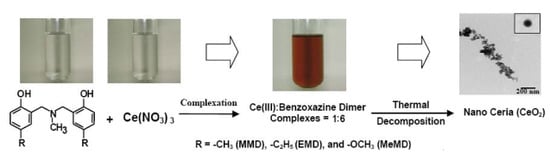
Novel Recovery of Nano-Structured Ceria (CeO2) from Ce(III)-Benzoxazine Dimer Complexes via Thermal Decomposition
Abstract
:1. Introduction
2. Results and Discussion
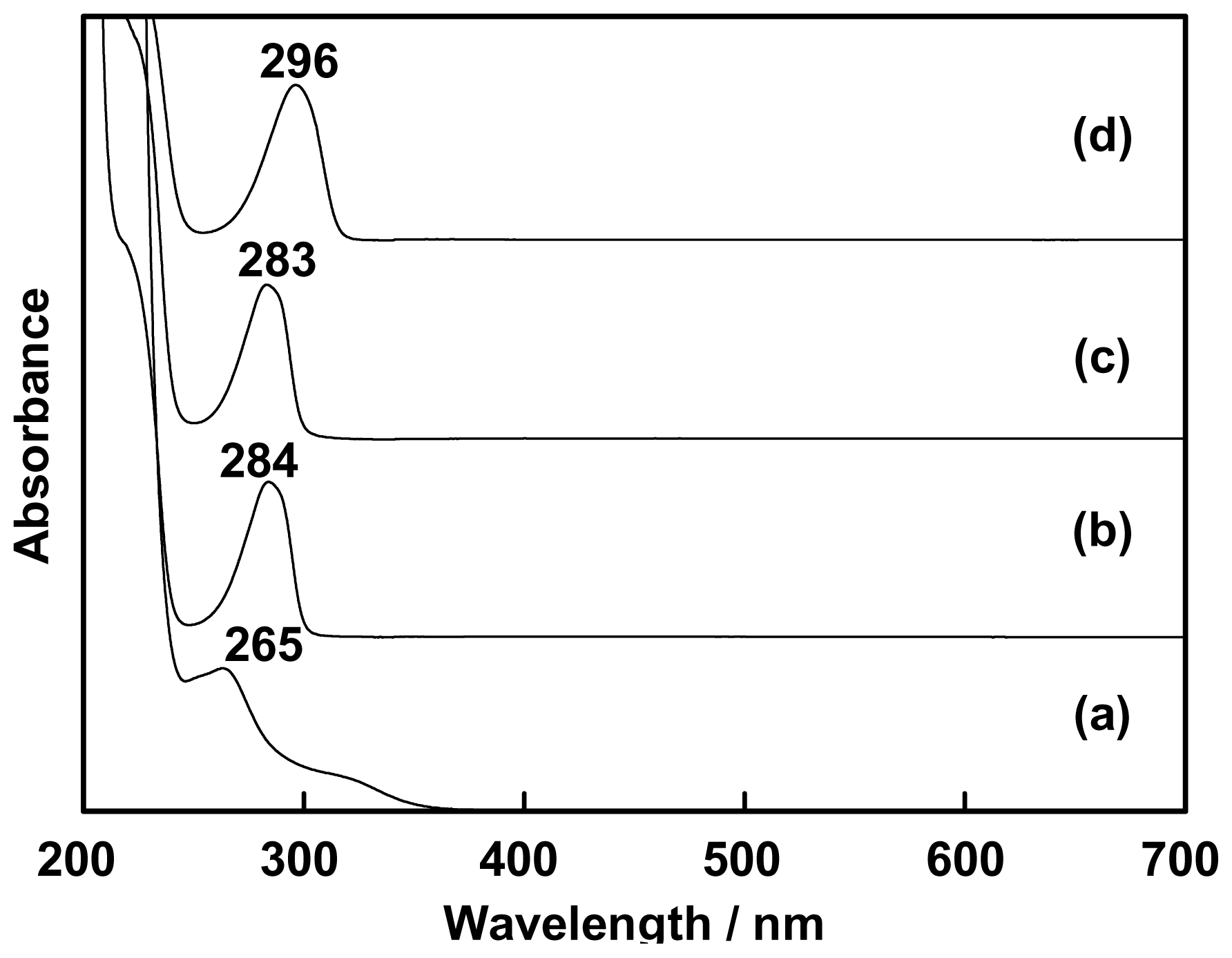
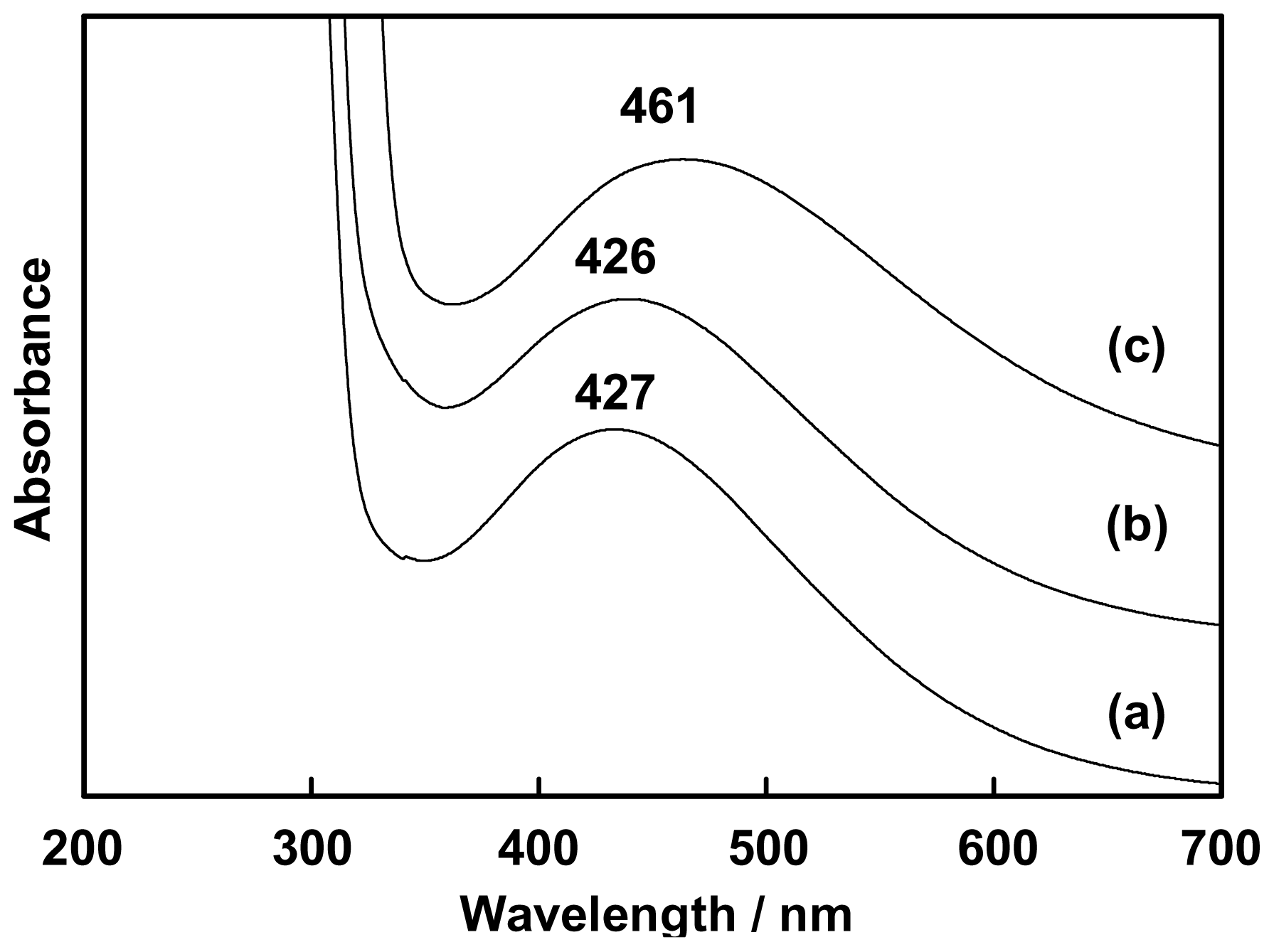
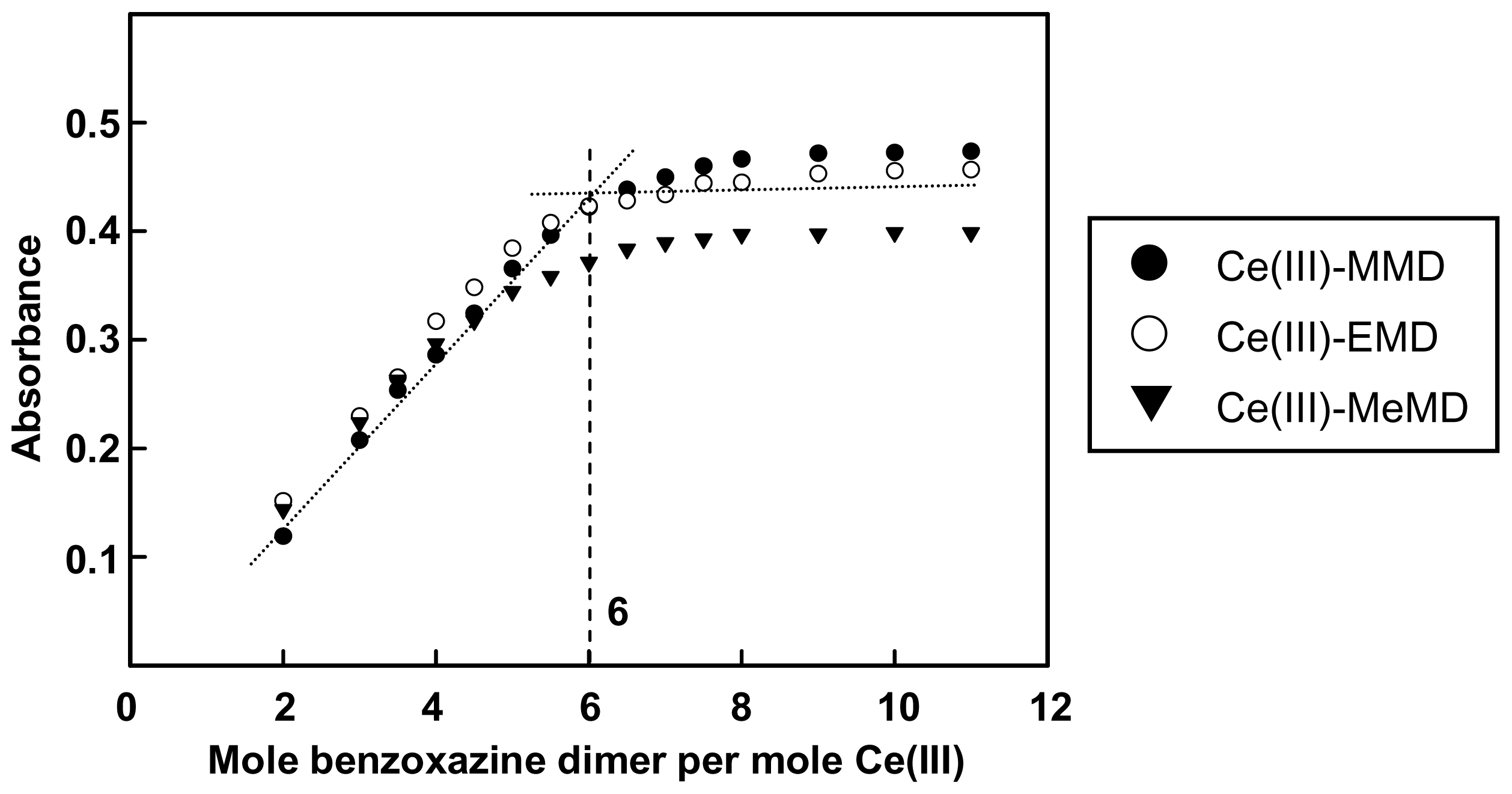
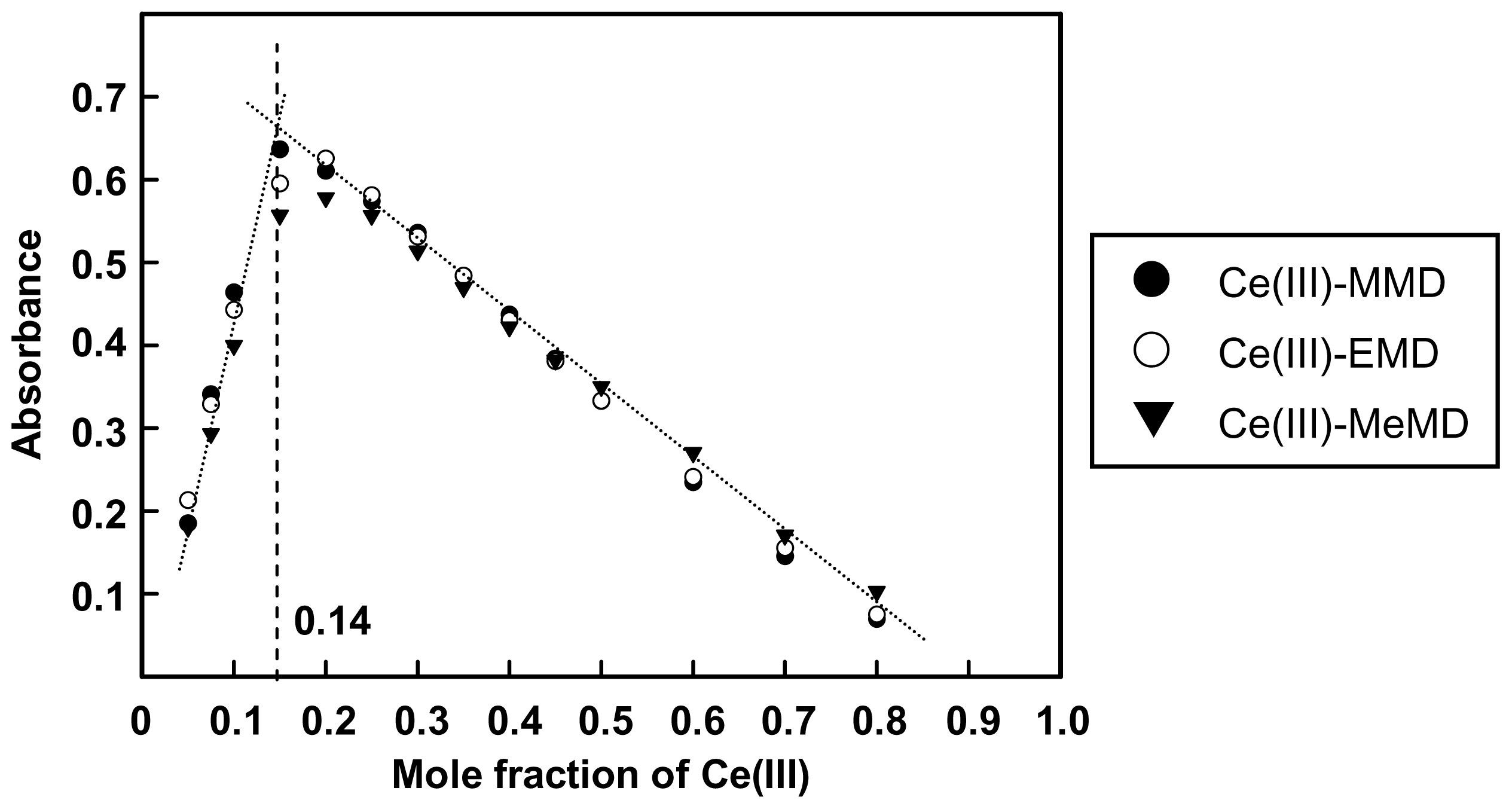
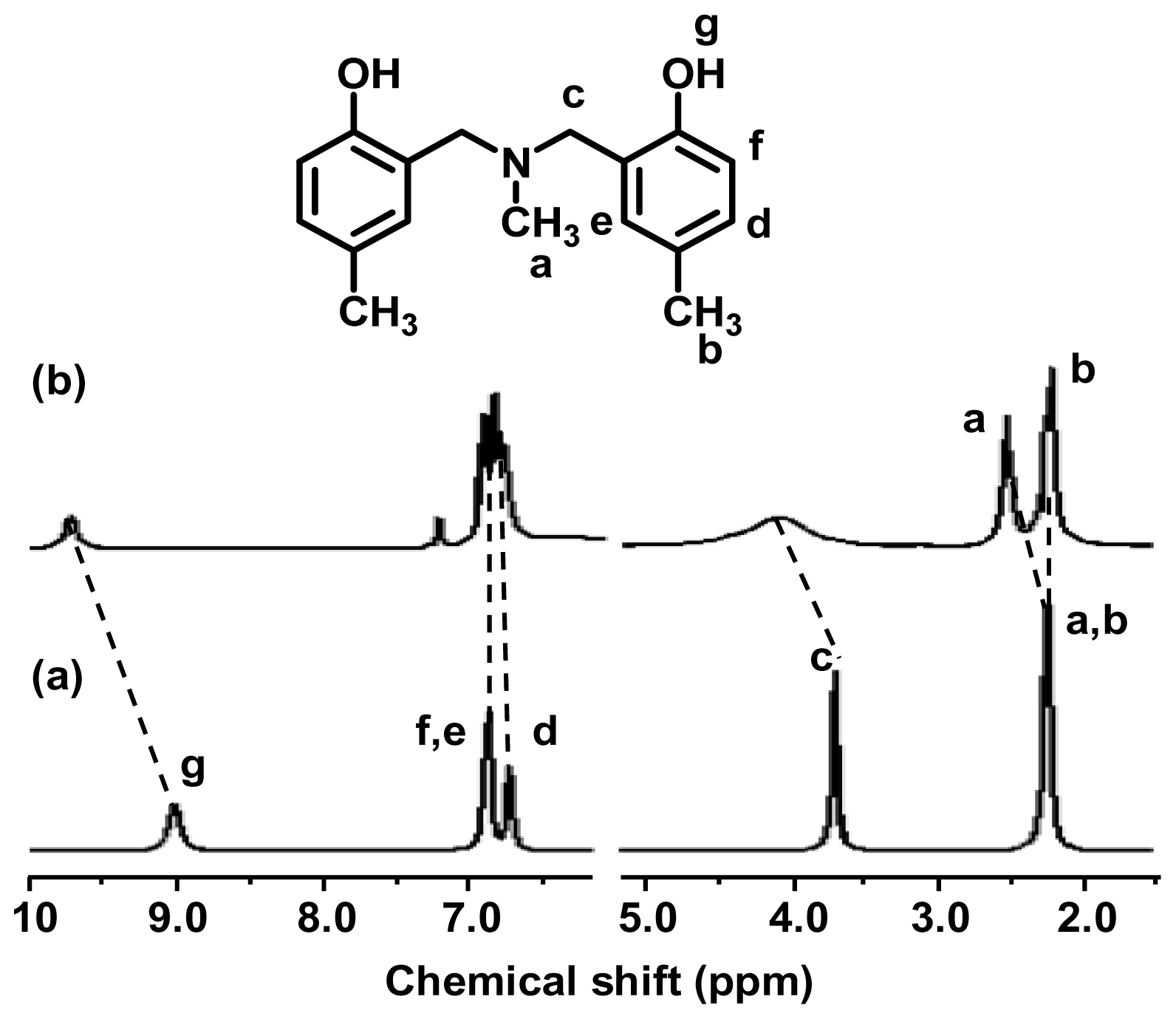
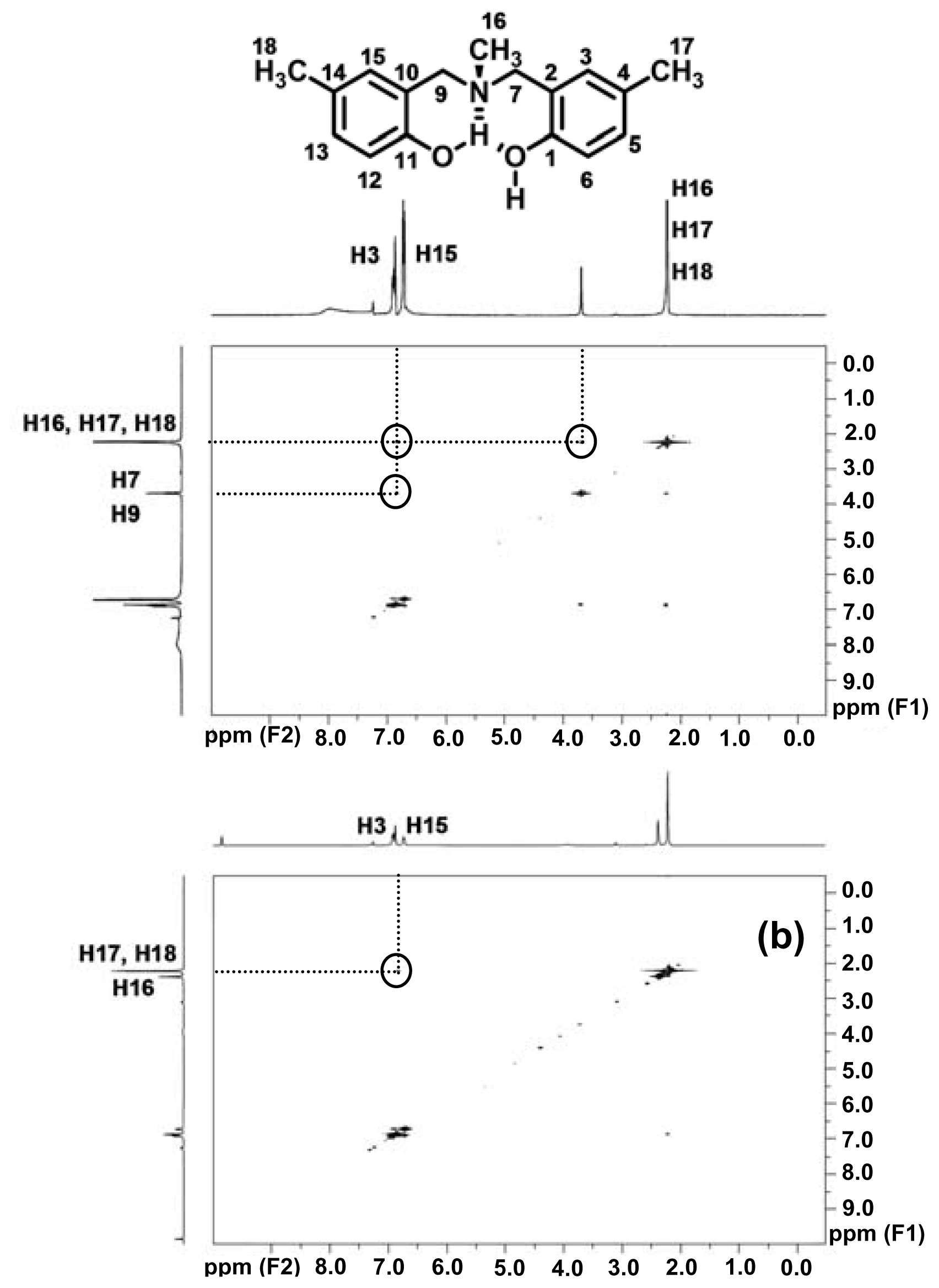
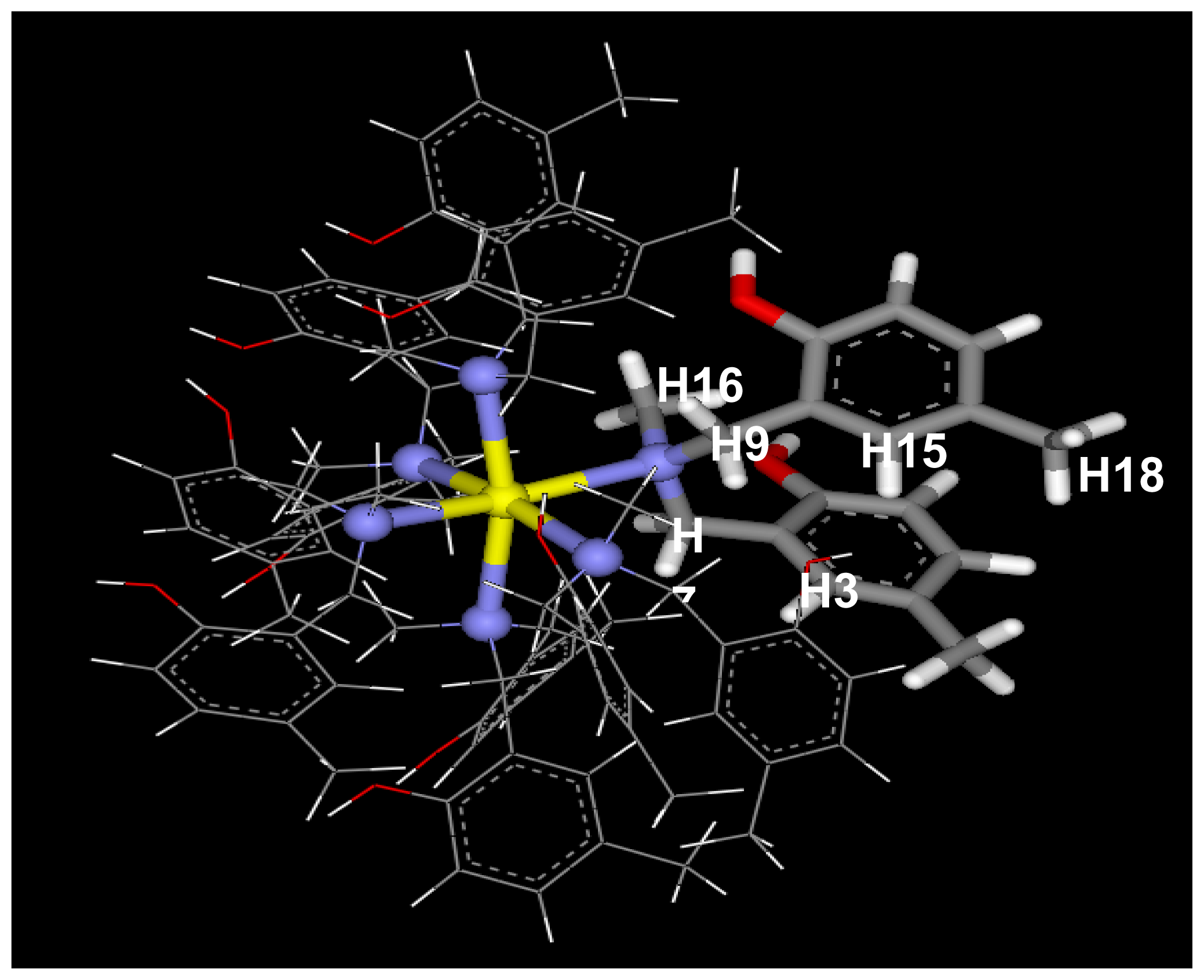
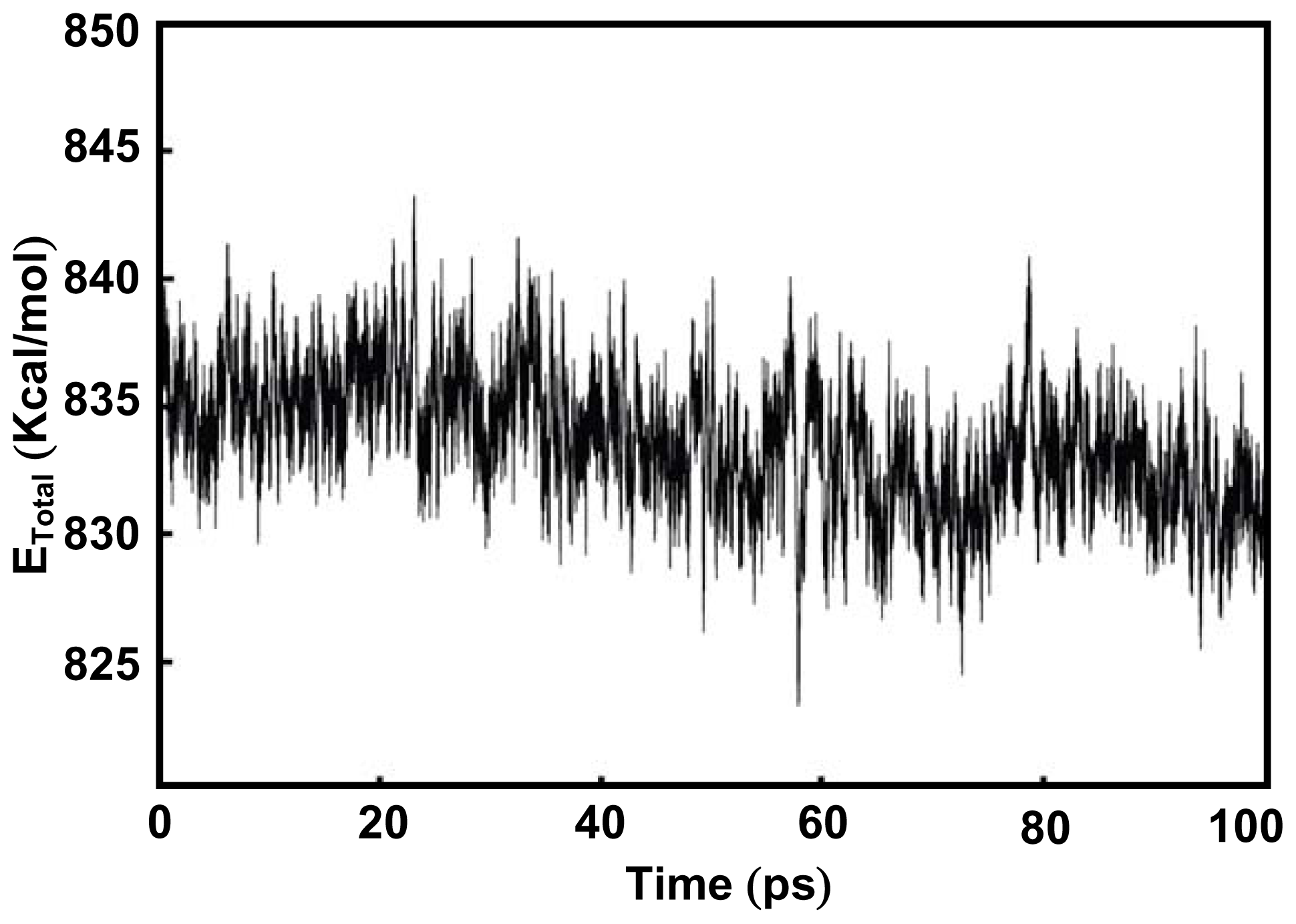
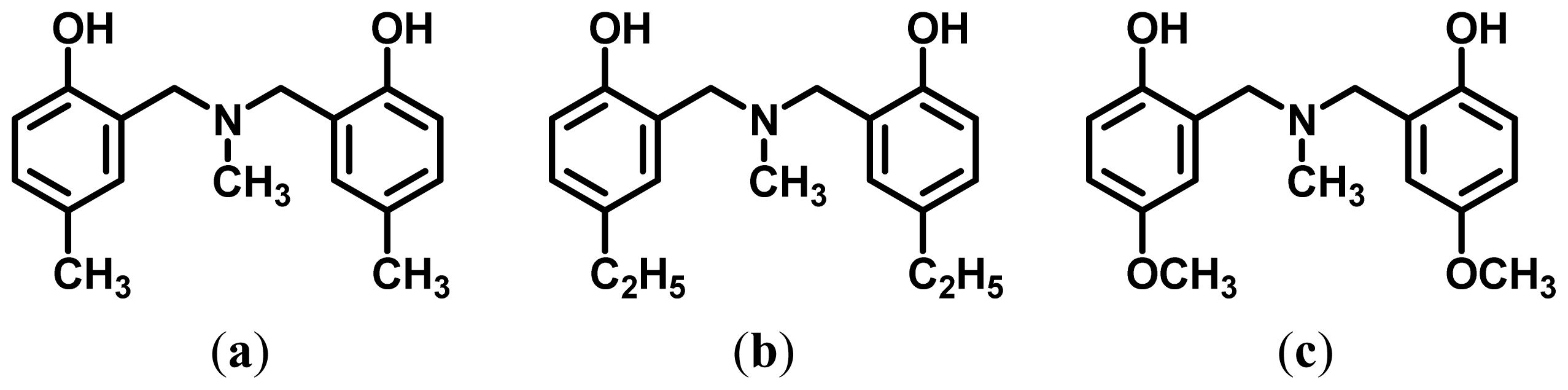
2.1. Complexation of Benzoxazine Dimers and Ce(III) Ion
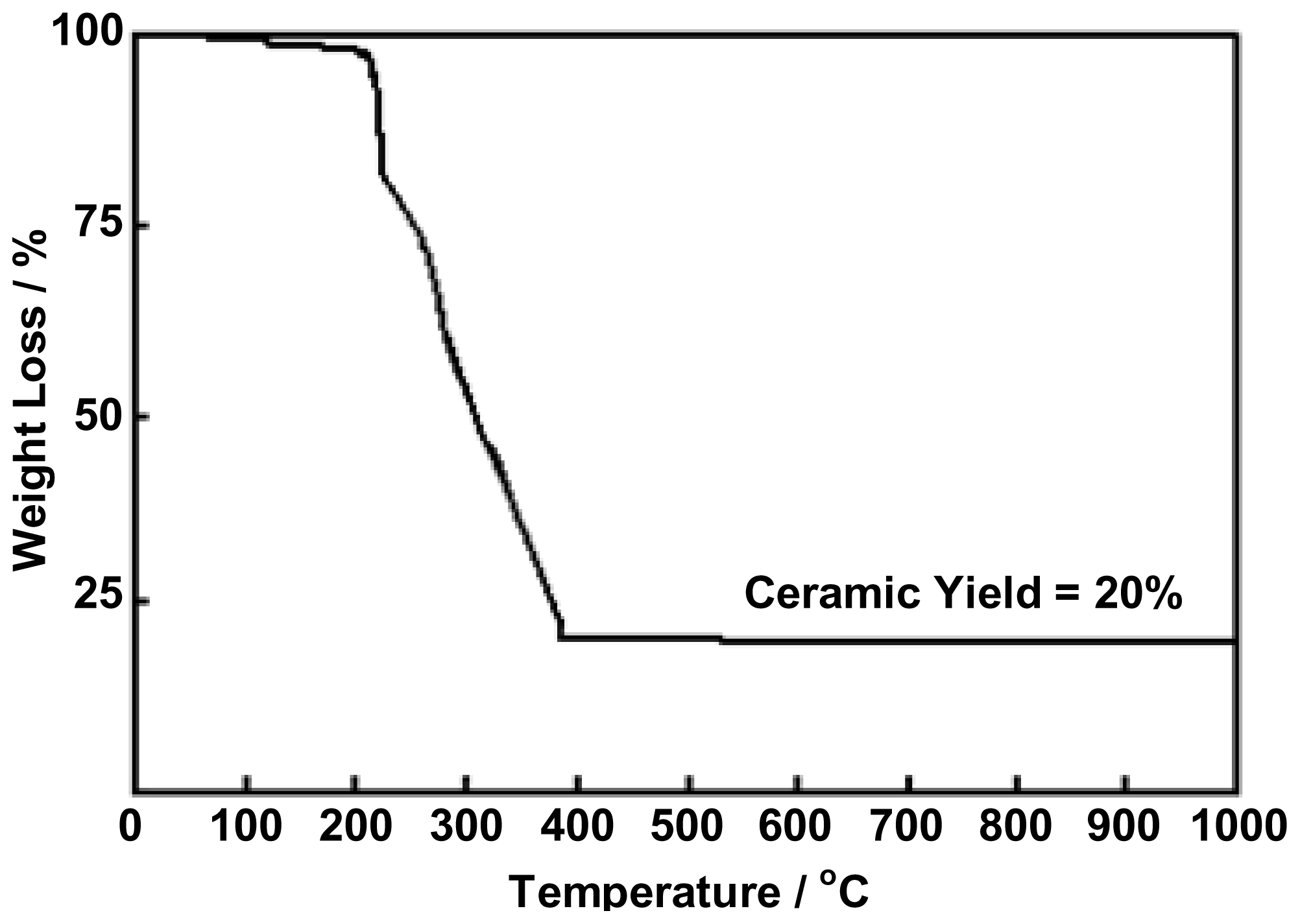
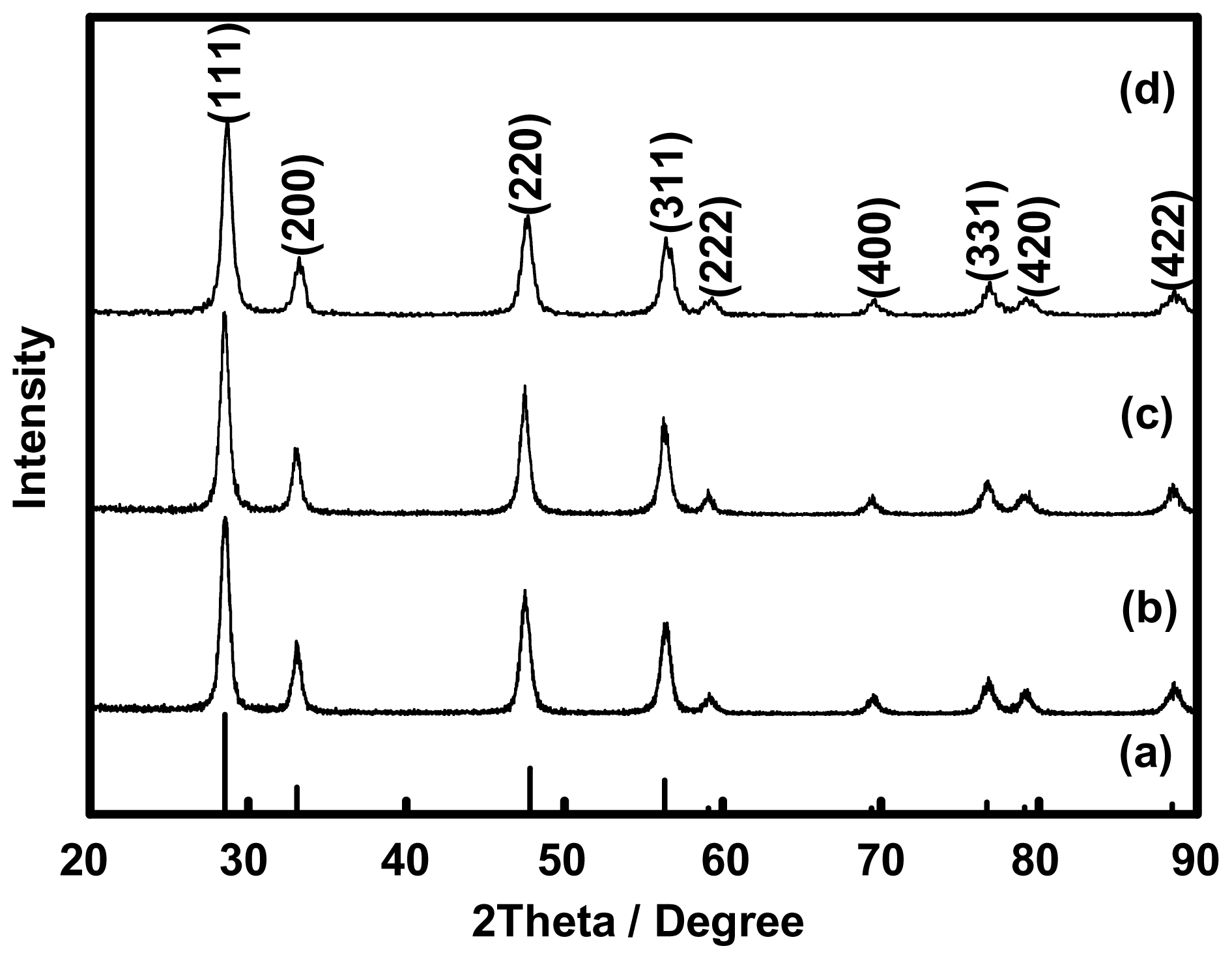
