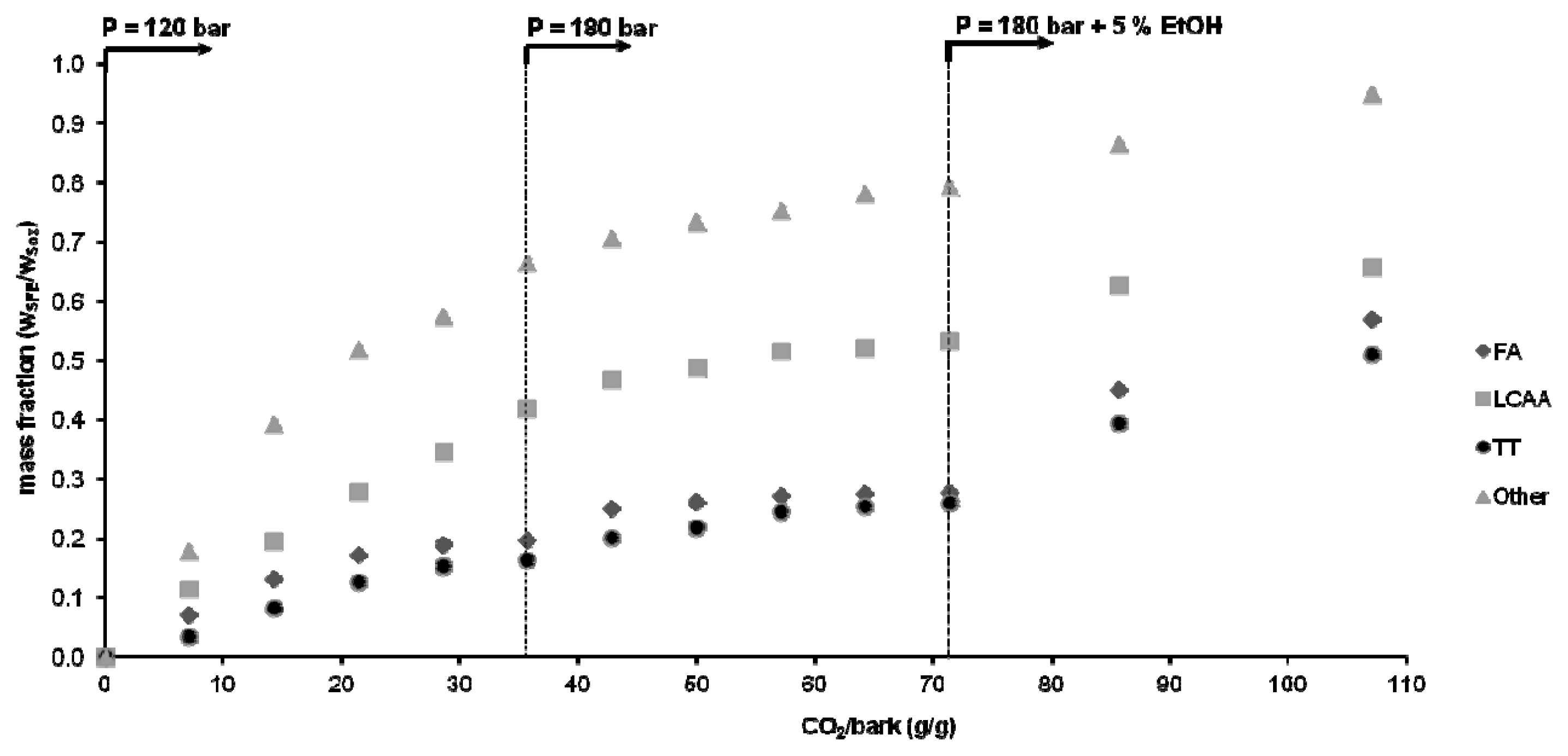
2.3. Stepwise SC-CO2 Extraction of Eucalyptus globulus Deciduous Bark
3. Experimental Section
3.1. Chemicals
3.2. Bark Samples
3.3. Soxhlet Extraction
3.4. Supercritical Fluid Extraction (SFE)
3.4.1. SFE Apparatus
3.4.2. SFE Procedure
3.5. GC-MS Analyses
4. Conclusions
Acknowledgments
References
- Clark, J.H.; Tavener, S.J. Alternative solvents: Shades of green. Org. Process Res. Dev 2007, 11, 149–155. [Google Scholar]
- Huang, H.J.; Ramaswamy, S.; Tschirner, U.W.; Ramarao, B.V. A review of separation technologies in current and future biorefineries. Sep. Purif. Technol 2008, 62, 1–21. [Google Scholar]
- Lucia, L.A.; Argyropoulos, D.S.; Adamopoulos, L.; Gaspar, A.R. Materials, Chemicals, and Energy From Forest Biomass: A Review. In Materials, Chemicals, and Energy from Forest Biomass; Argyropoulos, D.S., Ed.; American Chemical Society: Washington, DC, USA, 2007; Volume 954, p. 5. [Google Scholar]
- Sheldon, R.A. The E factor: Fifteen years on. Green Chem 2007, 9, 1273–1283. [Google Scholar]
- Clark, J.H.; Budarin, V.; Deswarte, F.E.I.; Hardy, J.J.E.; Kerton, F.M.; Hunt, A.J.; Luque, R.; Macquarrie, D.J.; Milkowski, K.; Rodriguez, A.; et al. Green chemistry and the biorefinery: A partnership for a sustainable future. Green Chem 2006, 8, 853–860. [Google Scholar]
- Fernando, S.; Adhikari, S.; Chandrapal, C.; Murali, N. Biorefineries: Current status, challenges, and future direction. Energy Fuels 2006, 20, 1727–1737. [Google Scholar]
- Gallezot, P. Process options for converting renewable feedstocks to bioproducts. Green Chem 2007, 9, 295–302. [Google Scholar]
- Kamm, B.; Kamm, M.; Schmidt, M.; Hirth, T.; Schulze, M. Lignocellulose-Based Chemical Products and Product Family Trees. In Biorefinerie—Industrial Processes and Products: Status Quo and Future Directions; Kamm, B., Gruber, P., Kamm, M., Eds.; Wiley-VCH: Weinheim, Germany, 2006; pp. 97–150. [Google Scholar]
- Jenck, J.F.; Agterberg, F.; Droescher, M.J. Products and processes for a sustainable chemical industry: A review of achievements and prospects. Green Chem 2004, 6, 544–556. [Google Scholar]
