A Novel Chemometric Method for the Prediction of Human Oral Bioavailability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dataset Construction
2.2. Molecular Descriptors
2.3. Database Division
2.4. Design of Training and Test Sets
2.5. MLR
2.6. Partial Least Squares Analysis (PLS)
2.7. Support Vector Regression (SVR)
3. Results and Discussion
3.1. Dataset Division
3.2. Design of Training and Test Sets
3.3. Model Building
3.3.1. The Results of MLR
3.3.2. The Results of PLS
3.3.3. The Results of SVR
3.4. Comparison of the MLR, PLS and SVR Models
4. Conclusions
Acknowledgement
References
- O’Brien, S.E.; de Groot, M.J. Greater than the sum of its parts: Combining models for useful ADMET prediction. J. Med. Chem 2005, 48, 1287–1291. [Google Scholar]
- Beresford, A.P.; Selick, H.E.; Tarbit, M.H. The emerging importance of predictive ADME simulation in drug discovery. Drug Discov. Today 2002, 7, 109–116. [Google Scholar]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of drug absorption using multivariate statistics. J. Med. Chem 2000, 43, 3867–3877. [Google Scholar]
- Chen, M.L.; Shah, V.; Patnaik, R.; Adams, W.; Hussain, A.; Conner, D.; Mehta, M.; Malinowski, H.; Lazor, J.; Huang, S.M.; et al. Bioavailability and bioequivalence: An FDA regulatory overview. Pharm. Res 2001, 18, 1645–1650. [Google Scholar]
- Hou, T.; Li, Y.; Zhang, W.; Wang, J. Recent developments of in silico predictions of intestinal absorption and oral bioavailability. Comb. Chem. High Throughput Scr 2009, 12, 497–506. [Google Scholar]
- Hou, T.; Wang, J. Structure-ADME relationship: Still a long way to go? Expert Opin. Drug Metab. Toxicol 2008, 4, 759–770. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliver. Rev 1997, 23, 3–25. [Google Scholar]
- Aller, S.G.; Yu, J.; Ward, A.; Weng, Y.; Chittaboina, S.; Zhuo, R.; Harrell, P.M.; Trinh, Y.T.; Zhang, Q.; Urbatsch, I.L.; et al. Structure of P-Glycoprotein reveals a molecular basis for poly-Specific Drug Binding. Science 2009, 323, 1718–1722. [Google Scholar]
- Yoshida, F.; Topliss, J.G. QSAR model for drug human oral bioavailability. J. Med. Chem 2000, 43, 2575–2585. [Google Scholar]
- Hou, T.J.; Wang, J.M.; Zhang, W.; Xu, X.J. ADME evaluation in drug discovery. 6. Can oral bioavailability in humans be effectively predicted by simple molecular property-based rules? J. Chem. Inf. Model 2007, 47, 460–463. [Google Scholar]
- Wang, Z.; Yan, A.X.; Yuan, Q.P.; Gasteiger, J. Explorations into modeling human oral bioavailability. Eur. J. Med. Chem 2008, 43, 2442–2452. [Google Scholar]
- Ma, C.Y.; Yang, S.Y.; Zhang, H.; Xiang, M.L.; Huang, Q.; Wei, Y.Q. Prediction models of human plasma protein binding rate and oral bioavailability derived by using GA-CG-SVM method. J. Pharma. Biomed 2008, 47, 677–682. [Google Scholar]
- Tian, S.; Li, Y.Y.; Wang, J.M.; Zhang, J.; Hou, T.J. ADME evaluation in drug discovery. 9. prediction of oral bioavailability in humans based on molecular properties and structural fingerprints. Mol. Phar 2011, 8, 841–851. [Google Scholar]
- Hou, T.; Li, Y.; Zhang, W.; Wang, J. Recent developments of in silico predictions of intestinal absorption and oral bioavailability. Comb. Chem. High Throughput Scr 2009, 12, 497–506. [Google Scholar]
- Chan, L.M.; Lowes, S.; Hirst, B.H. The ABCs of drug transport in intestine and liver: Efflux proteins limiting drug absorption and bioavailability. Eur. J. Pharm. Sci 2004, 21, 25–51. [Google Scholar]
- Doherty, M.M.; Charman, W.N. The mucosa of the small intestine: How clinically relevant as an organ of drug metabolism? Clin. Pharmacokinet 2002, 41, 235–253. [Google Scholar]
- Benet, L.Z.; Wu, C.Y.; Hebert, M.F.; Wacher, V.J. Intestinal drug metabolism and antitransport processes: A potential paradigm shift in oral drug delivery. J. Control Rel 1996, 39, 139–143. [Google Scholar]
- Borchardt, R.T.; Smith, P.; Wilson, G. Models for Assessing Drug Absorption and Metabolism; Plenum Press: New York, NY, USA, 1996. [Google Scholar]
- Hou, T.J.; Xu, X.J. ADME evaluation in drug discovery. 1. Applications of genetic algorithms on the prediction of blood-brain partitioning of a large set drugs from structurally derived descriptors. J. Mol. Model 2002, 8, 337–349. [Google Scholar]
- Chemical Book Database. Available online: http://www.chemicalbook.com/ accessed on 5 June 2012.
