Glucose-Modulated Mitochondria Adaptation in Tumor Cells: A Focus on ATP Synthase and Inhibitor Factor 1
Abstract
:1. Introduction
2. Results and Discussion
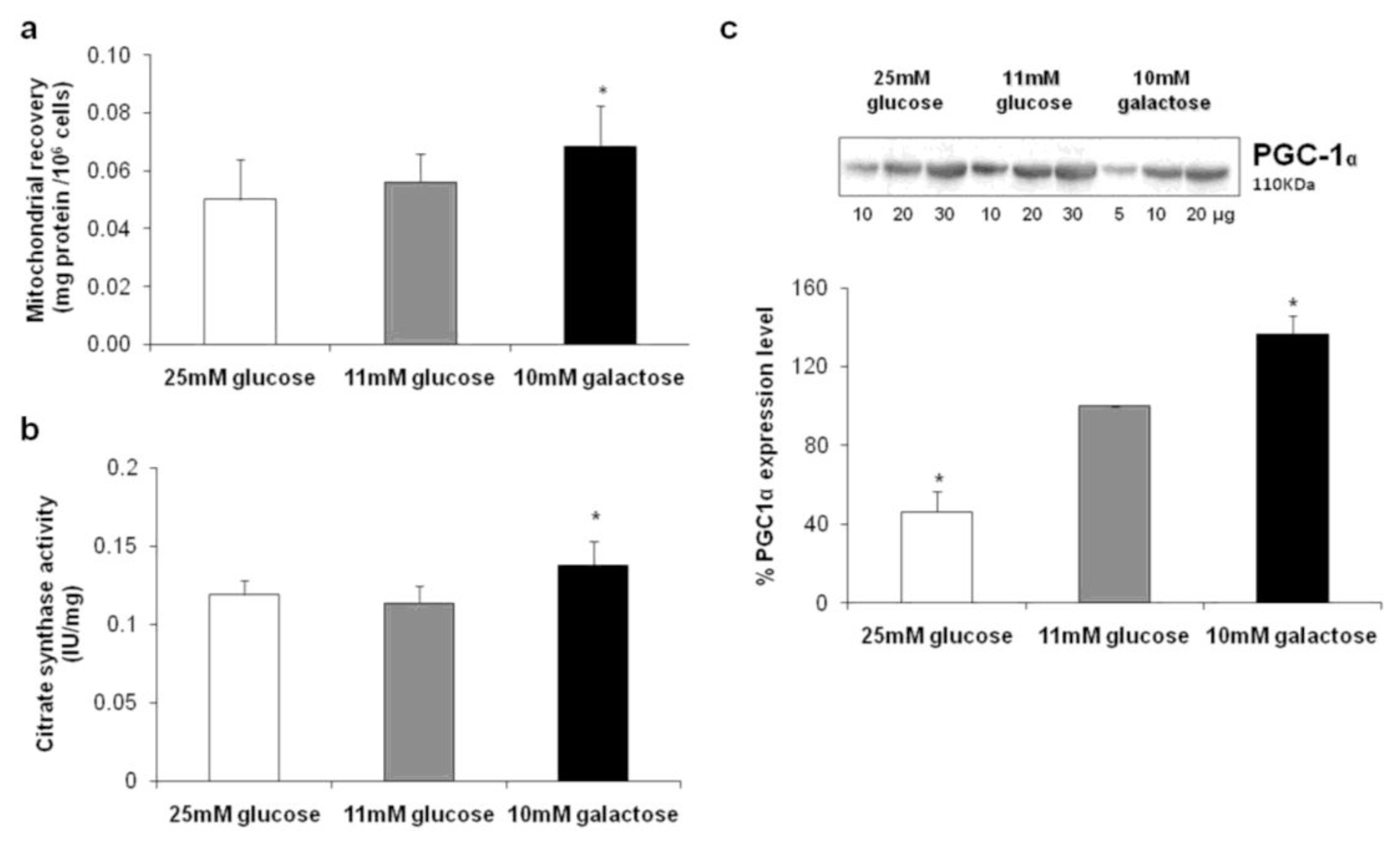
2.1. Cell Proliferation and Mitochondrial Content/Biogenesis
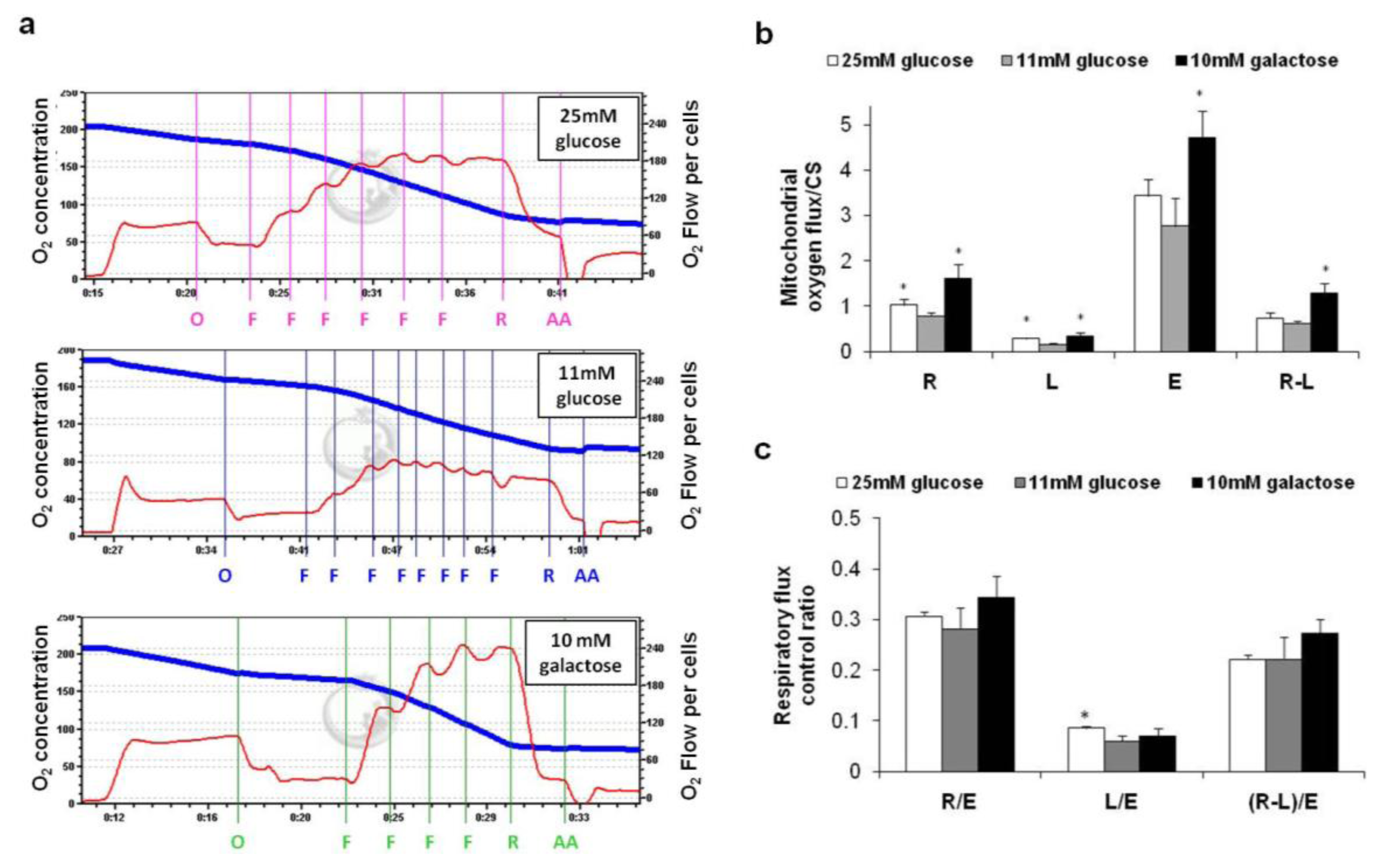
2.2. Oxygen Consumption in Intact Cells
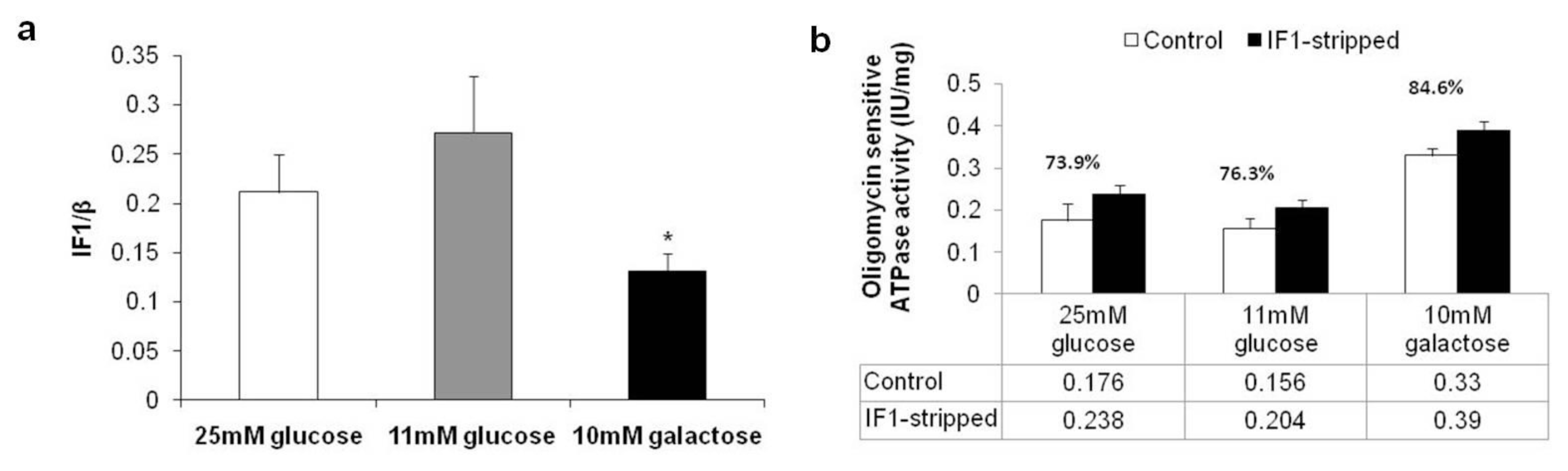
2.3. Expression Levels of Complex II, ATP Synthase and Inhibitor Factor 1
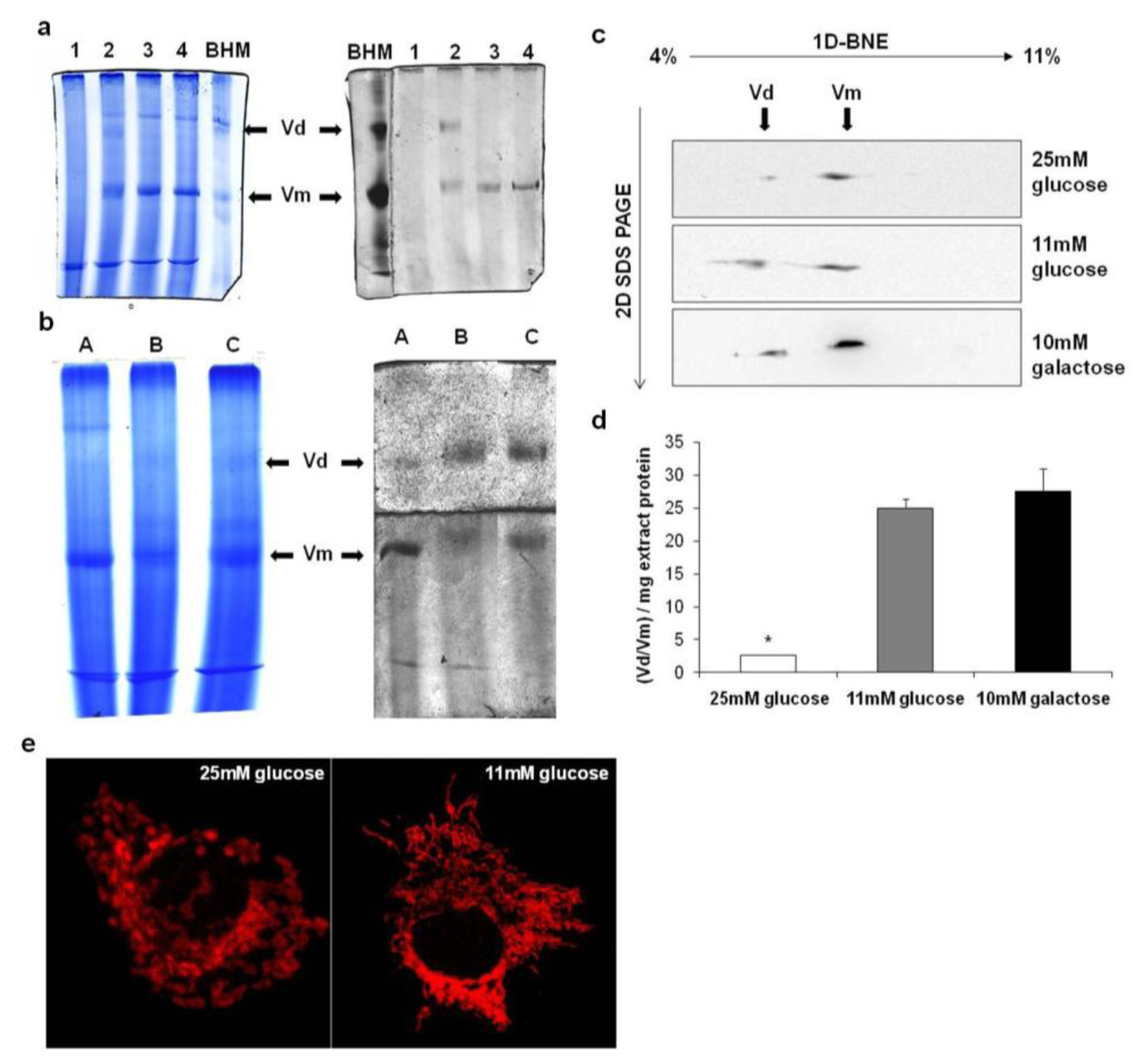
2.4. Supramolecular Organization of Mitochondrial ATP Synthase in Membranes
2.5. Proteomic and Functional Analysis of IF1 Association with ATP Synthase
3. Experimental Section
3.1. Cell Culture Conditions
3.2. Determination of Mitochondrial Mass
3.3. Polarographic Measurement of Respiration
3.4. Preparation of Crude Mitochondria Fractions
3.5. Western Blot Analysis of Cell and Mitochondrial Lysates
3.6. 2D-Immunoblotting Analysis with Blue Native Electrophoresis as a First-Dimension
3.7. Confocal Microscopy
3.8. ATP Synthase Immunoprecipitation and IF1 Immunodetection
3.9. Mitochondrial ATP Synthase Activity
3.10. Statistical Analyses
4. Conclusions
Acknowledgments
- Conflict of InterestThere is no conflict of interest.
References
- Warburg, O. On respiratory impairment in cancer cells. Science 1956, 124, 269–270. [Google Scholar]
- Moreno-Sanchez, R.; Rodriguez-Enriquez, S.; Saavedra, E.; Marin-Hernandez, A.; Gallardo-Perez, J.C. The bioenergetics of cancer: Is glycolysis the main ATP supplier in all tumor cells? Biofactors 2009, 35, 209–225. [Google Scholar]