- Deswarte, F.E.I.; Clark, J.H.; Hardy, J.J.E. Extraction of High-value Chemicals from Wheat Straw by Supercritical Carbon Dioxide. Proceedings of the 230th National Meeting of the American Chemical Society, Washington, DC, USA, 28 August–1 September 2005; American Chemical Society: Washington, DC, USA, 2005; pp. U61–U62. [Google Scholar]
- Eckert, C.; Liotta, C.; Ragauskas, A.; Hallett, J.; Kitchens, C.; Hill, E.; Draucker, L. Tunable solvents for fine chemicals from the biorefinery. Green Chem 2007, 9, 545–548. [Google Scholar]
- Fernandes, P.; Cabral, J.M.S. Phytosterols: Applications and recovery methods. Bioresour. Technol 2007, 98, 2335–2350. [Google Scholar]
- Hamunen, A. Process for the Purification of β-sitosterol Isolated from the Unsaponifiables in Crude Soap from the Sulphate Cellulose Process. U.S. Patent 4422974, 1983. [Google Scholar]
- Niemela, K. Low-molecular-weigth Organic Compounds in Brich Kraft Black Liquor. Ph.D Dissertation, Helsinki University of Technology, Helsinki, Finland, 1990. [Google Scholar]
- Pietarinen, S.P.; Willfor, S.M.; Ahotupa, M.O.; Hemming, J.E.; Holmbom, B.R. Knotwood and bark extracts: Strong antioxidants from waste materials. J. Wood Sci 2006, 52, 436–444. [Google Scholar]
- Willfor, S.; Nisula, L.; Hemming, J.; Reunanen, M.; Holmbom, B. Bioactive phenolic substances in industrially important tree species. Part 2: Knots and stemwood of fir species. Holzforschung 2004, 58, 650–659. [Google Scholar]
- Willfor, S.; Nisula, L.; Hemming, J.; Reunanen, M.; Holmbom, B. Bioactive phenolic substances in industrially important tree species. Part 1: Knots and stemwood of different spruce species. Holzforschung 2004, 58, 335–344. [Google Scholar]
- Kolomitsyn, I.V.; Holy, J.; Perkins, E.; Krasutsky, P.A. Analysis and antiproliferative activity of bark extractives of Betula neoalaskana and B. papyrifera. Synthesis of the most active extractive component—betulin 3-caffeate. Nat. Prod. Commun 2007, 2, 17–26. [Google Scholar]
- Krasutsky, P.A. Birch bark research and development. Nat. Prod. Rep 2006, 23, 919–942. [Google Scholar]