- Wang, X.; Yang, W.; Xu, X.; Zhang, H.; Li, Y.; Wang, Y.H. Studies of benzothiadiazine derivatives as hepatitis C virus NS5B polymerase inhibitors using 3D-QSAR, molecular docking and molecular dynamics. Curr. Med. Chem 2010, 17, 2788–2803. [Google Scholar]
- Hancock, T.; Put, R.; Coomans, D.; vander Heyden, Y.; Everingham, Y. A performance comparison of modern statistical techniques for molecular descriptor selection and retention prediction in chromatographic QSRR studies. Chemometr. Intell. Lab 2005, 76, 185–196. [Google Scholar]
- Saíz-Urra, L.; González, M.P.; Teijeira, M. QSAR studies about cytotoxicity of benzophenazines with dual inhibition toward both topoisomerases I and II: 3D-MoRSE descriptors and statistical considerations about variable selection. Bioorg. Med. Chem 2006, 14, 7347–7358. [Google Scholar]
- Khajeh, A.; Modarress, H. Quantitative structure–property relationship for surface tension of some common alcohols. J. Chemometr 2011, 25, 333–339. [Google Scholar]
- Talete, S. Dragon for windows (software for molecular descriptor calculations), version 5.4. Available online: http://www.talete.mi.it accessed on 20 May 2011.