- Jose, C.; Bellance, N.; Rossignol, R. Choosing between glycolysis and oxidative phosphorylation: A tumor’s dilemma? Biochim. Biophys. Acta 2011, 1807, 552–561. [Google Scholar]
- Gogvadze, V.; Zhivotovsky, B.; Orrenius, S. The Warburg effect and mitochondrial stability in cancer cells. Mol. Aspects Med 2010, 31, 60–74. [Google Scholar]
- Rossignol, R.; Gilkerson, R.; Aggeler, R.; Yamagata, K.; Remington, S.J.; Capaldi, R.A. Energy substrate modulates mitochondrial structure and oxidative capacity in cancer cells. Cancer Res 2004, 64, 985–993. [Google Scholar]
- Smolkova, K.; Bellance, N.; Scandurra, F.; Genot, E.; Gnaiger, E.; Plecita-Hlavata, L.; Jezek, P.; Rossignol, R. Mitochondrial bioenergetic adaptations of breast cancer cells to aglycemia and hypoxia. J. Bioenerg. Biomembr 2010, 42, 55–67. [Google Scholar]
- Plecita-Hlavata, L.; Lessard, M.; Santorova, J.; Bewersdorf, J.; Jezek, P. Mitochondrial oxidative phosphorylation and energetic status are reflected by morphology of mitochondrial network in INS-1E and HEP-G2 cells viewed by 4Pi microscopy. Biochim. Biophys. Acta 2008, 1777, 834–846. [Google Scholar]
- Weber, K.; Ridderskamp, D.; Alfert, M.; Hoyer, S.; Wiesner, R.J. Cultivation in glucose-deprived medium stimulates mitochondrial biogenesis and oxidative metabolism in HepG2 hepatoma cells. Biol. Chem 2002, 383, 283–290. [Google Scholar]
- Rodríguez-Enríquez, S.; Carreño-Fuentes, L.; Gallardo-Pérez, J.C.; Saavedra, E.; Quezada, H.; Vega, A.; Marín-Hernández, A.; Olín-Sandoval, V.; Torres-Márquez, M.E.; Moreno-Sánchez, R. Oxidative phosphorylation is impaired by prolonged hypoxia in breast and possibly in cervix carcinoma. Int. J. Biochem. Cell B 2010, 42, 1744–1751. [Google Scholar]