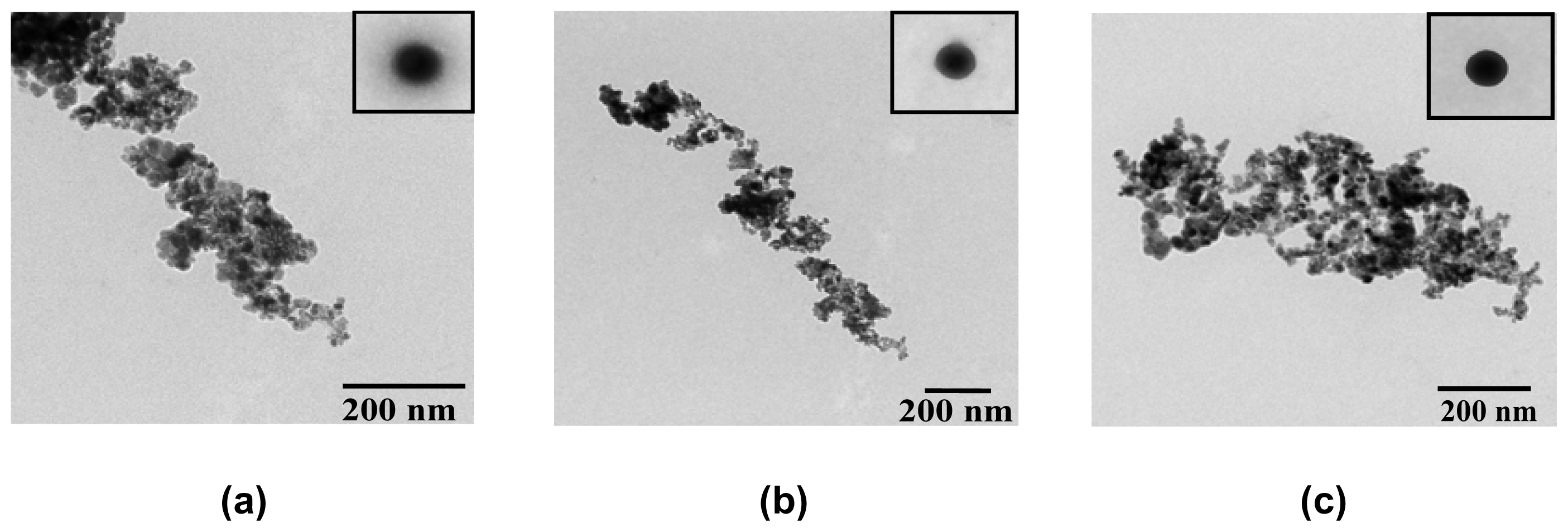
2.2. Preparation of Ceria (CeO2) from Ce(III)-Benzoxazine Dimer Complexes
3. Experimental Section
3.1. Chemicals
3.2. Instruments
3.3. Complexation of Benzoxazine Dimers and Ce(III) Ion
3.4. Computational Simulation
3.5. Preparation of Ceria (CeO2) from Ce(III)-benzoxazine Dimer Complexes
4. Conclusions
Supplementary Materials
ijms-12-04365-s001.pdfAcknowledgements
References
- Badwal, SPS; Foger, K. Solid oxide electrolyte fuel cell review. Ceram. Int 1996, 22, 257–265. [Google Scholar]
- Stambouli, AB; Traversa, E. Solid oxide fuel cells (SOFCs): A review of an environmentally clean and efficient source of energy. Renew. Sustain. Energ. Rev 2002, 6, 433–455. [Google Scholar]
