Biodegradable Tri-Block Copolymer Poly(lactic acid)-poly(ethylene glycol)-poly(L-lysine)(PLA-PEG-PLL) as a Non-Viral Vector to Enhance Gene Transfection
Abstract
:1. Introduction
2. Results and Discussion
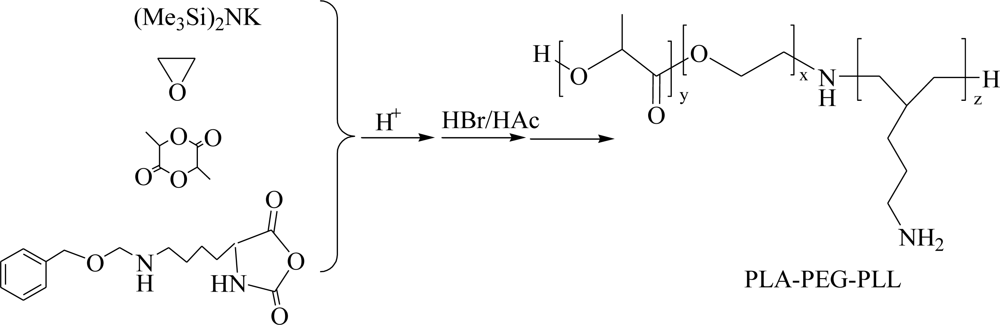
2.1. Synthesis of PLA-PEG-PLL
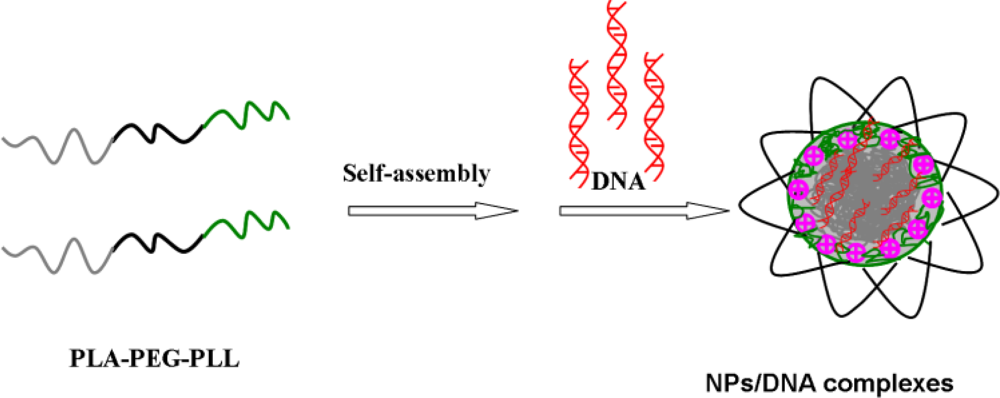
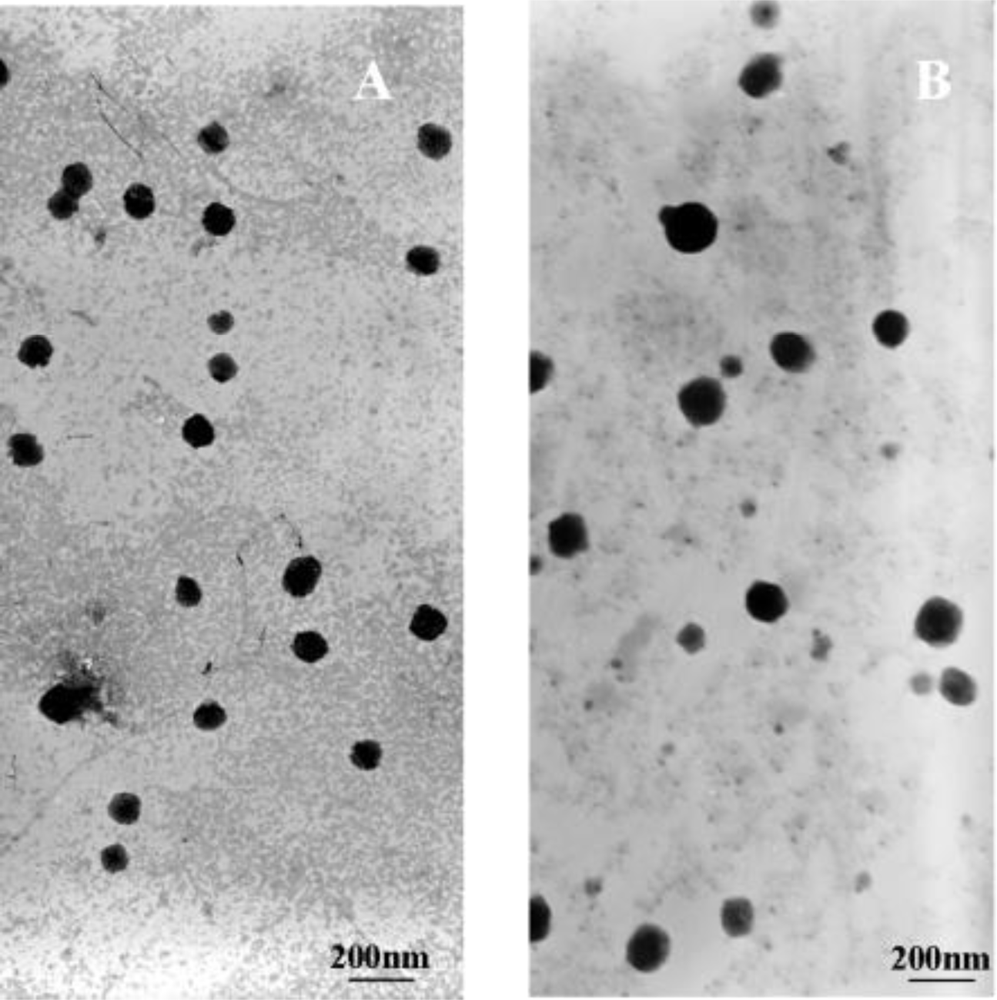
2.2. Formation and Physicochemical Characterization of Cationic PLA-PEG-PLL NPs and PLA-PEG-PLL NPs/DNA Complexes
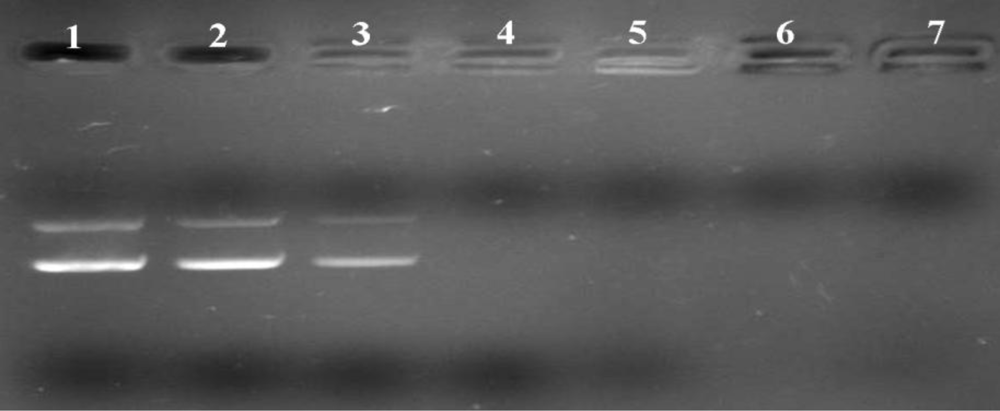
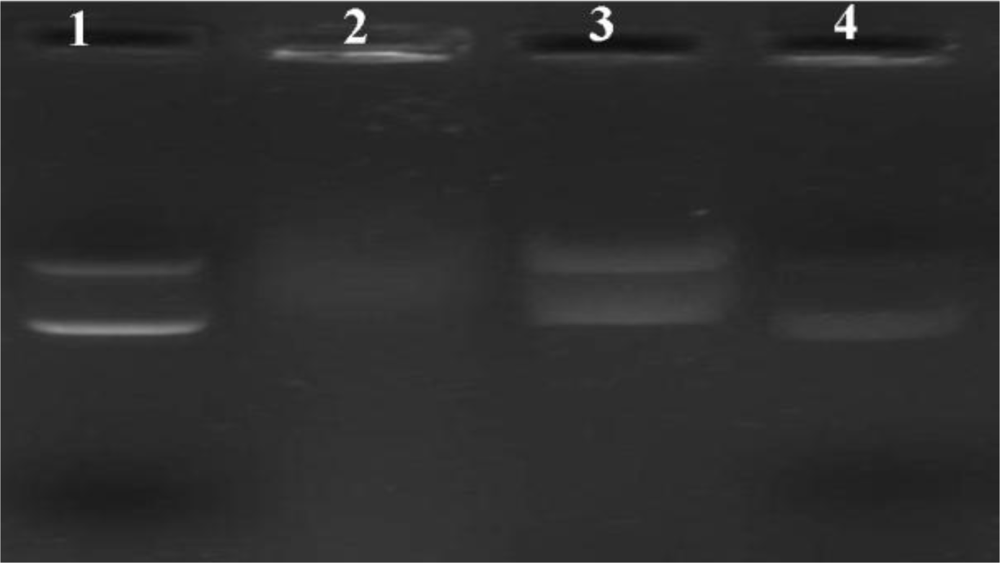
2.3. Gel Retardation Assay
2.4. Heparin Replacement
2.5. Stability of PLA-PEG-PLL NPs/DNA Complexes in Plasma
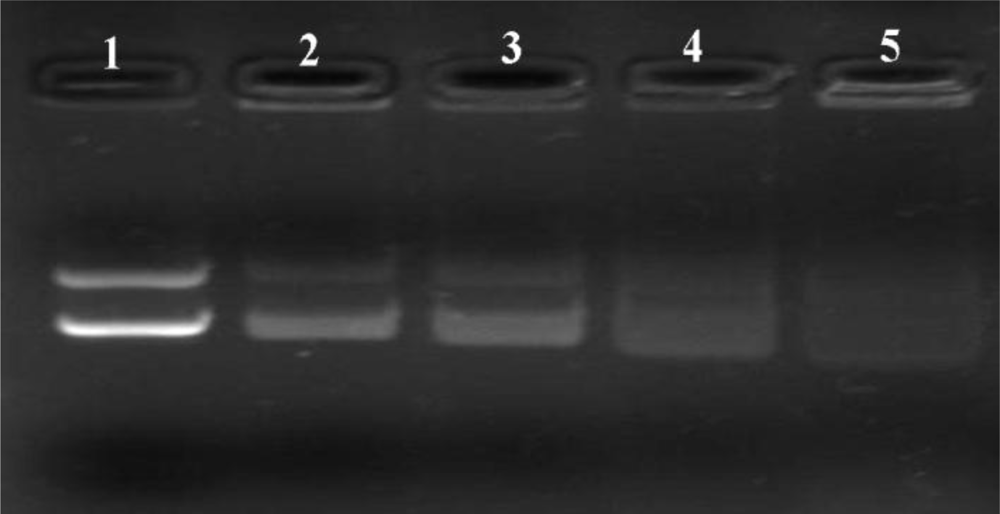
2.6. DNase I Protection Assays
2.7. Cytotoxicity of PLA-PEG-PLL NPs/DNA Complexes
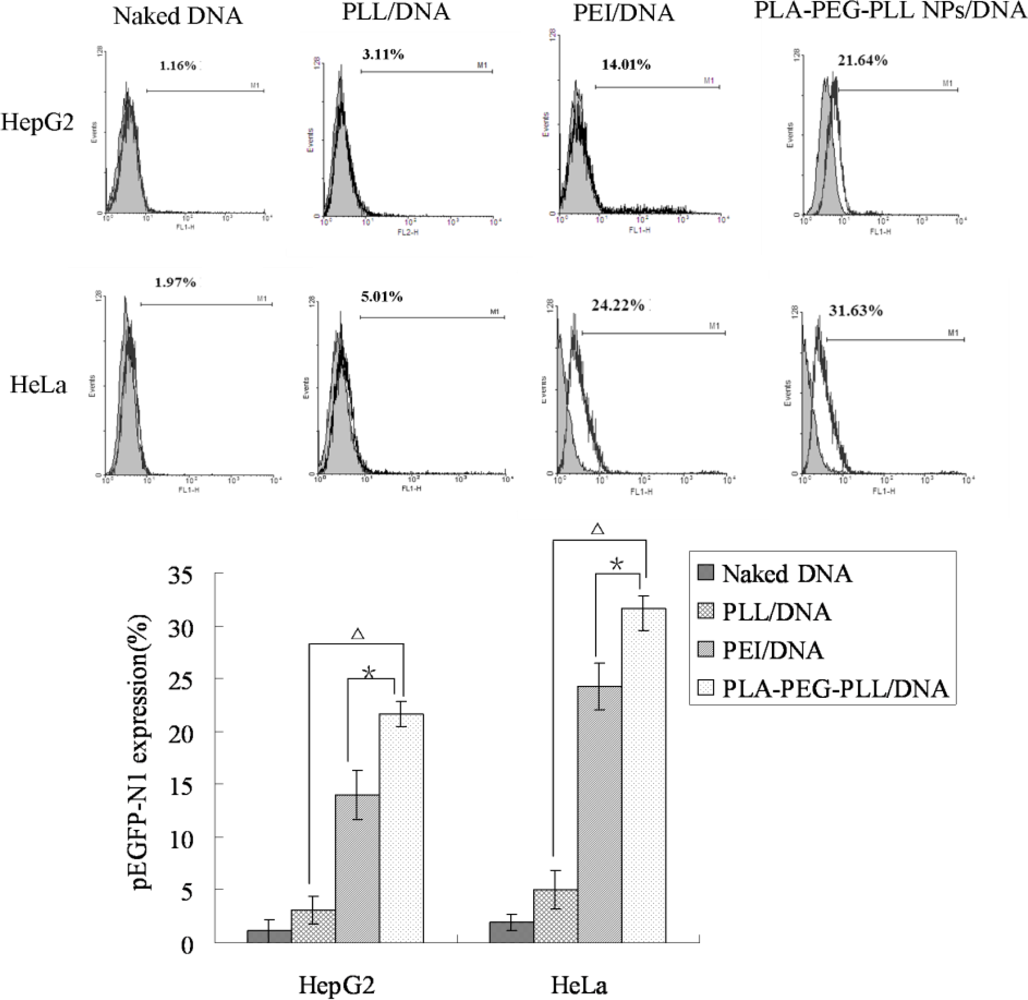
2.8. In Vitro Transfection Studies
3. Experimental Section
3.1. Material
3.2. Synthesis of PLA-PEG-PLL
3.3. Preparation of the Blank PLA-PEG-PLL Nanoparticles
3.4. Preparation of Gene Loaded PLA-PEG-PLL Nanoparticles
3.5. Agarose Gel Electrophoresis of NPs/DNA Complexes
3.6. Morphology, Particle Size and Zeta Potential
3.7. Integrity Analysis of DNA
3.8. Stability Study in Human Plasma
3.9. DNase I Protection Study
3.10. In Vitro Cell Viability
3.11. In Vitro Transfection Efficiency
4. Conclusions
Acknowledgments
References
- Thomas, M; Klibanov, AM. Non-viral gene therapy: polycation-mediated DNA delivery. Appl. Microbiol. Biotechnol 2003, 62, 27–34. [Google Scholar]
- Wolff, JA; Rozema, DB. Breaking the bonds: Non-viral vectors become chemically dynamic. Mol. Ther 2008, 16, 8–15. [Google Scholar]