- Ristow, M.; Pfister, M.F.; Yee, A.J.; Schubert, M.; Michael, L.; Zhang, C.Y.; Ueki, K.; Michael, M.D.; Lowell, B.B.; Kahn, C.R. Frataxin activates mitochondrial energy conversion and oxidative phosphorylation. Proc. Natl. Acad. Sci. USA 2000, 97, 12239–12243. [Google Scholar]
- Fantin, V.R.; St-Pierre, J.; Leder, P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 2006, 9, 425–434. [Google Scholar]
- Loiseau, D.; Morvan, D.; Chevrollier, A.; Demidem, A.; Douay, O.; Reynier, P.; Stepien, G. Mitochondrial bioenergetic background confers a survival advantage to HepG2 cells in response to chemotherapy. Mol. Carcinog 2009, 48, 733–741. [Google Scholar] [Green Version]
- Domenis, R.; Comelli, M.; Bisetto, E.; Mavelli, I. Mitochondrial bioenergetic profile and responses to metabolic inhibition in human hepatocarcinoma cell lines with distinct differentiation characteristics. J. Bioenerg. Biomembr 2011, 43, 493–505. [Google Scholar]
- Chernyak, B.V.; Dukhovich, V.F.; Khodjaev, E. Regulation of ATP hydrolysis in hepatoma 22a mitochondria. Arch. Biochem. Biophys 1991, 286, 604–609. [Google Scholar]
- Luciakova, K.; Kuzela, S. Increased content of natural ATPase inhibitor in tumor mitochondria. FEBS Lett 1984, 177, 85–88. [Google Scholar]
- Sanchez-Cenizo, L.; Formentini, L.; Aldea, M.; Ortega, A.D.; Garcia-Huerta, P.; Sanchez-Arago, M.; Cuezva, J.M. Up-regulation of the ATPase inhibitory factor 1 (IF1) of the mitochondrial H+-ATP synthase in human tumors mediates the metabolic shift of cancer cells to a Warburg phenotype. J. Biol. Chem 2010, 285, 25308–25313. [Google Scholar]