- Balazs, GB; Glass, RS. AC Impedance studies of rare earth oxide doped ceria. Solid State Ion 1995, 76, 155–162. [Google Scholar]
- Kuharuangrong, S. Ionic conductivity of Sm, Gd, Dy and Er-doped ceria. J. Power Sour 2007, 171, 506–510. [Google Scholar]
- Torrens, RS; Sammes, NM; Tompsett, GA. Characterisation of (CeO2)0.8(GdO1.5)0.2 synthesised using various techniques. Solid State Ion 1998, 111, 9–15. [Google Scholar]
- Tok, AIY; Luo, LH; Boey, FYC. Carbonate Co-precipitation of Gd2O3-doped CeO2 solid solution nano-particles. Mater. Sci. Eng. A 2004, 383, 229–234. [Google Scholar]
- Fu, YP; Wen, SB; Lu, CH. Preparation and characterization of samaria-doped ceria electrolyte materials for solid oxide fuel cells. J. Am. Ceram. Soc 2008, 91, 127–131. [Google Scholar]
- Ruifeng, G; Zongqiang, M. Sintering of Ce0.8Sm0.2O1.9. J. Rare Earths 2007, 25, 364–367. [Google Scholar]
- Dikmen, S; Shuk, P; Greenblatt, M; Goomez, H. Hydrothermal synthesis and properties of Ce1-xGdxO2-δ solid solutions. Solid State Sci 2002, 4, 585–590. [Google Scholar]
- Fuentes, RO; Baker, RT. Synthesis and properties of Gadolinium-doped ceria solid solutions for IT-SOFC electrolytes. Int. J. Hydrog. Energ 2008, 33, 3480–3483. [Google Scholar]
- Chirachanchai, S; Laobuthee, A; Phongtamrag, S. Self termination of ring opening reaction of p-substituted phenol-based benzoxazines: An obstructive effect via intramolecular hydrogen bond. J. Heterocycl. Chem 2009, 46, 714–721. [Google Scholar]