- Jain, A.N. Surflex: Fully automatic flexible molecular docking using a molecular similarity-based search engine. J. Med. Chem 2003, 46, 499–511. [Google Scholar]
- RCSB Protein Data Bank. Available online: http://www.rcsb.org accessed on 5 June 2012.
- Xu, X.; Fu, J.X.; Wang, H.; Zhang, B.D.; Wang, X.; Wang, Y.H. Influence of P-glycoprotein on embryotoxicity of the antifouling biocides to sea urchin (Strongylocentrotus intermedius). Ecotoxicology 2011, 20, 419–428. [Google Scholar]
- Xue, Y.; Guoyin, C.; Guan, Y.N.; Cracknell, A.P.; Jiakui, T. Iterative self-consistent approach for Earth surface temperature determination. Int. J. Remote Sens 2005, 26, 185–192. [Google Scholar]
- Vesanto, J.; Alhoniemi, E. Clustering of the self-organizing map. IEEE Trans. Neural Networks 2000, 11, 586–600. [Google Scholar]
- Wang, Y.; Li, Y.; Ding, J.; Wang, Y.; Chang, Y. Prediction of binding affinity for estrogen receptor α modulators using statistical learning approaches. Mol. Divers 2008, 12, 93–102. [Google Scholar]
- Höskuldsson, A. PLS regression methods. J. Chemometr 1988, 2, 211–228. [Google Scholar]
