Synthesis and Characterization of Polyethylene Glycol Mediated Silver Nanoparticles by the Green Method
Abstract
:1. Introduction
2. Results and Discussion
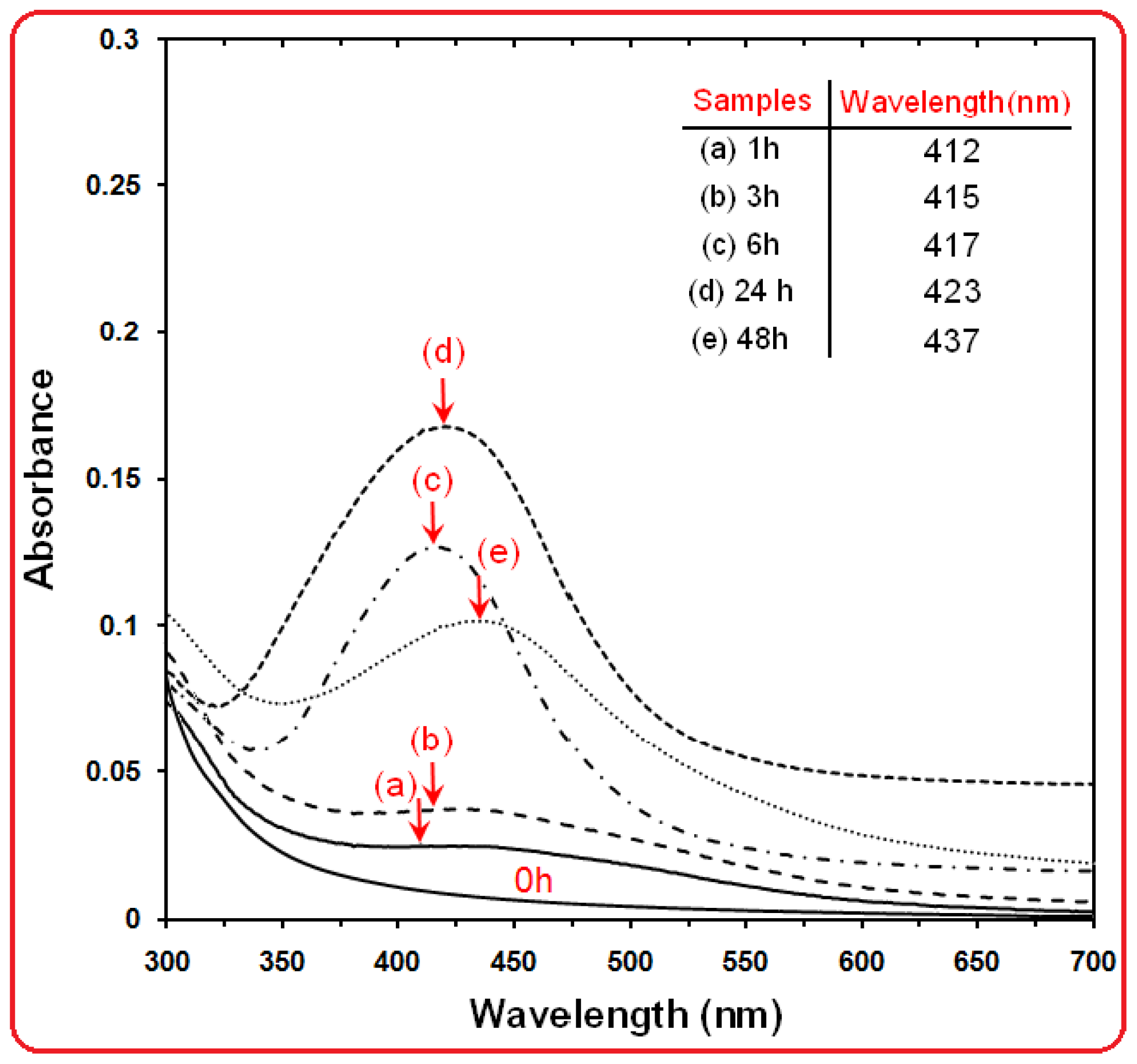
2.1. UV-Visible Spectroscopy
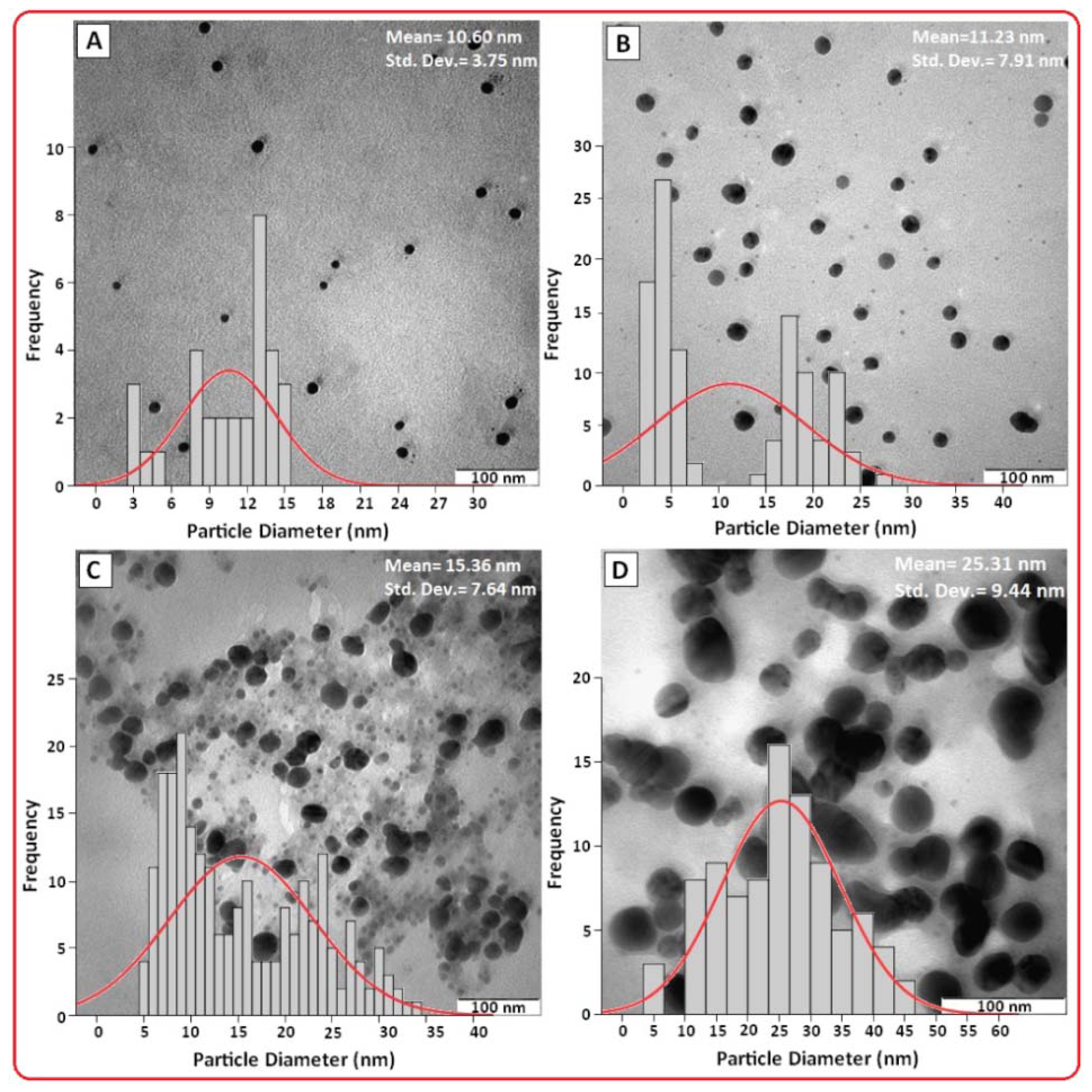
2.2. Morphologie Study
2.3. Powder X-ray Diffraction
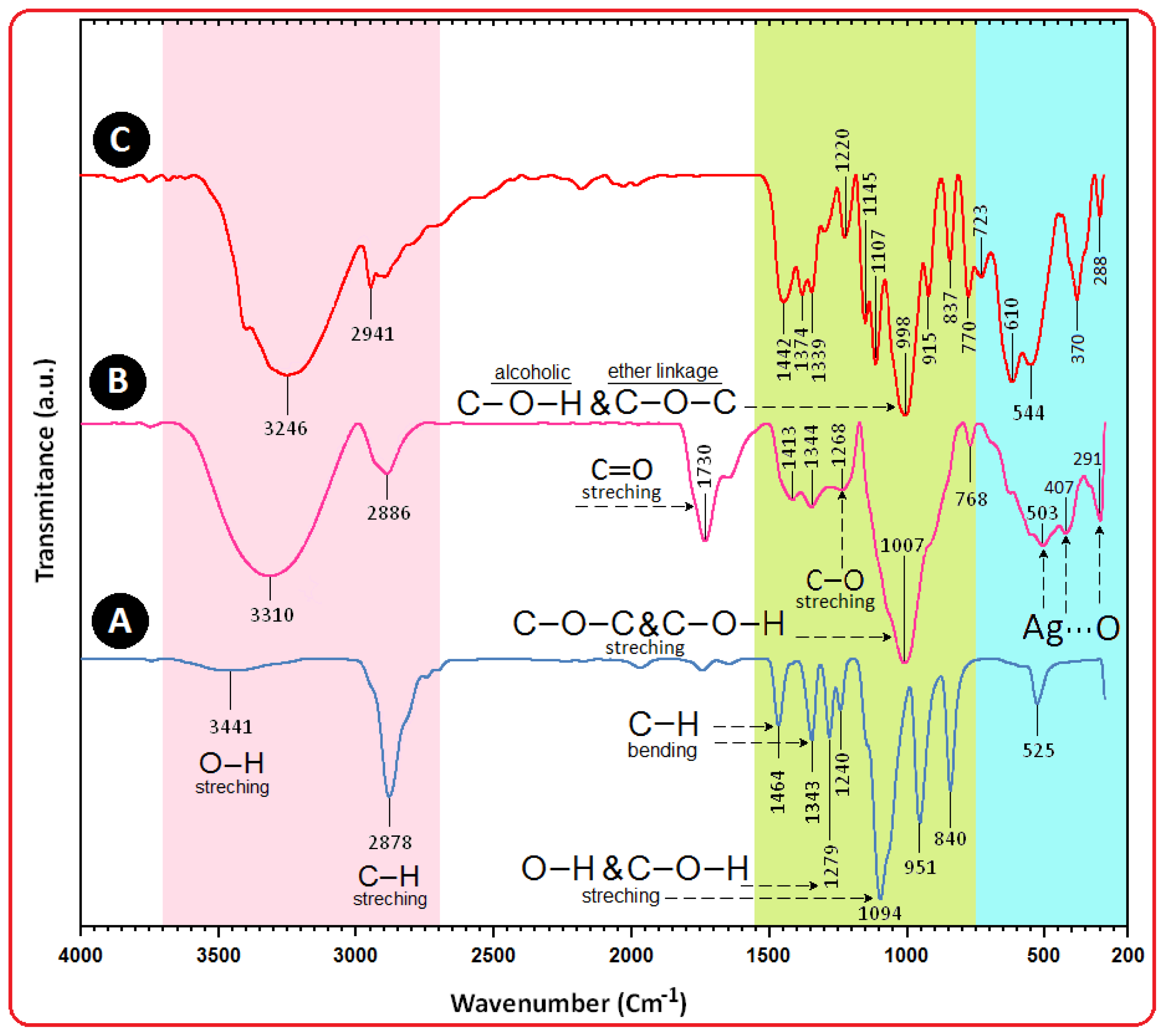
2.4. Surface Chemistry (FT-IR)
2.5. Zeta Potential Measurement
3. Experimental Section
3.1. Materials and Method
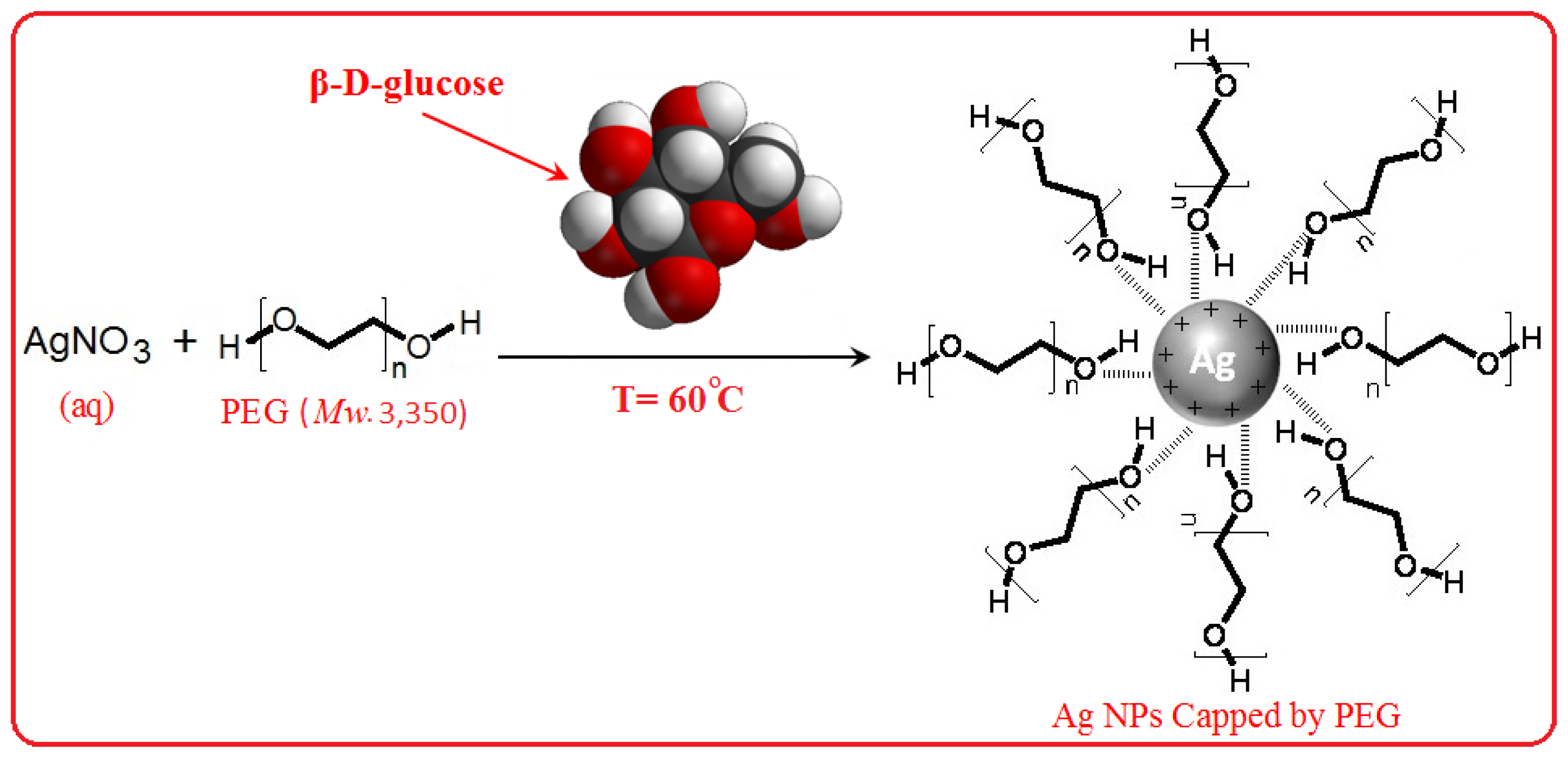
3.2. Synthesis of Ag NPs by Using Green Method
3.3. Characterization Methods and Instruments
4. Conclusions
Acknowledgements
References
- Murphy, C.J. Sustainability as an emerging design criterion in nanoparticle synthesis and applications. J. Mater. Chem 2008, 18, 2173–2176. [Google Scholar]
- Aihara, N.; Torigoe, K.; Esumi, K. Preparation and characterization of gold and silver nanoparticles in layered laponite suspensions. Langmuir 1998, 14, 4945–4949. [Google Scholar]
- Lin, X.Z.; Teng, X.; Yang, H. Direct synthesis of narrowly dispersed silver nanoparticles using a single-source precursor. Langmuir 2003, 19, 10081–10085. [Google Scholar]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci 2009, 145, 83–96. [Google Scholar]
- Papp, S.; Patakfalvi, R.; Dekany, I. Metal nanoparticle formation on layer silicate lamellae. Colloid Polym. Sci 2008, 286, 3–14. [Google Scholar]