- Domingues, R.M.A.; Patinha, D.J.S.; Sousa, G.D.A.; Villaverde, J.J.; Silva, C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Eucalyptus biomass residues from agro-forest and pulping industries as sources of high-value triterpenic compounds. Cell. Chem. Technol 2011, 45, 475–481. [Google Scholar]
- Domingues, R.M.A.; Sousa, G.D.A.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Eucalyptus globulus biomass residues from pulping industry as a source of high value triterpenic compounds. Ind. Crop. Prod 2010, 31, 65–70. [Google Scholar]
- Domingues, R.M.A.; Sousa, G.D.A.; Silva, C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. High value triterpenic compounds from the outer barks of several Eucalyptus species cultivated in Brazil and in Portugal. Ind. Crops Prod 2011, 33, 158–164. [Google Scholar]
- Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Cavaleiro, J.A.S. Lipophilic extractives of the inner and outer barks of Eucalyptus globulus. Holzforschung 2002, 56, 372–379. [Google Scholar]
- Santos, S.A.O.; Freire, C.S.R.; Domingues, M.R.M.; Silvestre, A.J.D.; Neto, C.P. Characterization of phenolic components in polar extracts of Eucalyptus globulus labill. bark by high-performance liquid chromatography-mass spectrometry. J. Agric. Food. Chem 2011, 59, 9386–9393. [Google Scholar]
- Santos, S.A.O.; Villaverde, J.J.; Freire, C.S.R.; Domingues, M.R.M.; Neto, C.P.; Silvestre, A.J.D. Phenolic composition and antioxidant activity of Eucalyptus grandis, E. urograndis (E. grandis × E. urophylla) and E. maidenii bark extracts. Ind. Crop. Prod 2012, 39, 120–127. [Google Scholar]
- Patinha, D.J.S.; Domingues, R.M.A.; Villaverde, J.J.; Silva, A.M.S.; Silva, C.M.; Freire, C.S.R.; Neto, C.P.; Silvestre, A.J.D. Lipophilic extractives from the bark of Eucalyptus grandis × globulus, a rich source of methyl morolate: Selective extraction with supercritical CO2. Ind. Crop. Prod 2012, (in press).. [Google Scholar]
- Laszczyk, M.N. pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med 2009, 75, 1549–1560. [Google Scholar]
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Markova, L.; Urban, M.; Sarek, J. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat. Prod. Rep 2006, 23, 394–411. [Google Scholar]