- Chin, W.W.; Marcolin, B.L.; Newsted, P.R. A partial least squares latent variable modeling approach for measuring interaction effects: Results from a monte carlo simulation study and voice mail emotion/adoption study. Inf. Syst. Res 2003, 14, 189–217. [Google Scholar]
- Wold, S.; Ruhe, A.; Wold, H.; Dunn, W.J.J. The collinearity problem in linear regression. The partial least squares (PLS) approach to generalized inverses. Sci. Stat. Comput 1984, 5, 735–743. [Google Scholar]
- Vapnik, V.; Golowich, S.; Smola, A. Support Vector Method for Function Approximation, Regression Estimation, and Signal Processing. In Advances in Neural Information Processing Systems 9, Proceedings of the 1996 Neural Information Processing Systems Conference NIPS 1996; The MIT Press: Cambridge, MA, USA, 1997; pp. 281–287. [Google Scholar]
- Benet, L.Z.; Cummins, C.L.; Wu, C.Y. Unmasking the dynamic interplay between efflux transporters and metabolic enzymes. Int. J. Pharm 2004, 277, 3–9. [Google Scholar]
- Szakács, G.; Váradi, A.; Özvegy-Laczka, C.; Sarkadi, B. The role of ABC transporters in drug absorption, distribution, metabolism, excretion and toxicity (ADME–Tox). Drug Discov. Today 2008, 13, 379–393. [Google Scholar]