- Palmeira, C.M.; Rolo, A.P.; Berthiaume, J.; Bjork, J.A.; Wallace, K.B. Hyperglycemia decreases mitochondrial function: The regulatory role of mitochondrial biogenesis. Toxicol. Appl. Pharm 2007, 225, 214–220. [Google Scholar]
- Marroquin, L.D.; Hynes, J.; Dykens, J.A.; Jamieson, J.D.; Will, Y. Circumventing the Crabtree effect: Replacing media glucose with galactose increases susceptibility of HepG2 cells to mitochondrial toxicants. Toxicol. Sci 2007, 97, 539–547. [Google Scholar]
- Derdak, Z.; Mark, N.M.; Beldi, G.; Robson, S.C.; Wands, J.R.; Baffy, G. The mitochondrial uncoupling protein-2 promotes chemoresistance in cancer cells. Cancer Res 2008, 68, 2813–2819. [Google Scholar]
- Herst, P.M.; Berridge, M.V. Cell surface oxygen consumption: a major contributor to cellular oxygen consumption in glycolytic cancer cell lines. Biochim. Biophys. Acta 2007, 1767, 170–177. [Google Scholar]
- Di Pietro, A.; Penin, F.; Julliard, J.H.; Godinot, C.; Gautheron, D.C. IF1 inhibition of mitochondrial F1-ATPase is correlated to entrapment of four adenine- or guanine-nucleotides including at least one triphosphate. Biochem. Biophys. Res. Commun 1988, 152, 1319–1325. [Google Scholar]
- Campanella, M.; Casswell, E.; Chong, S.; Farah, Z.; Wieckowski, M.R.; Abramov, A.Y.; Tinker, A.; Duchen, M.R. Regulation of mitochondrial structure and function by the F1F0-ATPase inhibitor protein, IF1. Cell Metab 2008, 8, 13–25. [Google Scholar]
- Paumard, P.; Vaillier, J.; Coulary, B.; Schaeffer, J.; Soubannier, V.; Mueller, D.M.; Brethes, D.; di Rago, J.P.; Velours, J. The ATP synthase is involved in generating mitochondrial cristae morphology. EMBO J 2002, 21, 221–230. [Google Scholar]