- David, D.; Evaboff, J.; Chumanov, G. Syntheis and optical properties of silver nanoparticles and arrays. Chemphyschem 2005, 6, 1221–1231. [Google Scholar]
- Hadad, L.; Perkas, N.; Gofer, Y.; Calderon-Moreno, J.; Ghule, A.; Gedanken, A. Sonochemical deposition of silver nanoparticles on wool fibers. J. Appl. Polym. Sci 2007, 104, 1732–1737. [Google Scholar]
- Kapoor, S. Preparation, characterization, and surface modification of silver particles. Langmuir 1998, 14, 1021–1025. [Google Scholar]
- Ahmad, M.B.; Tay, M.Y.; Shameli, K.; Hussein, M.Z.; Lim, J.J. Green synthesis and characterization of silver/chitosan/polyethylene glycol nanocomposites without any reducing agent. Int. J. Mol. Sci 2011, 12, 4872–4884. [Google Scholar]
- Shameli, K.; Ahmad, M.B.; Yunus, W.M.Z.W.; Ibrahim, N.A.; Rahman, R.A.; Jokar, M. Silver/poly(lactic acid) nanocomposites: Preparation, characterization, and antibacterial activity. Int. J. Nanomedicine 2010, 5, 573–579. [Google Scholar]
- Shameli, K.; Ahmad, M.B.; Yunus, W.M.Z.W.; Ibrahim, N.A. Synthesis and characterization of silver/talc nanocomposites using the wet chemical reduction method. Int. J. Nanomedicine 2010, 5, 743–751. [Google Scholar]
- Tsuji, T.; Thang, D.H.; Okazaki, Y.; Nakanishi, M.; Tsuboi, Y.; Tsuji, M. Preparation of silver nanoparticles by laser ablation in polyvinylpyrrolidone solutions. Appl. Surf. Sci 2008, 254, 5224–5230. [Google Scholar]