- Niven, R; Zhang, Y; Smith, J. Toward development of a non-viral gene therapeutic. Adv. Drug Deliv. Rev 1997, 26, 135–150. [Google Scholar]
- Piskin, E; Dincer, S; Turk, M. Gene delivery: Intelligent but just at the beginning. J. Biomater. Sci.-Polym. Ed 2004, 15, 1181–1202. [Google Scholar]
- Panyam, J; Labhasetwar, V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev 2003, 55, 329–347. [Google Scholar]
- Niu, X; Zou, W; Liu, C; Zhang, N; Fu, C. Modified nanoprecipitation method to fabricate DNA-loaded PLGA nanoparticles. Drug Dev. Ind. Pharm 2009, 35, 1375–1383. [Google Scholar]
- Jeon, O; Lim, HW; Lee, M; Song, SJ; Kim, BS. Poly(l-lactide-co-glycolide) nanospheres conjugated with a nuclear localization signal for delivery of plasmid DNA. J. Drug Target 2007, 15, 190–198. [Google Scholar]
- Zou, W; Liu, C; Chen, Z; Zhang, N. Studies on bioadhesive PLGA nanoparticles: A promising gene delivery system for efficient gene therapy to lung cancer. Int. J. Pharm 2009, 370, 187–195. [Google Scholar]
- Chen, J; Tian, B; Yin, X; Zhang, Y; Hu, D; Hu, Z; Liu, M; Pan, Y; Zhao, J; Li, H; Hou, C; Wang, J. Preparation, characterization and transfection efficiency of cationic PEGylated PLA nanoparticles as gene delivery systems. J. Biotechnol 2007, 130, 107–113. [Google Scholar]
- Li, X; Yang, Z; Yang, K; Zhou, Y; Chen, X; Zhang, Y; Wang, F; Liu, Y; Ren, L. Self-Assembled Polymeric Micellar Nanoparticles as Nanocarriers for Poorly Soluble Anticancer Drug Ethaselen. Nanoscale Res. Lett 2009, 4, 1502–1511. [Google Scholar]
- Prabha, S; Labhasetwar, V. Critical determinants in PLGA/PLA nanoparticle-mediated gene expression. Pharm. Res 2004, 21, 354–364. [Google Scholar]