- Bisetto, E.; Di Pancrazio, F.; Simula, M.P.; Mavelli, I.; Lippe, G. Mammalian ATPsynthase monomer versus dimer profiled by blue native PAGE and activity stain. Electrophoresis 2007, 28, 3178–3185. [Google Scholar]
- Yu, T.; Robotham, J.L.; Yoon, Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc. Natl. Acad. Sci. USA 2006, 103, 2653–2658. [Google Scholar]
- Velours, J.; Dautant, A.; Salin, B.; Sagot, I.; Brèthes, D. Mitochondrial F1F0-ATP synthase and organellar internal architecture. Int. J. Biochem. Cell B 2009, 41, 1783–1789. [Google Scholar]
- Solaini, G.; Sgarbi, G.; Baracca, A. Oxidative phosphorylation in cancer cells. Biochim. Biophys. Acta 2011, 1807, 534–542. [Google Scholar]
- Weidemann, A.; Johnson, R.S. Biology of HIF-1alpha. Cell Death Differ 2008, 15, 621–627. [Google Scholar]
- Huang, L.J.; Chuang, I.C.; Dong, H.P.; Yang, R.C. Hypoxia-inducible factor 1α regulates the expression of the mitochondrial ATPase inhibitor protein (IF1) in rat liver. Shock 2011, 36, 90–96. [Google Scholar]
- Marusich, M.F.; Murray, J.; Xie, J.; Capaldi, R.A. Novel antibody-based strategies for the rapid diagnosis of mitochondrial disease and dysfunction. Int. J. Biochem. Cell B 2009, 41, 2081–2088. [Google Scholar]
- Rouslin, W.; Broge, C.W. IF1 function in situ in uncoupler-challenged ischemic rabbit, rat, and pigeon hearts. J. Biol. Chem 1996, 271, 23638–23641. [Google Scholar]
- Tomasetig, L.; Di Pancrazio, F.; Harris, D.A.; Mavelli, I.; Lippe, G. Dimerization of F0F1ATP synthase from bovine heart is independent from the binding of the inhibitor protein IF1. Biochim. Biophys. Acta 2002, 1556, 133–141. [Google Scholar]