- Shameli, K.; Ahmad, M.B.; Yunus, W.M.Z.W.; Rustaiyan, A.; Ibrahim, N.A.; Zargar, M.; Abdollahi, Y. Green synthesis of silver/montmorillonite/chitosan bionanocopmosites using the UV irradiation method and evaluation of antibacterial activity. Int. J. Nanomedicine 2010, 5, 875–887. [Google Scholar]
- Shameli, K.; Ahmad, M.B.; Yunus, W.M.Z.W.; Ibrahim, N.A.; Gharayebi, Y.; Sedaghat, S. Synthesis of silver/montmorillonite nanocomposites using γ-irradiation. Int. J. Nanomedicine 2010, 5, 1067–1077. [Google Scholar]
- Eustis, S.; El-Sayed, M.A. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shape. Chem. Soc. Rev 2006, 35, 209–217. [Google Scholar]
- Donescu, D.; Nistor, C.L.; Purcar, C.; Petcu, C.; Serban, S.; Corobea, M.C.; Ghiurea, M. Formation and dissolution of silver nanoparticles. J. Optoelectron. Adv. Mater. 2009, 1, 44–48. [Google Scholar]
- Tada, H.; Teranishi, K.; Ito, S. Additive effect of sacrificial electron donors on Ag/TiO2 photocatalytic reduction of bis(2-dipyridyl)-disulfide to 2-mercaptopyridine in aqueous media. Langmuir 1999, 15, 7084–7087. [Google Scholar]
- Chimentao, R.J.; Kirm, I.; Medina, F.; Rodriguez, X.; Cesteros, Y.; Salagre, P.; Sueiras, J.E.; Fierro, J.L.G. Sensitivity of styrene oxidation reaction to the catalyst structure of silver nanoparticles. Appl. Surf. Sci 2005, 252, 793–800. [Google Scholar]
