Expression Patterns of Genes Involved in the Defense and Stress Response of Spiroplasma citri Infected Madagascar Periwinkle Catharanthus roseus
Abstract
:1. Introduction
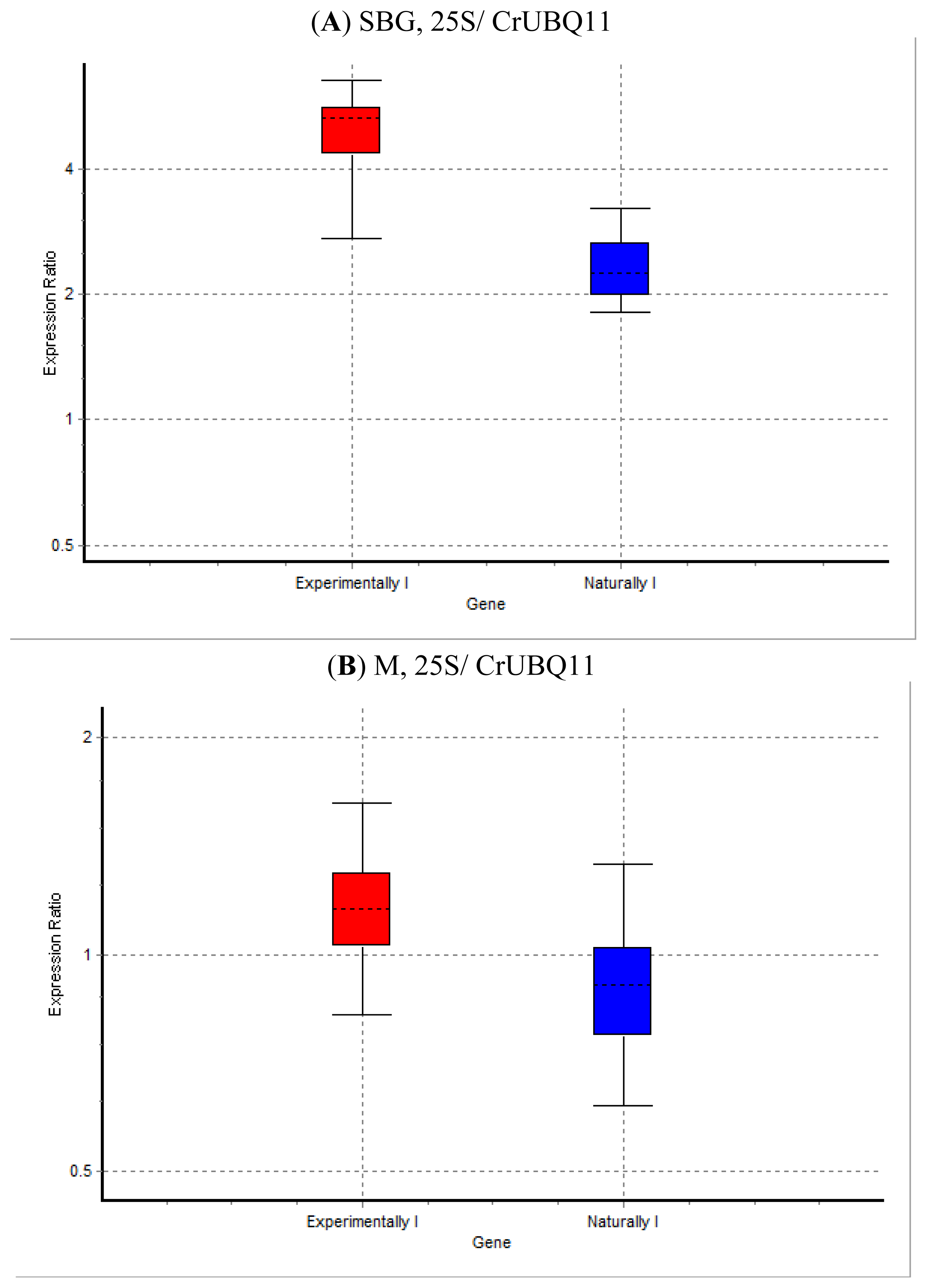
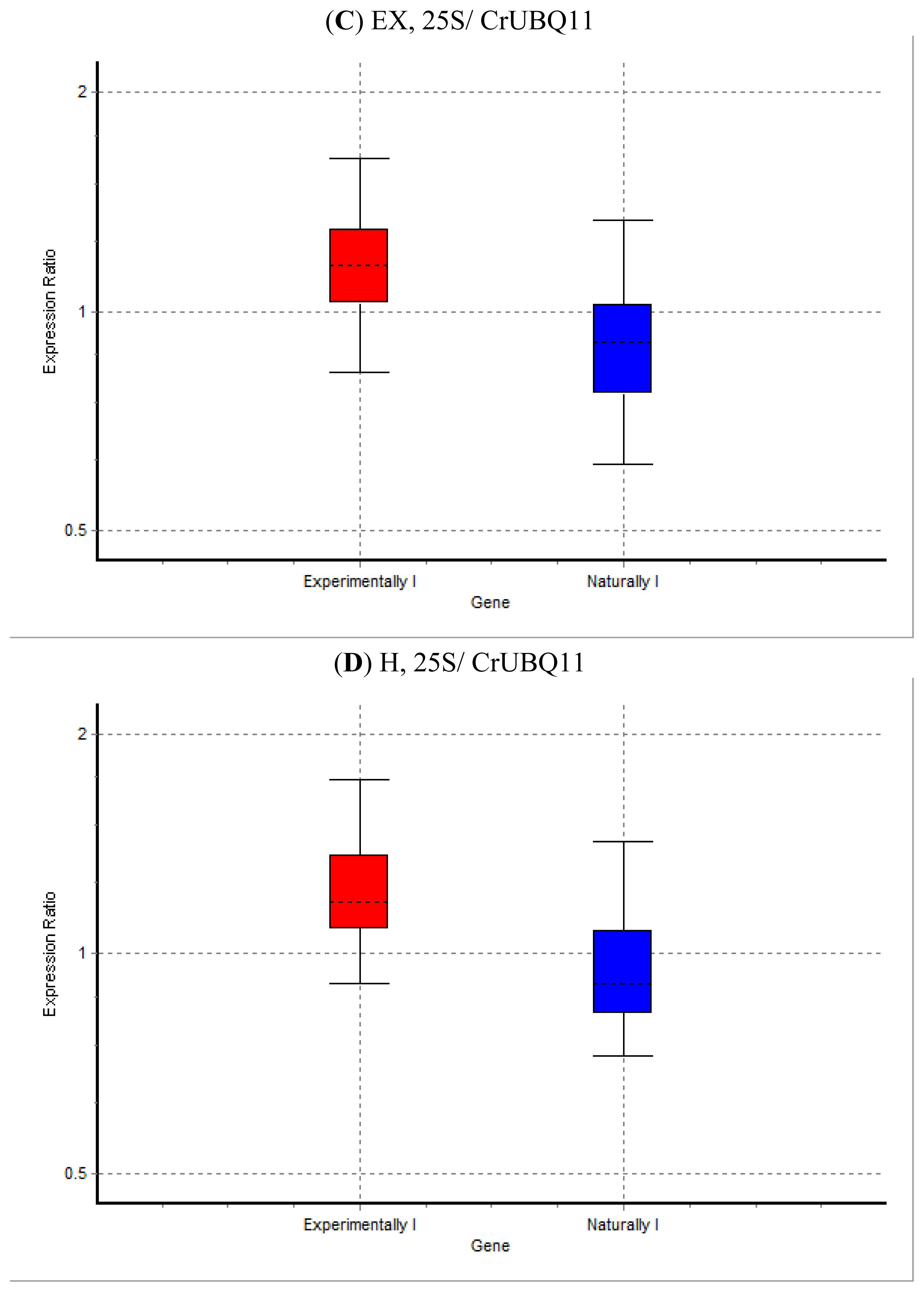
2. Results and Discussion
3. Experimental Section
3.1. Plant Material
3.2. Spiroplasma citri Detection
3.3. RNA Isolation
3.4. Primer Designing
3.5. Real-Time PCR Analysis
4. Conclusions
Acknowledgments
References
- Canto-Canche, B.B.; Loyola-Vargas, V.M. Multiple forms of NADPH-cytichrome P450 oxidoreductases in the Madagascar periwinkle Catharanthus roseus. In Vitro Cell. Dev. Biol. Plant 2001, 37, 622–628. [Google Scholar]
- Schröder, G.; Beck, M.; Eichel, J.; Vetter, H.-P.; Schröder, L. HSP90 homologue from Madagascar periwinkle (Catharanthus roseus): CDNA sequence, regulation of protein expression and location in the endoplasmic reticulum. Plant Mol. Biol 1993, 23, 583–594. [Google Scholar]
- Sottomayor, M.; Lopes Cardoso, I.; Pereira, L.G.; Ros Barceló, A. Peroxidase and the biosynthesis of terpenoid indole alkaloids in the medicinal plant Catharanthus roseus (L.) G. Don. Phytochem. Rev 2004, 3, 159–171. [Google Scholar]
- Luijendijk, T.J.C.; van der Meijden, E.; Verpoorte, R. Involvement of strictosidine as a defensive chemical in Catharanthus roseus. J. Chem. Ecol 1996, 22, 1355–1366. [Google Scholar]
- Ahrens, U.; Seemüller, R.E. Detection of DNA of plant pathogenic mycoplasma-like organisms by a polymerase chain reaction that amplifies a sequence of the 16S rRNA gene. Phytopathology 1992, 82, 828–832. [Google Scholar]
- Davis, R.E.; Lee, I.-M. Pathogenicity of Spiroplasmas, Mycoplasma-Like Organisms, and Vascular-Limited Fastidious Wallet Bacteria. In Phytopathogenic Prokaryotes; Mount, M., Lacy, G., Eds.; Academic Press: New York NY, USA, 1982; Volume I, pp. 491–505. [Google Scholar]
- Deng, S.; Hiruki, C. Genetic relatedness between two nonculturable mycoplasma-like organisms revealed by nucleic acid hybridization and polymerase chain reaction. Phytopathology 1991, 81, 1475–1479. [Google Scholar]
- Firrao, G.; Gobbi, E.; Loci, R. Use of polymerase chain reaction to produce oligonucleotide probes for mycoplasma-like organisms. Phytopathology 1993, 83, 602–606. [Google Scholar]
- Jagoueix-Eveillard, S.; Tarendeau, F.; Guolter, K.; Danet, J.L.; Bové, J.M.; Garnier, M. Catharanthus roseus genes regulated differentially by mollicute infections. Mol. Plant Microbe Interact 2001, 14, 225–233. [Google Scholar]