- Kataoka, K; Harada, A; Nagasaki, Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv. Drug Deliv. Rev 2001, 47, 113–131. [Google Scholar]
- Rizkalla, N; Range, C; Lacasse, FX; Hildgen, P. Effect of various formulation parameters on the properties of polymeric nanoparticles prepared by multiple emulsion method. J. Microencapsul 2006, 23, 39–57. [Google Scholar]
- Mok, HJ; Park, TG. Direct plasmid DNA encapsulation within PLGA nanospheres by single oil-in-water emulsion method. Eur. J. Pharm. Biopharm 2008, 68, 105–111. [Google Scholar]
- Fay, F; Quinn, DJ; Gilmore, BF; McCarron, PA; Scott, CJ. Gene delivery using dimethyldidodecylammonium bromide-coated PLGA nanoparticles. Biomaterials 2010, 31, 4214–4222. [Google Scholar]
- Zou, W; Liu, C; Chen, Z; Zhang, N. Preparation and Characterization of Cationic PLA-PEG Nanoparticles for Delivery of Plasmid DNA. Nanoscale Res. Lett 2009, 4, 982–992. [Google Scholar]
- Lv, H; Zhang, S; Wang, B; Cui, S; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar]
- Sun, X; Zhang, N. Cationic polymer optimization for efficient gene delivery. Mini Rev Med Chem 10, 108–125.
- Yu, H; Guo, X; Qi, X; Liu, P; Shen, X; Duan, Y. Synthesis and characterization of arginineglycine-aspartic peptides conjugated poly(lactic acid-co-l-lysine) diblock copolymer. J. Mater. Sci. Mater. Med 2008, 19, 1275–1281. [Google Scholar]
- Lu, Z; Song, JB; Zhang, GZ. Amphiphilic block copolymer, nano-particles thereof and method for preparing same. Chinese Patent. CN 101565497 2009. [Google Scholar]
- Liufu, S; Xiao, H; Li, YP. Investigation of PEG adsorption on the surface of zinc oxide nanoparticles. Powder Technol 2004, 145, 20–24. [Google Scholar]