- Sultana, N.; Ata, A. Oleanolic acid and related derivatives as medicinally important compounds. J. Enzym. Inhib. Med. Chem 2008, 23, 739–756. [Google Scholar]
- Yogeeswari, P.; Sriram, D. Betulinic acid and its derivatives: A review on their biological properties. Curr. Med. Chem 2005, 12, 657–666. [Google Scholar]
- Pitzer, K.S.; Schreiber, D.R. Improving equation-of-state accuracy in the critical region; equations for carbon dioxide and neopentane as examples. Fluid Phase Equilib 1988, 41, 1–17. [Google Scholar]
- Galicia-Luna, L.A.; Ortega-Rodriguez, A.; Richon, D. New apparatus for the fast determination of high-pressure vapor-liquid equilibria of mixtures and of accurate critical pressures. J. Chem. Eng. Data 2000, 45, 265–271. [Google Scholar]
- Güçlü-Üstünda, O.; Temelli, F. Solubility behavior of ternary systems of lipids, cosolvents and supercritical carbon dioxide and processing aspects. J. Supercrit. Fluids 2005, 36, 1–15. [Google Scholar]
- Moreau, R.A.; Whitaker, B.D.; Hicks, K.B. Phytosterols, phytostanols, and their conjugates in foods: Structural diversity, quantitative analysis, and health-promoting uses. Prog. Lipid Res 2002, 41, 457–500. [Google Scholar]
- Ostlund, R.E. Phytosterols in human nutrition. Annu. Rev. Nutr 2002, 22, 533–549. [Google Scholar]
- Budzikiewicz, H.; Wilson, J.M.; Djerassi, C. Mass spectrometry in structural and stereochemical problems. 32 pentacyclic triterpenes. J. Am. Chem. Soc 1963, 85, 3688–3699. [Google Scholar]
- Burnoufradosevich, M.; Delfel, N.E.; England, R. Gas chromatography-mass spectrometry of oleanane-type and ursane-type triterpenes—Application chenopodium-quinoa triterpenes. Phytochemistry 1985, 24, 2063–2066. [Google Scholar]
- Gutierrez, A.; del Rio, J.C.; Gonzalez-Vila, F.J.; Martin, F. Chemical composition of lipophilic extractives from Eucalyptus globulus Labill. wood. Holzforschung 1999, 53, 481–486. [Google Scholar]
- Pelillo, M.; Lafelice, G.; Marconi, E.; Caboni, M. Identification of plant sterols in hexaploid and tetraploid wheats using gas chromatography with mass spectrometry. Rapid Commun. Mass Spectrom 2003, 17, 2245–2252. [Google Scholar]