- Lee, I.-M.; Gundersen-Rindal, D.E.; Bertaccini, A. Phytoplasma: Ecology and genomic diversity. Phytopathology 1998, 88, 1359–1366. [Google Scholar]
- Prince, J.P.; Davis, R.E.; Wolf, T.K.; Lee, I.-M.; Mogen, B.; Dally, E.; Bertaccini, A.; Credi, R.; Barba, M. Molecular detection of diverse mycoplasma-like organisms (MLOs) associated with grapevine yellows and their classification with aster yellows, X-disease and elm yellows MLOs. Phytopathology 1993, 83, 1130–1137. [Google Scholar]
- Bove, J.M.; Renaudin, J.; Saillard, C.; Foissac, X.; Garnier, M. Spiroplasma citri, a plant pathogenic mollicute: Relationships with its two hosts, the plant and the leafhopper vector. Annu. Rev. Phytopathol 2003, 41, 483–500. [Google Scholar]
- Saglio, P.; L’Hospital, M.; Lafléche, D.; Dupont, G.; Bové, J.M.; Tully, J.G.; Freundt, E.A. Spiroplasma citri gen. and sp. n.: A mycoplasma-like organism associated with stubborn disease of citrus. Int. J. Syst. Bacteriol 1973, 23, 191–204. [Google Scholar]
- Chang, C.J. Pathogenicity of aster yellows phytoplasma and Spiroplasma citri on periwinkle. Phytopathology 1998, 88, 1347–1350. [Google Scholar]
- Daniels, M.J. Mechanisms of Spiroplasma Pathogenicity. In The Mycoplasmas; Whitcomb, R.F., Tully, J.G., Eds.; Academic Press: New York NY, USA, 1979; Volume 3, pp. 209–227. [Google Scholar]
- Markham, P.G.; Townsend, R. Transmission of Spiroplasma citri to plants. Colloq. INSERM 1974, 33, 201–206. [Google Scholar]
- Nejat, N.; Vadamalai, G.; Sijam, K.; Dickinson, M. First report of Spiroplasma citri associated with periwinkle lethal yellows in Southeast Asia. Plant Dis 2011, 95, 1312. [Google Scholar]
- Whiteside, J.O.; Garney, S.M.; Timmer, L.W. Compendium of Citrus Diseases; APS Press: St. Paul, MN, USA, 1988. [Google Scholar]
- Choi, Y.H.; Tapias, E.C.; Kim, H.K.; Lefeber, A.W.M.; Erkelens, C.; Verhoeven, J.T.J.; Brzin, J.; Zel, J.; Verpoorte, R. Metabolic discrimination of Catharanthus roseus leaves infected by phytoplasma using 1H-NMR spectroscopy and multivariate data analysis. Plant Physiol 2004, 135, 2398–410. [Google Scholar]
- Lepka, P.; Stitt, M.; Moll, E.; Seemüller, E. Effect of phytoplasmal infection on concentration and translocation of carbohydrates and amino acids in periwinkle and tobacco. Physiol. Mol. Plain Pathol 1999, 5, 59–68. [Google Scholar]
- Machenaud, J.; Raphaël, H.; Dieuaide-Noubhani, M.; Pracros, P.; Renaudin, J.; Eveillard, S. Gene expression and enzymatic activity of invertases and sucrose synthase in Spiroplasma citri or stolbur phytoplasma infected plants. Bull Insectol 2007, 60, 219–220. [Google Scholar]