- Sauvanet, C.; Duvezin-Caubet, S.; di Rago, J.P.; Rojo, M. Energetic requirements and bioenergetic modulation of mitochondrial morphology and dynamics. Semin. Cell Dev. Biol 2010, 21, 558–565. [Google Scholar]
- Bravo, C.; Minauro-Sanmiguel, F.; Morales-Rios, E.; Rodriguez-Zavala, J.S.; Garcia, J.J. Overexpression of the inhibitor protein IF(1) in AS-30D hepatoma produces a higher association with mitochondrial F(1)F(0) ATP synthase compared to normal rat liver: functional and cross-linking studies. J. Bioenerg. Biomembr 2004, 36, 257–264. [Google Scholar]

| Total O2 Consumption | Mitochondrial O2 Consumption | Non Mitochondrial O2 Consumption | |
|---|---|---|---|
| 25 mM Glucose | 73.3 ± 11.7 (100%) * | 41.1 ± 5.5 (56.2%) * | 32.1 ± 6.5 (43.8%) * |
| 10 mM Galactose | 107.7 ± 24.6 (100%) | 91.7 ± 17.2 (85.1%) | 16 ± 7.8 (14.9%) |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Domenis, R.; Bisetto, E.; Rossi, D.; Comelli, M.; Mavelli, I. Glucose-Modulated Mitochondria Adaptation in Tumor Cells: A Focus on ATP Synthase and Inhibitor Factor 1. Int. J. Mol. Sci. 2012, 13, 1933-1950. https://doi.org/10.3390/ijms13021933
Domenis R, Bisetto E, Rossi D, Comelli M, Mavelli I. Glucose-Modulated Mitochondria Adaptation in Tumor Cells: A Focus on ATP Synthase and Inhibitor Factor 1. International Journal of Molecular Sciences. 2012; 13(2):1933-1950. https://doi.org/10.3390/ijms13021933
Chicago/Turabian StyleDomenis, Rossana, Elena Bisetto, Davide Rossi, Marina Comelli, and Irene Mavelli. 2012. "Glucose-Modulated Mitochondria Adaptation in Tumor Cells: A Focus on ATP Synthase and Inhibitor Factor 1" International Journal of Molecular Sciences 13, no. 2: 1933-1950. https://doi.org/10.3390/ijms13021933