- Guha, R.; Serra, J.R.; Jurs, P.C. Generation of QSAR sets with a self-organizing map. J. Mol. Graph. Model. 2004, 1–14. [Google Scholar]
- Todeschini, R.; Gramatica, P.; Provenzani, R.; Marengo, E. Weighted holistic invariant molecular descriptors. Part 2. Theory development and applications on modeling physicochemical properties of polyaromatic hydrocarbons. Chemom. Intell. Lab. Syst 1995, 27, 221–229. [Google Scholar]
- Cristianini, N.; Shawe-Taylor, J. An Introduction to Support Vector Machines; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Schölkopf, B.; Burges, C.J.C.; Smola, A.J. Advances in Kernel Methods: Support Vector Learning; The MIT Press: Cambridge, MA, USA, 1999. [Google Scholar]
- Bhandare, P.; Mendelson, Y.; Peura, R.A.; Janatsch, G.; Kruse-Jarres, J.D.; Marbach, R.; Heise, H.M. Multivariate determination of glucose in whole blood using partial least-squares and artificial neural networks based on mid-infrared spectroscopy. Appl. Spectrosc. 1993, 47, 1214–1221. [Google Scholar]
- Goodarzi, M.; Freitas, M.P.; Richard, J. Feature selection and linear/nonlinear regression methods for the accurate prediction of glycogen synthase kinase-3B inhibitory activities. QSAR Comb. Sci 2008, 27, 1092–1098. [Google Scholar]
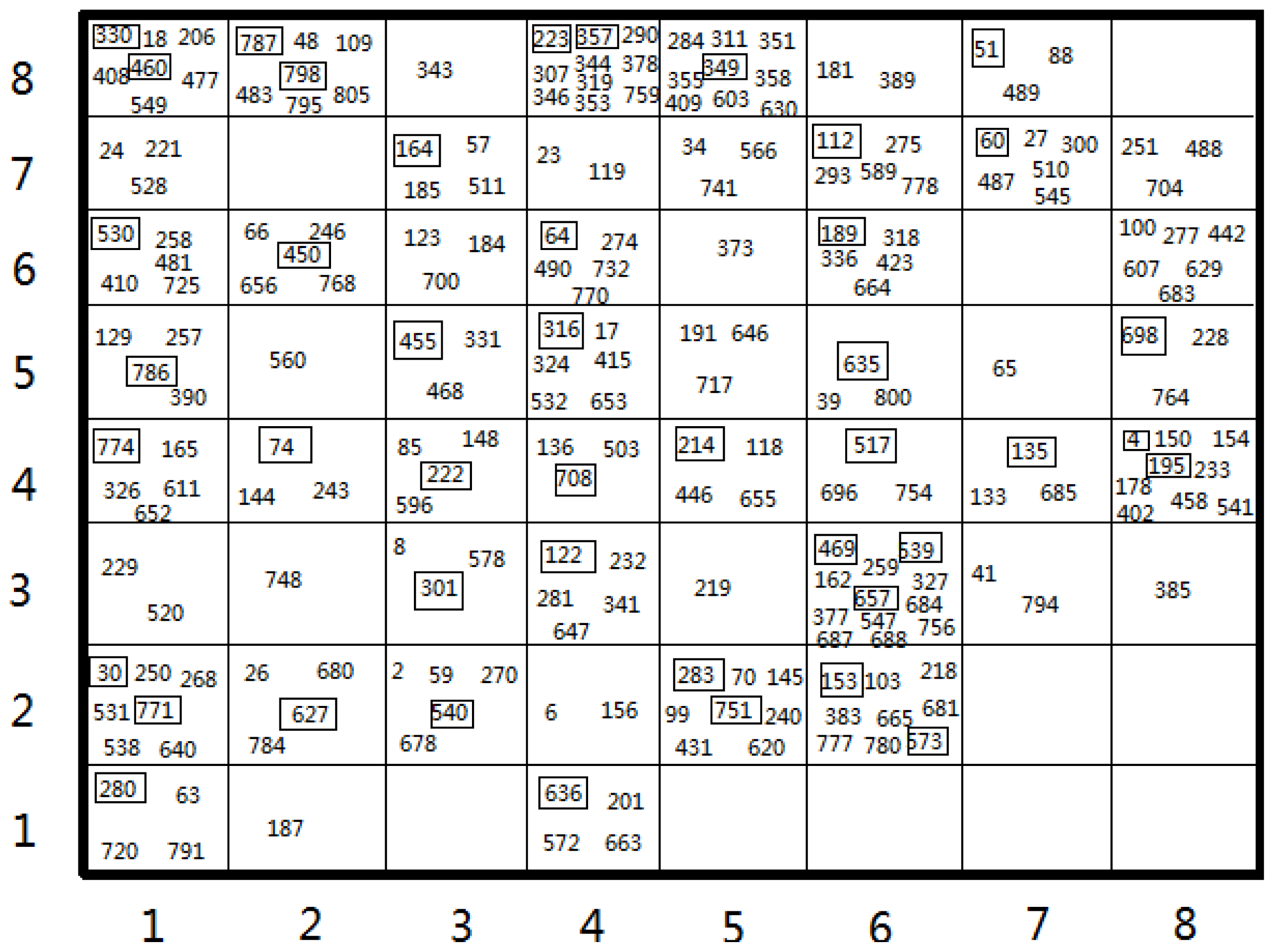
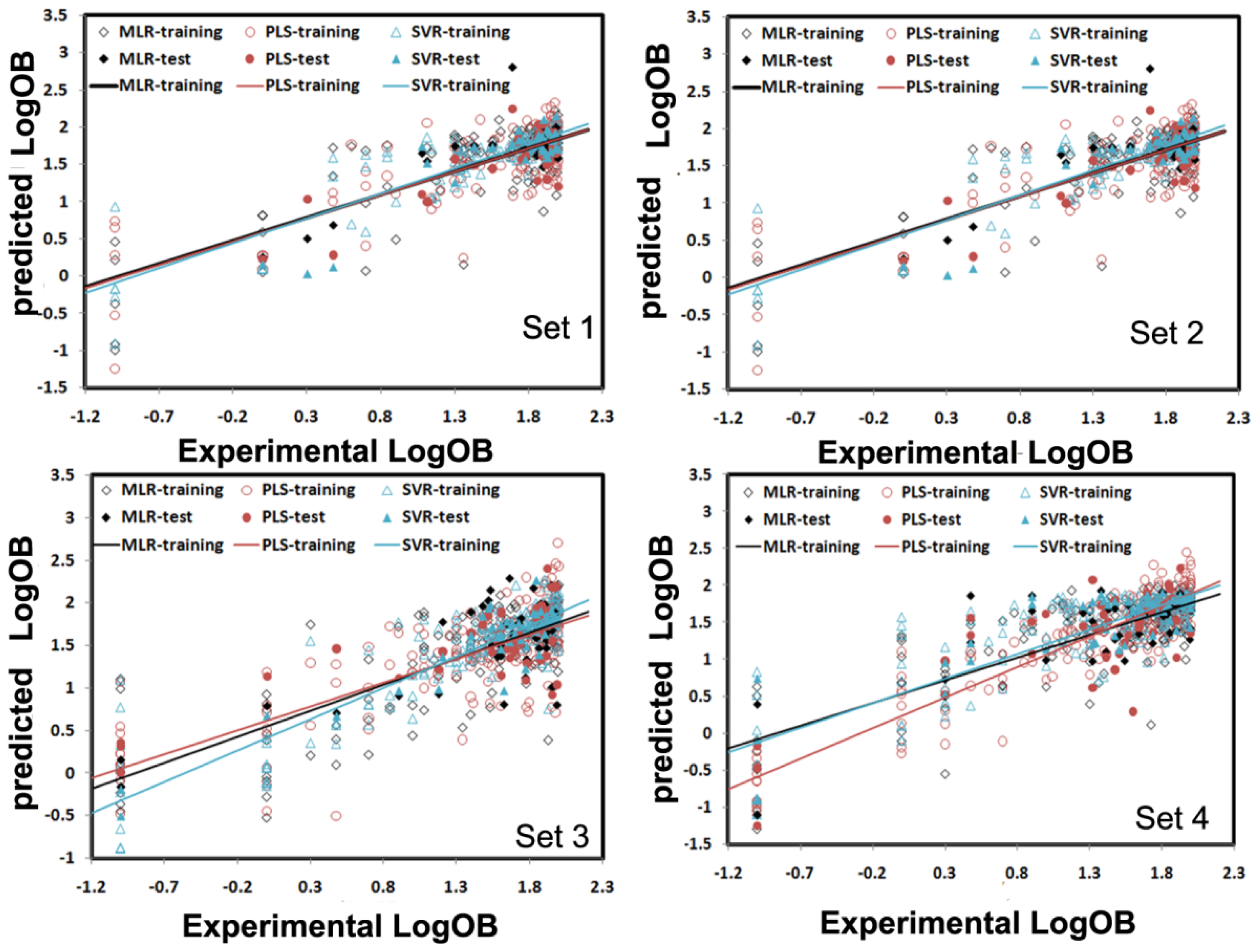
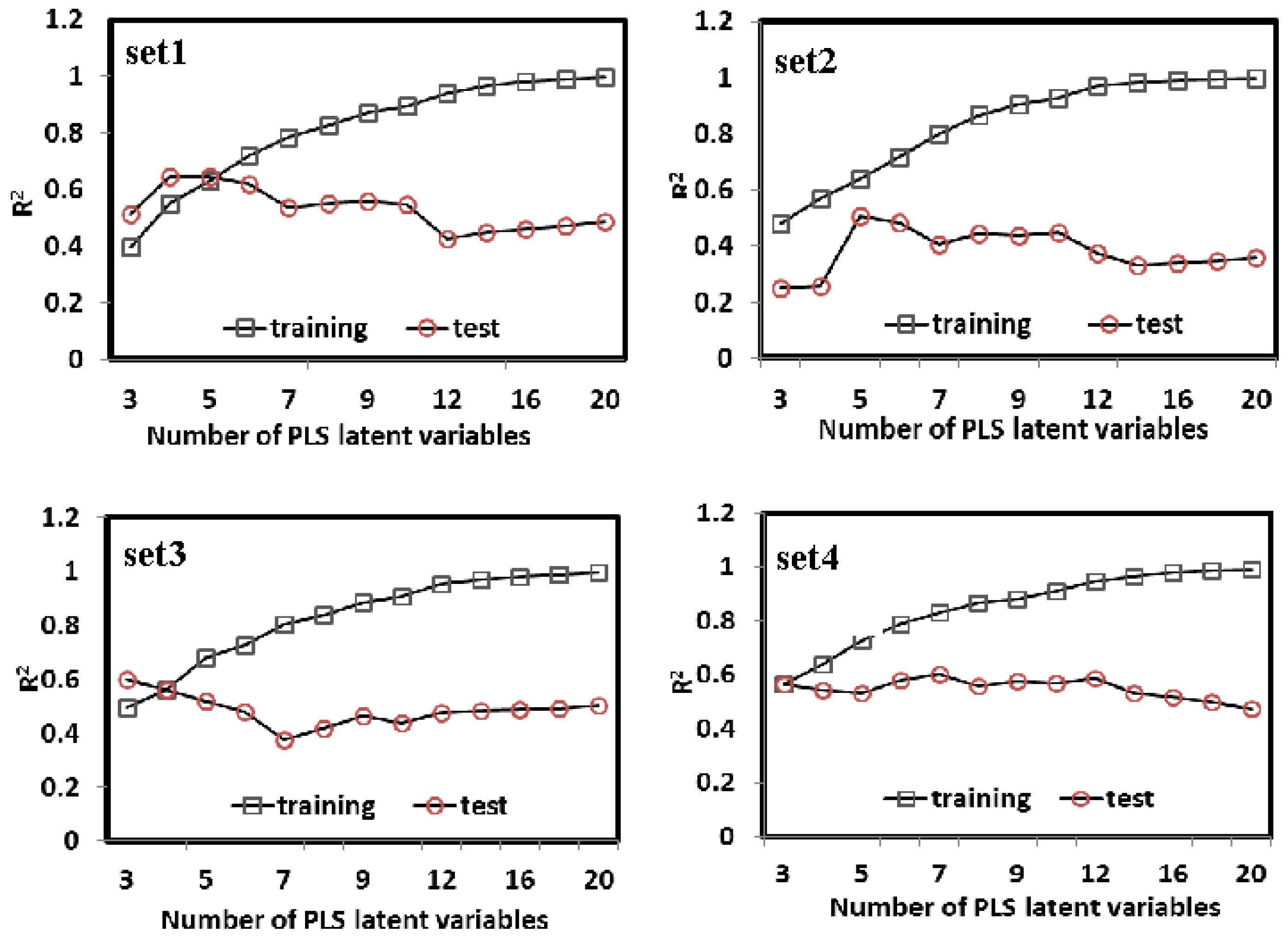

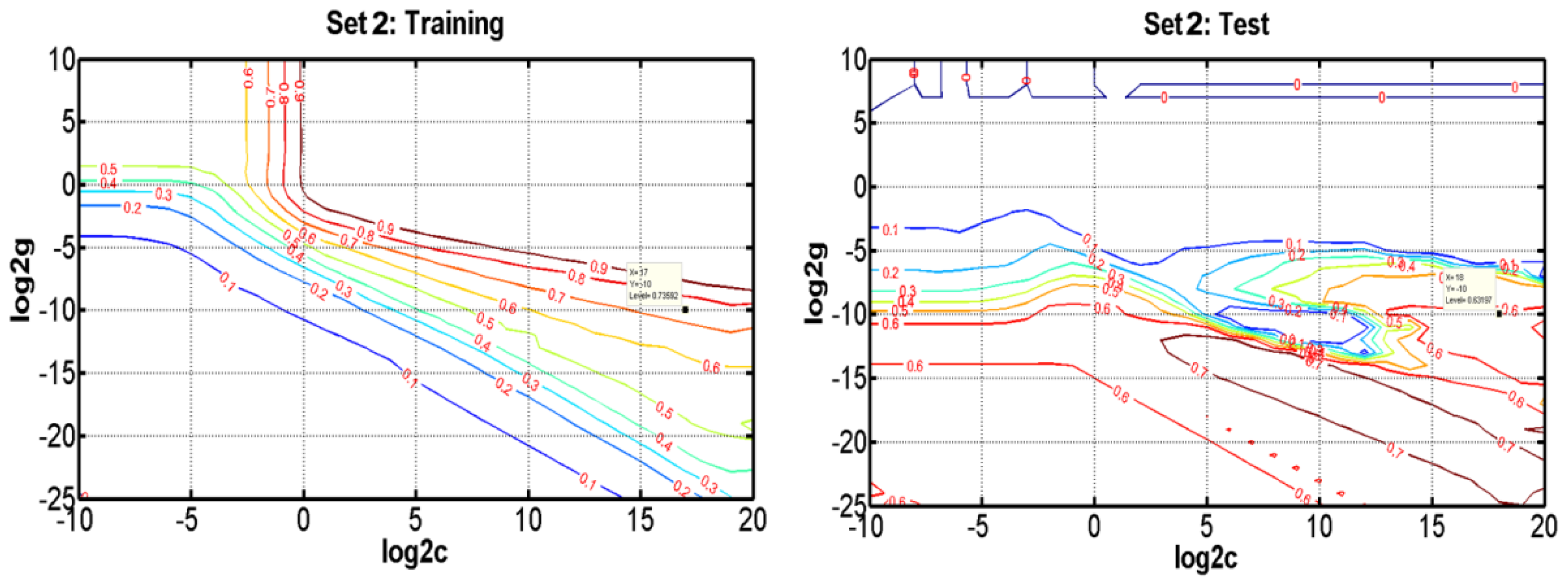
| Set 1 | Set 2 | Set 3 | Set 4 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Training size | Test size | Training size | Test size | Training size | Test size | Training size | Test size | |||||||||
| 156 | 36 | 122 | 27 | 180 | 44 | 197 | 43 | |||||||||
| R2 | SEE | Qex2 | SEP | R2 | SEE | Qex2 | SEP | R2 | SEE | Qex2 | SEP | R2 | SEE | Qex2 | SEP | |
| MLR | 0.621 | 0.411 | 0.612 | 0.311 | 0.521 | 0.400 | 0.541 | 0.482 | 0.610 | 0.492 | 0.612 | 0.48 | 0.61 | 0.482 | 0.622 | 0.480 |
| PLS | 0.631 | 0.390 | 0.651 | 0.311 | 0.643 | 0.331 | 0.511 | 0.470 | 0.561 | 0.500 | 0.561 | 0.521 | 0.831 | 0.312 | 0.600 | 0.490 |
| SVM | 0.800 | 0.311 | 0.720 | 0.220 | 0.750 | 0.280 | 0.630 | 0.772 | 0.780 | 0.361 | 0.800 | 0.361 | 0.690 | 0.421 | 0.682 | 0.461 |