- Chang, C.J. Nutrition and Cultivation of Spiroplasmas. In The Mycoplasma; Whitcomb, R.F., Tully, J.G., Eds.; Academic Press: New York NY, USA, 1989; Volume 5, pp. 201–241. [Google Scholar]
- André, A.; Maucourt, M.; Moing, A.; Rolin, D.; Renaudin, J. Sugar import and phytopathogenicity of Spiroplasma citri: Glucose and fructose play distinct roles. Mol. Plant Microbe Interact. 2005, 18, 33–42. [Google Scholar]
- Renaudin, J. Sugar metabolism and pathogenicity of Spiroplasma citri. J. Plant Pathol 2006, 88, 129–139. [Google Scholar]
- Carginale, V.; Luca, V.; Capasso, C.; Baldi, MR.; Maria, G.; Pastore, M.; Bertaccini, A.; Carrara, L.; Capasso, A. Effect of pear decline phytoplasma on gene expression in Catharanthus roseus. Bull Insectol 2007, 60, 213–214. [Google Scholar]
- Carginale, V.; Maria, G.; Capasso, C.; Ionata, E.; la Cara, F.; Pastore, M.; Bertaccini, A.; Capasso, A. Identification of genes expressed in response to phytoplasma infection in leaves of Primus armeniaca by messenger RNA differential display. Gene 2004, 332, 29–34. [Google Scholar]
- Chen, W.Y.; Lin, C.P. Characterization of Catharanthus roseus genes regulated differentially by peanut witches’ broom phytoplasma infection. J. Phytopathol. 2011, 159, 505–510. [Google Scholar]
- Hren, M.; Ravnikar, M.; Brzin, J.; Ermacora, P.; Carraro, L.; Bianco, P.A.; Casati, P.; Borgo, M.; Angelini, E.; Rotter, A.; Gruden, K. Induced expression of sucrose synthase and alcohol dehydrogenase I genes in phytoplasma-infected grapevine plants grown in the field. Plant Pathol 2009, 58, 170–180. [Google Scholar]
- Nicolaisen, M.; Horvath, D.P. A branch-inducing phytoplasma in Euphorbia pulcherrima is associated with changes in expression of host genes. J. Phytopathol 2008, 156, 403–407. [Google Scholar]
- Pracros, P.; Renaudin, J.; Eveillard, S.; Mouras, A.; Hernould, M. Tomato flower abnormalities induced by stolbur phytoplasma infection are associated with changes of expression of floral development genes. Mol. Plant Microbe Interact 2006, 19, 62–68. [Google Scholar]
- Vidhyasekaran, P. Concise Encyclopedia of Plant Pathology; Haworth Press: Philadelphia, PA, USA, 2004; pp. 511–517. [Google Scholar]
- Roberts, K.; Shirsat, A.H. Increased extensin levels in Arabidopsis affect inflorescence stem thickening and height. J. Exp. Bot 2006, 57, 537–545. [Google Scholar]
- Tierney, M.L.; Varner, J.E. The extensins. Plant Physiol 1987, 84, 1–2. [Google Scholar]