- Hua, SH; Li, YY; Liu, Y; Xiao, W; Li, C; Huang, FW; Zhang, XZ; Zhuo, RX. Self-Assembled Micelles Based on PEG-Polypeptide Hybrid Copolymers for Drug Delivery. Macromol. Rapid Commun 2010, 31, 81–86. [Google Scholar]
- Liu, J; Guo, S; Li, Z; Liu, L; Gu, J. Synthesis and characterization of stearyl protamine and investigation of their complexes with DNA for gene delivery. Colloids Surf. B. Biointerfaces 2009, 73, 36–41. [Google Scholar]
- Mansouri, S; Cuie, Y; Winnik, F; Shi, Q; Lavigne, P; Benderdour, M; Beaumont, E; Fernandes, JC. Characterization of folate-chitosan-DNA nanoparticles for gene therapy. Biomaterials 2006, 27, 2060–2065. [Google Scholar]
- Oster, CG; Kim, N; Grode, L; Barbu-Tudoran, L; Schaper, AK; Kaufmann, SH; Kissel, T. Cationic microparticles consisting of poly(lactide-co-glycolide) and polyethylenimine as carriers systems for parental DNA vaccination. J. Control. Release 2005, 104, 359–377. [Google Scholar]
- Cui, CJ; Schwendeman, SP. Surface entrapment of polylysine in biodegradable poly(dl-lactide-co-glycolide) microparticles. Macromolecules 2001, 34, 8426–8433. [Google Scholar]
- Blum, JS; Saltzman, WM. High loading efficiency and tunable release of plasmid DNA encapsulated in submicron particles fabricated from PLGA conjugated with poly-l-lysine. J. Control. Release 2008, 129, 66–72. [Google Scholar]
- Capan, Y; Woo, BH; Gebrekidan, S; Ahmed, S; DeLuca, PP. Influence of formulation parameters on the characteristics of poly(d,l-lactide-co-glycolide) microspheres containing poly(l-lysine) complexed plasmid DNA. J. Control. Release 1999, 60, 279–286. [Google Scholar]
