Effect of Interface Structure on Mechanical Properties of Advanced Composite Materials
Abstract
:1. Introduction
2. Hybrid Composite Materials
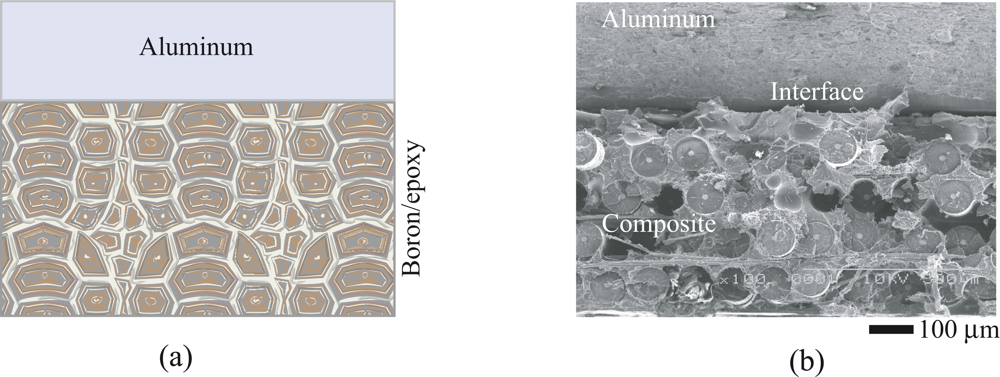
3. Polymer/Metal Bonded Composite Structures
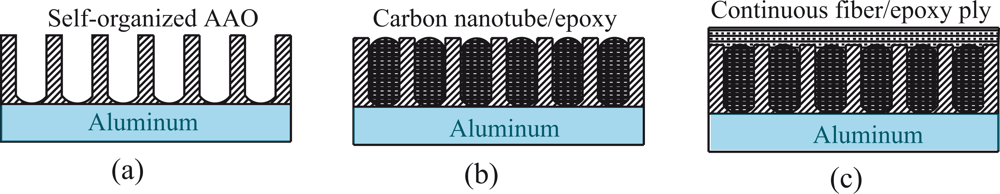
4. Nanoarchitectured Interfaces in Polymeric Composites
4.1. Formation of nanoporous surface structures
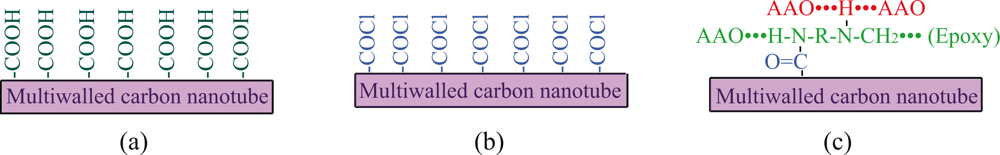
4.2. Addition of active carbon nanotubes
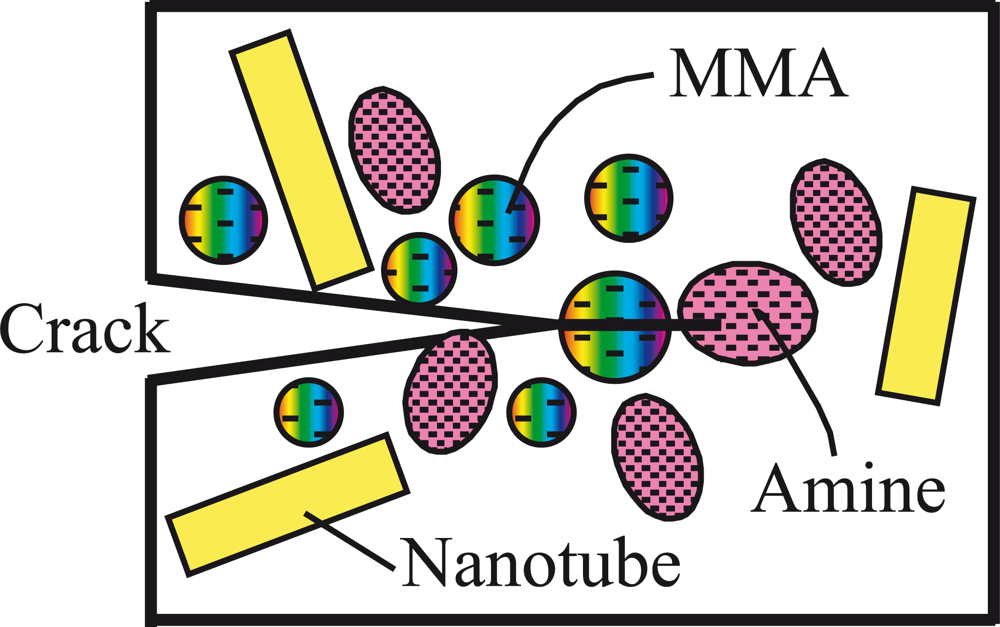
4.3. Addition of nanoscale self-healing capsules
5. Nonlinear Damage Model for Polymeric Nanocomposites
5.1. Modeling the nonlinearity
5.2. Application of the nonlinear damage model
6. Conclusions
Acknowledgments
References
- Afaghi-Khatibi, A; Lawcock, G; Ye, L; Mai, Y. On the fracture mechanical behaviour of fibre reinforced metal laminates (FRMLs). Comput. Methods Appl. Mech. Engrg 2000, 185, 173–190. [Google Scholar]
- Castrodeza, EM; Bastian, FL; Ipina, JEP. Critical fracture toughness, JC and δC, of unidirectional fibre-metal laminates. Thin-Walled Struct 2003, 41, 1089–1101. [Google Scholar]
- Vaidya, UK; Pillay, S; Bartus, S; Ulven, CA; Grow, DT; Mathew, B. Impact and post-impact vibration response of protective metal foam composite sandwich plates. Mater. Sci. Eng. A 2006, 428, 59–66. [Google Scholar]
- Callister, WD. Materials Science and Engineering An Introduction, 6th ed; John Wiley & Sons, Inc: New York, NY, USA, 2005; pp. 659–662. [Google Scholar]
- Sekercioglu, T; Gulsoz, A; Rende, H. The effects of bonding clearance and interference fit on the strength of adhesively bonded cylindrical components. Mater. Design 2005, 26, 377–381. [Google Scholar]
- Ghosh, A; Schiraldi, DA. Improving interfacial adhesion between thermoplastic polyurethane and copper foil using amino carboxylic acids. J. Appl. Polym. Sci 2009, 112, 1738–1744. [Google Scholar]
- Friedrich, J; Mix, R; Wettmarshausen, S. A new concept for adhesion promotion in metal-polymer systems by introduction of covalently bonded spacers at the interface. J. Adhesion Sci. Technol 2008, 22, 1123–1143. [Google Scholar]
- Kulkarni, RR; Chawla, KK; Vaidya, UK; Sands, JM. Thermal stresses in aluminum 6061 and nylon 66 long fiber thermoplastic (LFT) composite joint in a tailcone. J. Mater. Sci 2007, 42, 7389–7396. [Google Scholar]
- van Tijum, R; Vellinga, WP; De Hosson, JTM. Surface roughening of metal-polymer systems during plastic deformation. Acta Mater 2007, 55, 2757–2764. [Google Scholar]