- Coyle, P.; Philcox, J.C.; Carey, L.C.; Rofe, A.M. Metallothionein: The multipurpose protein. Cell. Mol. Lefe Sci 2002, 59, 627–647. [Google Scholar]
- English, T.E.; Storey, K.B. Freezing and anoxia stresses induce expression of metallothionein in the foot muscle and hepatopancreas of the marine gastropod Littorina littorea. J. Exp. Biol 2003, 206, 2517–2524. [Google Scholar]
- Ukamaka, A.M.; Obinnaya, C.L.; Adebayo, O.; Miriam, I.-E. Metallothionein induction in edible mangrove periwinkles, Tympanotonus fuscatus var radula and Pachymelania aurita exposed to oily drill cuttings. J. Am. Sci 2010, 6, 89–97. [Google Scholar]
- Viarengo, A.; Burlando, B.; Cavaletto, M.; Marchi, B.; Ponzano, E.; Blasco, J. Role of metallothionein against oxidative stress in the mussel Mytilus galloprovincialis. Am. J. Physiol 1999, 277, R1612–R1619. [Google Scholar]
- Gupta, R.S. Phylogenetic analysis of the 90 KD heat shock family of protein sequences and an examination of the relationship among animals, plants, and fungi species. Mol. Biol. Evol 1995, 12, 1063–1073. [Google Scholar]
- Koide, T.; Vêncio, R.Z.N.; Gomes, S.L. Global gene expression analysis of the heat shock response in the phytopathogen Xylella fastidiosa. J. Bacteriol 2006, 188, 5821–5830. [Google Scholar]
- Csermely, P.; Schnaider, T.; Soti, C.; Prohaszka, Z.; Nardai, G. The 90-kDa molecular chaperone family: Structure, function, and clinical applications, a comprehensive review. Pharmacol. Ther 1998, 79, 129–168. [Google Scholar]
- Lindquist, S.; Craig, E.A. The heat-shock proteins. Annu. Rev. Genet 1988, 22, 631–677. [Google Scholar]
- Liu, D.; Zhang, X.; Cheng, Y.; Takano, T.; Liu, S. rHsp90 gene expression in response to several environmental stresses in rice (Oryza sativa L.). Plant Physiol. Biochem 2006, 44, 380–386. [Google Scholar]
- Faure, D. The family-3 glycoside hydrolases: From housekeeping functions to host-microbe interactions. Appl. Environ. Microbiol 2002, 68, 1485–1490. [Google Scholar]
- Henrissat, B.; Coutinho, P.M.; Davies, G.J. A census of carbohydrate-active enzymes in the genome of Arabidopsis thaliana. Plant Mol. Biol 2001, 7, 55–72. [Google Scholar]
- Luca, V.D.; Capasso, C.; Capasso, A.; Pastore, M.; Carginale, V. Gene expression profiling of phytoplasma-infected Madagascar periwinkle leaves using differential display. Mol. Biol. Rep 2011, 38, 2993–3000. [Google Scholar]
- Minic, Z. Physiological roles of plant glycoside hydrolases. Planta 2008, 227, 723–740. [Google Scholar]