- Pereira, S.I.; Freire, C.S.R.; Neto, C.P.; Silvestre, A.J.D.; Silva, A.M.S. Chemical composition of the epicuticular wax from the fruits of Eucalyptus globulus. Phytochem. Anal 2005, 16, 364–369. [Google Scholar]
- Rencoret, J.; Gutierrez, A.; del Rio, J.C. Lipid and lignin composition of woods from different eucalypt species. Holzforschung 2007, 61, 165–174. [Google Scholar]
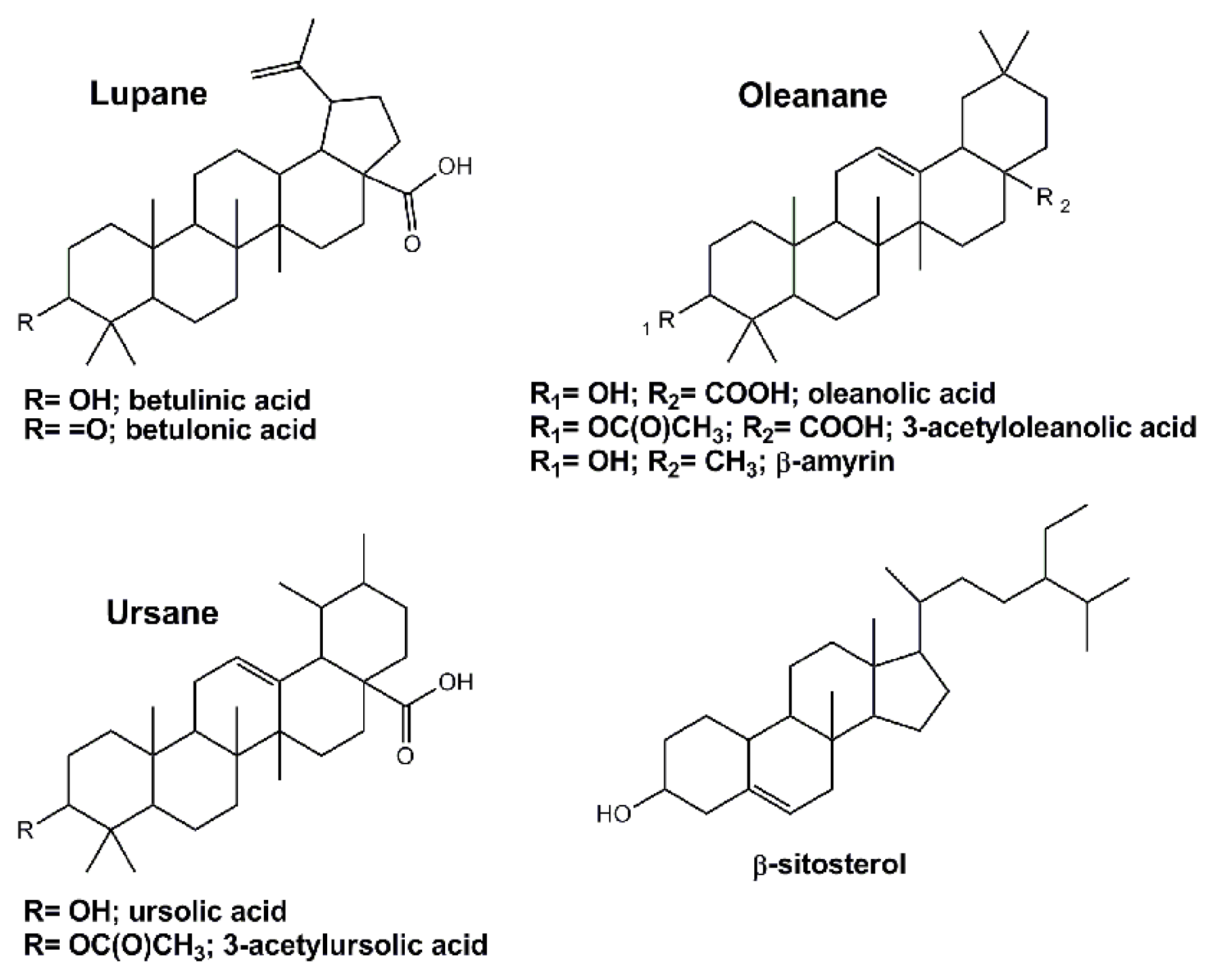




| tr (min) | Compound | Content (mg/kg of bark) |
|---|---|---|
| Fatty acids (FA) | 482.8 | |
| 29.81 | tetradecanoic acid | 40.6 |
| 34.50 | hexadecanoic acid | 109.2 |
| 38.15 | linoleic acid | 16.7 |
| 38.82 | oleic acid | 22.3 |
| 42.10 | octadecanoic acid | 21.6 |
| 48.61 | docosanoic acid | 27.6 |
| 50.15 | tetracosanoic acid | 114.0 |
| 55.03 | hexacosanoic acid | 109.3 |
| 59.87 | octacosanoic acid | 21.6 |
| Long chain aliphatic alcohols (LCAA) | 445.5 | |
| 32.73 | hexadecan-1-ol | 96.6 |
| 36.49 | Z-9-octadecen-1-ol | 81.9 |
| 37.19 | E-9-octadecen-1-ol | 23.6 |
| 37.94 | octadecan-1-ol | 62.6 |
| 49.99 | tetracosan-1-ol | 25.6 |
| 53.58 | hexacosan-1-ol | 85.9 |
| 59.37 | octacosan-1-ol | 69.1 |
| Sterols (ST) | 242.5 | |
| 60.13 | β-sitosterol | 242.5 |
| Triterpenoids (TT) | 9,001.7 | |
| 60.07 | β-amyrin | 313.7 |
| 64.21 | betulonic acid | 797.7 |
| 64.96 | oleanolic acid | 712.5 |
| 65.86 | betulinic acid | 618.7 |
| 66.18 | ursolic acid | 2,771.9 |
| 66.68 | 3-acetyloleanolic acid | 691.2 |
| 66.97 | 3-acetylbetulinic acid | 55.1 |
| 68.03 | 3-acetylursolic acid | 2,635.1 |
| unidentified triterpenoids | 405.8 | |
| Other compounds/unidentified compounds | 566.5 | |
| Total detected compounds | 10,738.9 | |
| Supercritical Fluid Extraction b | Soxhlet | |||||
|---|---|---|---|---|---|---|
| P (bar) | 100 | 160 | 220 | 160 | 160 | Dichloromethane 7 h |
| Ethanol (%, wt) | 0 | 0 | 0 | 5 | 8 | |
| Fatty acids | 243.8 | 189.5 | 374.8 | 174.8 | 351.9 | 482.8 |
| Long chain aliphatic alcohols | 246.2 | 173.7 | 226.5 | 345.3 | 253.1 | 445.5 |
| Triterpenoids | 1,193.4 | 2,063.3 | 3,273.0 | 5,174.4 | 6,534.1 | 9,244.2 |
| β-sitosterol | 190.6 | 131.1 | 236.8 | 272.0 | 261.5 | 242.5 |