- Chirachanchai, S; Rungsimanon, T; Phongtamrag, S; Miyata, M; Laobuthee, A. Selective Macrocyclization: A Model Case Study from N,N-bis(2-hydroxy-3,5-dimethylbenzyl)alkylamine. Tetrahedron 2009, 65, 5855–5861. [Google Scholar]
- Laobuthee, A; Ishida, H; Chirachanchai, S. Metal ion guest responsive benzoxazine dimers and inclusion phenomena of cyclic derivatives. J. Incl. Phenom. Macrocycl. Chem 2003, 47, 179–185. [Google Scholar]
- Chirachanchai, S; Phongtamrag, S; Laobuthee, A. A simple, effective, and selective route without template effect (Part II) for [2 + 2] difunctional 28-membered macrocyclic ethers based on benzoxazine dimers and its inclusion phenomena with metal ions. Chem. Lett 2003, 5, 432–433. [Google Scholar]
- Laobuthee, A; Chirachanchai, S. A simple, effective, and selective synthesis route for difunctional 30-membered macrocyclic ester and linear oligoester derived from benzoxazine dimers. Chem. Lett 2002, 6, 613–614. [Google Scholar]
- Rakthin, T; Veranitisagul, C; Koonsaeng, N; Traversa, E; Laobuthee, A. Preparation of Ceria Powder via Metal-Organic Complex Method. Proceedings of Pure and Applied Chemistry International Conference: Chemistry for Sufficiency and Sustainability, Amsterdam, The Netherlands, 22–23 February 2008; Parasuk, W, Ed.; Chemical Society of Thailand: Bangkok, Thailand, 2008; pp. 312–316. [Google Scholar]
- Hyper Chem Program Release 7.5 for Windows; Hypercube, Inc.: Gainesville, FL, USA, 2002.