- Morant, A.V.; Jorgensen, K.; Jorgensen, C.; Paquette, S.M.; Sanchez-Perez, R.; Moller, B.L.; Bak, S. beta-glucosidases as detonators of plant chemical defense. Phytochemistry 2008, 69, 1795–1813. [Google Scholar]
- Kreps, J.A.; Wu, Y.; Chang, H.S.; Zhu, T.; Wang, X.; Harper, J.F. Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol 2002, 130, 2129–2141. [Google Scholar]
- Barleban, L.; Panjikar, S.; Ruppert, M.; Koepke, J.; Stöckigt, J. Molecular architecture of strictosidine glucosidase: The gateway to the biosynthesis of the monoterpenoid indole alkaloid family. Plant Cell 2007, 19, 2886–2897. [Google Scholar]
- Czjzek, M.; Cicek, M.; Zamboni, V.; Burmeister, W.P.; Bevan, D.R.; Henrissat, B.; Esen, A. Crystal structure of a monocotyledon (maize ZMGlu1) betaglucosidase and a model of its complex with p-nitrophenyl beta-D-thioglucoside. Biochem. J 2001, 354, 37–46. [Google Scholar]
- Niemeyer, H.M. Hydroxamic acids (4-hydroxy-1,4-Benzoxazin-3-Ones), defense chemicals in the gramineae. Phytochemistry 1988, 27, 3349–3358. [Google Scholar]
- Poulton, J.E. Cyanogenesis in plants. Plant Physiol 1990, 94, 401–405. [Google Scholar]
- Guirimand, G.; Courdavault, V.; Lanoue, A.; Mahroug, S.; Guihur, A.; Blanc, N.; Giglioli-Guivarc’h, N.; St-Pierre, B.; Burlat, V. Strictosidine activation in Apocynaceae: Towards a “nuclear time bomb”. BMC Plant Biol 2010, 10. [Google Scholar] [CrossRef]
- Stoeckigt, J.; Zenk, M.H. Strictosidine (isovincoside): The key intermediate in the biosynthesis of monoterpenoid indole alkaloids. J. Chem. Soc. Chem. Commun 1977, 18, 646–648. [Google Scholar]
- Treimer, J.F.; Zenk, M.H. Purification and properties of strictosidine synthase, the key enzyme in indole alkaloid formation. Eur. J. Biochem 1979, 101, 225–233. [Google Scholar]
- Geerling, A.; Ibañez, M.M.L.; Memelink, J.; Heijden, R.; Verpoorte, R. Molecular cloning and analysis of strictosidine β-D-glucosidase, an enzyme in terpnoid indol alkaloids biosynthesis in Catharanthus roseus. J. Biol. Chem 2000, 275, 3051–3056. [Google Scholar]
- Wei, S. Methyl jasmonic acid induced expression pattern of terpenoid indole alkaloid pathway genes in Catharanthus roseus seedlings. Plant Growth Regul 2010, 61, 243–251. [Google Scholar]
- Albertazzi, G.; Milc, J.; Caffagni, A.; Francia, E.; Roncaglia, E.; Ferrari, F.; Tagliafico, E.; Stefani, E.; Pecchioni, N. Gene expression in grapevine cultivars in response to Bois Noir phytoplasma infection. Plant Sci 2009, 176, 792–804. [Google Scholar]