- Zargar, M.; Hamid, A.A.; Bakar, F.B.; Shamsudin, M.N.; Shameli, K.; Jahanshiri, F.; Farahani, F. Green synthesis and antibacterial effect of silver nanoparticles using Vitex negundo L. Molecules 2011, 16, 6667–6676. [Google Scholar]
- Zhang, Y.; Zhang, K.; Ma, H. Electrochemical DNA biosensor based on silver nanoparticles/poly(3-(3-pyridyl) acrylic acid)/carbon nanotubes modified electrode. Anal. Biochem 2009, 387, 13–19. [Google Scholar]
- Zheng, J.; Ding, Y.; Tian, B.; Wang, Z.L.; Zhuang, X. Luminescent and raman active silver nanoparticles with polycrystalline structure. J. Am. Chem. Soc 2008, 130, 10472–10473. [Google Scholar]
- Nickel, U.; Castell, A.Z.; Poppl, K.; Schneider, S. A silver colloid produced by reduction with hydrazine as support for highly sensitive surface enhanced Raman spectroscopy. Langmuir 2000, 16, 9087–9091. [Google Scholar]
- Pal, T. Gelatin-A compound for all reasons. J. Chem. Educ 1994, 71, 679–681. [Google Scholar]
- Popa, M.; Pradell, T.; Crespo, D.; Calderón-Moreno, J.M. Stable silver colloidal dispersions using short chain polyethylene glycol. Colloids Surf. A Physicochem. Eng. Asp 2007, 303, 184–190. [Google Scholar]
- Luo, C.; Zhang, Y.; Zeng, X.; Zeng, Y.; Wang, Y. The role of poly(ethylene glycol) in the formation of silver nanoparticles. J. Colloid Interface Sci 2005, 288, 444–448. [Google Scholar]