- Castello, X; Estefen, SF. Limit strength and reeling effects of sandwich pipes with bonded layers. Int. J. Mech. Sci 2007, 49, 577–588. [Google Scholar]
- van Tijum, R; Vellinga, WP; De Hosson, JTM. Adhesion along metal-polymer interfaces during plastic deformation. J. Mater. Sci 2007, 42, 3529–3536. [Google Scholar]
- Li, T; Suo, Z. Ductility of thin metal films on polymer substrates modulated by interfacial adhesion. Int. J. Solids Struct 2007, 44, 1696–1705. [Google Scholar]
- Lu, N; Wang, X; Suo, ZG; Vlassak, J. Failure by simultaneous grain growth, strain localization, and interface debonding in metal films on polymer substrates. J. Mater. Res 2009, 24, 379–385. [Google Scholar]
- Zeng, F; Lv, F; Zong, RL; Wen, SP; Zhu, XY; Pan, F. Tensile properties of Cr inserted amorphous Co85Zr9Nb6 films deposited on polymer substrate. J. Alloys Compounds 2009, 477, 239–242. [Google Scholar]
- Li, Y; Wang, XS; Meng, XK. Buckling behavior of metal film/substrate structure under pure bending. Appl. Phys. Lett 2008, 92, 131902. [Google Scholar]
- Oehlers, DJ; Liu, IST; Seracino, R. Shear deformation debonding of adhesively bonded plates. Proc. Inst. Civ. Eng. Struc. Build 2005, 158, 77–84. [Google Scholar]
- Tantikom, K; Aizawa, T; Mukai, T. Symmetric and asymmetric deformation transition in the regularly cell-structured materials. Part II: Theoretical study. Int. J. Solids Struct 2005, 42, 2211–2224. [Google Scholar]
- Cognard, JY; Davies, P; Gineste, B; Sohier, L. Development of an improved adhesive test method for composite assembly design. Comp. Sci. Technol 2005, 65, 359–368. [Google Scholar]
- Pires, I; Quintino, L; Miranda, RM. Performance of 2024-T3 aluminium adhesive bonded joints. Mater. Manufact. Proc 2005, 20, 175–185. [Google Scholar]
- DiFelice, RA; Dillard, JG; Yang, D. An investigation of plasma processes in titanium(iv) isobutoxide: the formation of films on Ti and Si. Int. J. Adhesion Adhesives 2005, 25, 277–287. [Google Scholar]
- Collaud, M; Groening, P; Nowak, S; Schlapbach, L. Plasma teatment of polymers-the effect of the plasma parameters on the chemical, physical and morphological states of polymer surface and on the metal-polymer interface. J. Adhesion Sci. Technol 1994, 8, 1115–1127. [Google Scholar]
- Laniog, BN; Ramos, HJ; Wada, M; Mena, MG; Flauta, RE. Surface modification of epoxy resin based PCB substrates using argon and oxygen plasmas. Proc. 2006 Int. Conf. Electronic Mater. Packaging, Shanghai, China, August 26–29, 2006; 1–3, pp. 857–862.