| β-amyrin | 137.6 | 91.8 | 171.7 | 151.1 | 168.6 | 313.7 |
| betulonic acid | 198.6 | 399.8 | 648.1 | 653.8 | 731.6 | 797.7 |
| oleanolic acid | ND a | 49.9 | 79.5 | 661.1 | 691.4 | 712.5 |
| betulinic acid | ND a | 123.7 | 177.5 | 572.4 | 633.0 | 618.7 |
| ursolic acid | ND a | 73.0 | 69.4 | 1,740.5 | 1,779.6 | 2,771.9 |
| 3-acetyloleanolic acid | 143.9 | 238.5 | 384.6 | 172.0 | 429.1 | 691.2 |
| 3-acetylursolic acid | 409.9 | 875.6 | 1,377.6 | 820.6 | 1,495.3 | 2,635.1 |
| Other/unidentified triterpenoids | 112.8 | 79.9 | 127.8 | 130.9 | 344.0 | 460.9 |
| Other compounds | 520.4 | 398.3 | 926.2 | 523.6 | 521.9 | 566.5 |
| Total detected | 2,203.8 | 2,824.8 | 4,800.5 | 6,218.2 | 7,661.0 | 10,739.0 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Domingues, R.M.A.; Oliveira, E.L.G.; Freire, C.S.R.; Couto, R.M.; Simões, P.C.; Neto, C.P.; Silvestre, A.J.D.; Silva, C.M. Supercritical Fluid Extraction of Eucalyptus globulus Bark—A Promising Approach for Triterpenoid Production. Int. J. Mol. Sci. 2012, 13, 7648-7662. https://doi.org/10.3390/ijms13067648
Domingues RMA, Oliveira ELG, Freire CSR, Couto RM, Simões PC, Neto CP, Silvestre AJD, Silva CM. Supercritical Fluid Extraction of Eucalyptus globulus Bark—A Promising Approach for Triterpenoid Production. International Journal of Molecular Sciences. 2012; 13(6):7648-7662. https://doi.org/10.3390/ijms13067648
Chicago/Turabian StyleDomingues, Rui M. A., Eduardo L. G. Oliveira, Carmen S. R. Freire, Ricardo M. Couto, Pedro C. Simões, Carlos P. Neto, Armando J. D. Silvestre, and Carlos M. Silva. 2012. "Supercritical Fluid Extraction of Eucalyptus globulus Bark—A Promising Approach for Triterpenoid Production" International Journal of Molecular Sciences 13, no. 6: 7648-7662. https://doi.org/10.3390/ijms13067648
APA StyleDomingues, R. M. A., Oliveira, E. L. G., Freire, C. S. R., Couto, R. M., Simões, P. C., Neto, C. P., Silvestre, A. J. D., & Silva, C. M. (2012). Supercritical Fluid Extraction of Eucalyptus globulus Bark—A Promising Approach for Triterpenoid Production. International Journal of Molecular Sciences, 13(6), 7648-7662. https://doi.org/10.3390/ijms13067648