- Alexis, F; Pridgen, E; Molnar, LK; Farokhzad, OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm 2008, 5, 505–515. [Google Scholar]
- Park, MR; Han, KO; Han, IK; Cho, MH; Nah, JW; Choi, YJ; Cho, CS. Degradable polyethylenimine-alt-poly(ethylene glycol) copolymers as novel gene carriers. J. Control. Release 2005, 105, 367–380. [Google Scholar]
- Barry, ME; Pinto-Gonzalez, D; Orson, FM; McKenzie, GJ; Petry, GR; Barry, MA. Role of endogenous endonucleases and tissue site in transfection and CpG-mediated immune activation after naked DNA injection. Hum. Gene Ther 1999, 10, 2461–2480. [Google Scholar]
- Pollard, H; Toumaniantz, G; Amos, JL; Avet-Loiseau, H; Guihard, G; Behr, JP; Escande, D. Ca2+-sensitive cytosolic nucleases prevent efficient delivery to the nucleus of injected plasmids. J. Gene Med 2001, 3, 153–164. [Google Scholar]
- Liu, C; Chen, Z; Yu, W; Zhang, N. Novel cationic 6-lauroxyhexyl lysinate modified poly(lactic acid)-poly(ethylene glycol) nanoparticles enhance gene transfection. J Colloid Interface Sci 354, 528–535.
- Choi, KC; Bang, JY; Kim, PI; Kim, C; Song, CE. Amphotericin B-incorporated polymeric micelles composed of poly(d,l-lactide-co-glycolide)/dextran graft copolymer. Int. J. Pharm 2008, 355, 224–230. [Google Scholar]
- Yu, W; Liu, C; Ye, J; Zou, W; Zhang, N; Xu, W. Novel cationic SLN containing a synthesized single-tailed lipid as a modifier for gene delivery. Nanotechnology 2009, 20, 215102. [Google Scholar]
- Oster, CG; Wittmar, M; Bakowsky, U; Kissel, T. DNA nano-carriers from biodegradable cationic branched polyesters are formed by a modified solvent displacement method. J. Control. Release 2006, 111, 371–381. [Google Scholar]