- Allinger, NL. Conformational analysis. 130. MM2. A hydrocarbon force field utilizing V1 and V2 torsional terms. J. Am. Chem. Soc 1977, 99, 8127–8134. [Google Scholar]
| Ce(III)-Benzoxazine Dimer Complexes | SBET (m2/g) | DBET (nm) | Crystallite Size (nm) |
|---|---|---|---|
| Ce(III)-MMD | 60 | 13.86 | 25.96 |
| Ce(III)-EMD | 65 | 12.79 | 26.77 |
| Ce(III)-MeMD | 64 | 12.99 | 25.97 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Veranitisagul, C.; Kaewvilai, A.; Sangngern, S.; Wattanathana, W.; Suramitr, S.; Koonsaeng, N.; Laobuthee, A. Novel Recovery of Nano-Structured Ceria (CeO2) from Ce(III)-Benzoxazine Dimer Complexes via Thermal Decomposition. Int. J. Mol. Sci. 2011, 12, 4365-4377. https://doi.org/10.3390/ijms12074365
Veranitisagul C, Kaewvilai A, Sangngern S, Wattanathana W, Suramitr S, Koonsaeng N, Laobuthee A. Novel Recovery of Nano-Structured Ceria (CeO2) from Ce(III)-Benzoxazine Dimer Complexes via Thermal Decomposition. International Journal of Molecular Sciences. 2011; 12(7):4365-4377. https://doi.org/10.3390/ijms12074365
Chicago/Turabian StyleVeranitisagul, Chatchai, Attaphon Kaewvilai, Sarawut Sangngern, Worawat Wattanathana, Songwut Suramitr, Nattamon Koonsaeng, and Apirat Laobuthee. 2011. "Novel Recovery of Nano-Structured Ceria (CeO2) from Ce(III)-Benzoxazine Dimer Complexes via Thermal Decomposition" International Journal of Molecular Sciences 12, no. 7: 4365-4377. https://doi.org/10.3390/ijms12074365