- Dallas, P.; Sharma, V.K.; Zboril, R. Silver polymeric nanocomposites as advanced antimicrobial agents: Classification, synthetic paths, applications, and perspectives. Adv. Colloid Interface Sci 2011, 166, 119–135. [Google Scholar]
- Raveendran, P.; Fu, J.; Wallen, S.L. Completely “green” synthesis and stabilization of metal nanopartcieles. J. Am. Chem. Soc 2003, 125, 13940–13941. [Google Scholar]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar]
- Stepanov, A.L. Optical Properties of Metal nanoparticles synthesized in a polymer by ion implantation: A review. Technical. Phys 1997, 49, 143–153. [Google Scholar]
- Stamplecoskie, K.G.; Scaiano, J.C. Light emitting diode can control the morphology and optical properties of silver nanoparticles. J. Am. Chem. Soc 2010, 132, 1825–1827. [Google Scholar]
- Darroudi, M.; Ahmad, M.B.; Abdullah, A.H.; Ibrahim, N.A.; Shameli, K. Effect of accelerator in green synthesis of silver nanoparticles. Int. J. Mol. Sci 2010, 11, 3898–3905. [Google Scholar]
- Bhainsa, K.C.; D’Souza, S.F. Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigates. Colloids Surf. B Biointerfaces 2006, 47, 160–164. [Google Scholar]
- Peng, S.; McMahon, J.M.; Schatz, G.C.; Gray, S.K.; Sun, Y. Reversing the size-dependence of surface Plasmon resonances. Proc. Natl. Acad. Sci. USA 2010, 107, 14530–14534. [Google Scholar]