- Jiang, G; Jiang, Y; Shen, Y; Nam, KH; Lee, D; Gao, Z. DNA loaded carrier preferential extravasation from tumor blood vessel. Int. J. Pharm 2009, 369, 155–161. [Google Scholar]
- Oh, YK; Suh, D; Kim, JM; Choi, HG; Shin, K; Ko, JJ. Polyethylenimine-mediated cellular uptake, nucleus trafficking and expression of cytokine plasmid DNA. Gene Ther 2002, 9, 1627–1632. [Google Scholar]
- Peng, SF; Su, CJ; Wei, MC; Chen, CY; Liao, ZX; Lee, PW; Chen, HL; Sung, HW. Effects of the nanostructure of dendrimer/DNA complexes on their endocytosis and gene expression. Biomaterials 2010, 31, 5660–5670. [Google Scholar]




© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fu, C.; Sun, X.; Liu, D.; Chen, Z.; Lu, Z.; Zhang, N. Biodegradable Tri-Block Copolymer Poly(lactic acid)-poly(ethylene glycol)-poly(L-lysine)(PLA-PEG-PLL) as a Non-Viral Vector to Enhance Gene Transfection. Int. J. Mol. Sci. 2011, 12, 1371-1388. https://doi.org/10.3390/ijms12021371
Fu C, Sun X, Liu D, Chen Z, Lu Z, Zhang N. Biodegradable Tri-Block Copolymer Poly(lactic acid)-poly(ethylene glycol)-poly(L-lysine)(PLA-PEG-PLL) as a Non-Viral Vector to Enhance Gene Transfection. International Journal of Molecular Sciences. 2011; 12(2):1371-1388. https://doi.org/10.3390/ijms12021371
Chicago/Turabian StyleFu, Chunhua, Xiaoli Sun, Donghua Liu, Zhijing Chen, Zaijun Lu, and Na Zhang. 2011. "Biodegradable Tri-Block Copolymer Poly(lactic acid)-poly(ethylene glycol)-poly(L-lysine)(PLA-PEG-PLL) as a Non-Viral Vector to Enhance Gene Transfection" International Journal of Molecular Sciences 12, no. 2: 1371-1388. https://doi.org/10.3390/ijms12021371