- Pasquer, F.; Ochsner, U.; Zarn, J.; Keller, B. Common and distinct gene expression patterns induced by the herbicides 2,4-dichlorophenoxyacetic acid, conidon-ethyl and tribenuron-methyl in wheat. Pest Manag. Sci 2006, 62, 1155–1167. [Google Scholar]
- Lee, I.-M.; Bottner, K.D.; Munyaneza, J.E.; Davis, R.E.; Crosslin, J.M.; du Toit, L.J.; Crosby, T. Carrot purple leaf: A new spiroplasmal disease associated with carrots in Washington State. Plant Dis 2006, 90, 989–993. [Google Scholar]
- Kiefer, E.; Heller, W.; Ernst, D. A simple and efficient protocol for isolation of functional RNA from plant tissues rich in secondary metabolites. Plant Mol. Biol. Report 2000, 18, 33–39. [Google Scholar]
- Rosen, S.; Skaletsky, H. Primer 3 on the WWW for general Users and for Biologist Programmers. In Bioinformatics Methods and Protocols: Methods in Molecular Biology; Krawtez, S., Misener, S., Eds.; Humana: Totowa, NJ, USA, 2000; pp. 365–386. [Google Scholar]
| primer | Nucleotide sequence (5′-3′) | Size of PCR products (bp) | Accession number | Target gene | Reference |
|---|---|---|---|---|---|
| MF | CATGTCTTGCTCCTGTGGTG | 175 | DQ016341 | metallothionein | in this study |
| MR | ATGTCCTCCTTCTGCTCCAA | ||||
| HSPF | CGGCTCATGTACCAGACCGCA | 168 | L14594 | heat shock | in this study |
| HSPR | TGTGCCGGATTCAGCCTCAGC | protein 90 | |||
| EXF | CTCCACCATCAGTCCACAAA | 181 | D86853 | extensin | in this study |
| EXR | GGAGTGGGTGGGGGATATT | ||||
| BGF | TCACAAAGCTGCTGTGGAAG | 182 | AF112888 | β-glucosidase | in this study |
| BGR | CACCCGTTGTTAATGGCTCT |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nejat, N.; Vadamalai, G.; Dickinson, M. Expression Patterns of Genes Involved in the Defense and Stress Response of Spiroplasma citri Infected Madagascar Periwinkle Catharanthus roseus . Int. J. Mol. Sci. 2012, 13, 2301-2313. https://doi.org/10.3390/ijms13022301
Nejat N, Vadamalai G, Dickinson M. Expression Patterns of Genes Involved in the Defense and Stress Response of Spiroplasma citri Infected Madagascar Periwinkle Catharanthus roseus . International Journal of Molecular Sciences. 2012; 13(2):2301-2313. https://doi.org/10.3390/ijms13022301
Chicago/Turabian StyleNejat, Naghmeh, Ganesan Vadamalai, and Matthew Dickinson. 2012. "Expression Patterns of Genes Involved in the Defense and Stress Response of Spiroplasma citri Infected Madagascar Periwinkle Catharanthus roseus " International Journal of Molecular Sciences 13, no. 2: 2301-2313. https://doi.org/10.3390/ijms13022301