- Vidhu, V.K.; Aromal, A.; Philip, D. Green synthesis of silver nanoparticles using Macrotyloma uniflorum. Spectrochim. Acta A Mol. Biomol. Spectrosc 2011, 83, 392–397. [Google Scholar]
- Antonya, J.J.; Sivalingamb, S.; Sivaa, D. Comparative evaluation of antibacterial activity of silver nanoparticles synthesized using Rhizophora apiculata and glucose. Colloids Surf. B Biointerfaces 2011, 88, 134–140. [Google Scholar]
- Tunc, S.; Duman, O. The effect of different molecular weight of poly (ethylene glycol) on the electrokinetic and rheological properties of Na-bentonite suspensions. Colloids Surf. A Physicochem. Eng. Asp 2008, 37, 93–99. [Google Scholar]
- Philip, D. Honey mediated green synthesis of silver nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc 2010, 75, 1078–1081. [Google Scholar]
- Philip, D. Honey mediated green synthesis of gold nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc 2009, 73, 650–653. [Google Scholar]
- Gupta, K.; Jana, P.C.; Meikap, A.K. Optical and electrical transport properties of polyaniline-silver nanocomposites. Synth. Met. 2010, 160, 1566–1573. [Google Scholar]
- Jacobs, C.; Müller, R.H. Production and characterization of a budesonide nanosuspension for pulmonary administration. Pharm. Res 2002, 19, 189–194. [Google Scholar]


© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shameli, K.; Bin Ahmad, M.; Jazayeri, S.D.; Sedaghat, S.; Shabanzadeh, P.; Jahangirian, H.; Mahdavi, M.; Abdollahi, Y. Synthesis and Characterization of Polyethylene Glycol Mediated Silver Nanoparticles by the Green Method. Int. J. Mol. Sci. 2012, 13, 6639-6650. https://doi.org/10.3390/ijms13066639
Shameli K, Bin Ahmad M, Jazayeri SD, Sedaghat S, Shabanzadeh P, Jahangirian H, Mahdavi M, Abdollahi Y. Synthesis and Characterization of Polyethylene Glycol Mediated Silver Nanoparticles by the Green Method. International Journal of Molecular Sciences. 2012; 13(6):6639-6650. https://doi.org/10.3390/ijms13066639
Chicago/Turabian StyleShameli, Kamyar, Mansor Bin Ahmad, Seyed Davoud Jazayeri, Sajjad Sedaghat, Parvaneh Shabanzadeh, Hossein Jahangirian, Mahnaz Mahdavi, and Yadollah Abdollahi. 2012. "Synthesis and Characterization of Polyethylene Glycol Mediated Silver Nanoparticles by the Green Method" International Journal of Molecular Sciences 13, no. 6: 6639-6650. https://doi.org/10.3390/ijms13066639
APA StyleShameli, K., Bin Ahmad, M., Jazayeri, S. D., Sedaghat, S., Shabanzadeh, P., Jahangirian, H., Mahdavi, M., & Abdollahi, Y. (2012). Synthesis and Characterization of Polyethylene Glycol Mediated Silver Nanoparticles by the Green Method. International Journal of Molecular Sciences, 13(6), 6639-6650. https://doi.org/10.3390/ijms13066639