| SVMT | 0.840 | - | 0.731 | - | 0.731 | - | 0.310 | - | 0.970 | - | 0.590 | - | 0.990 | - | 0.561 | - |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xu, X.; Zhang, W.; Huang, C.; Li, Y.; Yu, H.; Wang, Y.; Duan, J.; Ling, Y. A Novel Chemometric Method for the Prediction of Human Oral Bioavailability. Int. J. Mol. Sci. 2012, 13, 6964-6982. https://doi.org/10.3390/ijms13066964
Xu X, Zhang W, Huang C, Li Y, Yu H, Wang Y, Duan J, Ling Y. A Novel Chemometric Method for the Prediction of Human Oral Bioavailability. International Journal of Molecular Sciences. 2012; 13(6):6964-6982. https://doi.org/10.3390/ijms13066964
Chicago/Turabian StyleXu, Xue, Wuxia Zhang, Chao Huang, Yan Li, Hua Yu, Yonghua Wang, Jinyou Duan, and Yang Ling. 2012. "A Novel Chemometric Method for the Prediction of Human Oral Bioavailability" International Journal of Molecular Sciences 13, no. 6: 6964-6982. https://doi.org/10.3390/ijms13066964
APA StyleXu, X., Zhang, W., Huang, C., Li, Y., Yu, H., Wang, Y., Duan, J., & Ling, Y. (2012). A Novel Chemometric Method for the Prediction of Human Oral Bioavailability. International Journal of Molecular Sciences, 13(6), 6964-6982. https://doi.org/10.3390/ijms13066964