- Mackova, A; Malinsky, P; Bocan, J; Svorcik, V; Pavlik, J; Stryhal, Z; Sajdl, P. Study of Ag and PE interface after plasma treatment. Physica Status Solidi C Curr. Topics Solid State Phys 2008, 5, 964–967. [Google Scholar]
- Kim, MH; Lee, KW. The effects of ion beam treatment on the interfacial adhesion of Cu/polyimide system. Met. Mater. Int 2006, 12, 425–433. [Google Scholar]
- Zaporojtchenko, V; Zekonyte, J; Faupel, F. Effects of ion beam treatment on atomic and macroscopic adhesion of copper to different polymer materials. Nucl. Instrum. Meth. Phys. Res. B 2007, 265, 139–145. [Google Scholar]
- Ratchev, BA; Was, GS; Booske, JH. Ion beam modification of metal-polymer interfaces for improved adhesion. Nucl. Instrum. Meth. Phys. Res. B 1995, 106, 68–73. [Google Scholar]
- Ghosh, A; Schiraldi, DA. Improving interfacial adhesion between thermoplastic polyurethane and copper foil using amino carboxylic acids. J. Appl. Polym. Sci 2009, 112, 1738–1744. [Google Scholar]
- David, E; Lazar, A; Armeanu, A. Surface modification of polytetrafluoroethylene for adhesive bonding. J. Mater. Proc. Technol 2004, 157, 284–289. [Google Scholar]
- Underhill, PR; Rider, AN. Hydrated oxide film growth on aluminium alloys immersed in warm water. Surf. Coat. Technol 2005, 192, 199–207. [Google Scholar]
- Shin, KC; Lee, JJ. Effects of thermal residual stresses on failure of co-cured lap joints with steel and carbon fiber-epoxy composite adherends under static and fatigue tensile loads. Composites Part A: Appl. Sci. Manufact 2006, 37, 476–487. [Google Scholar]
- Cognard, J. Some recent progress in adhesion technology. Comptes Rendus Chimie 2006, 9, 13–24. [Google Scholar]
- Barroso, A; Paris, F; Mantic, V. Representativity of the singular stress state in the failure of adhesively bonded joints between metals and composites. Comp. Sci. Technol 2009, 69, 1746–1755. [Google Scholar]
- Feraboli, P; Masini, A. Development of carbon/epoxy structural components for a high performance vehicle. Composites Part B: Eng 2004, 35, 323–330. [Google Scholar]
- Woerdeman, DL; Emerson, JA; Giunta, RK. JKR contact mechanics for evaluating surface contamination on inorganic substrates. Int. J. Adhesion Adhesives 2002, 22, 257–264. [Google Scholar]
- Roche, AA; Bouchet, J; Bentadjine, S. Formation of epoxy-diamine/metal interphases. Int. J. Adhesion Adhesives 2002, 22, 431–441. [Google Scholar]
- Ageorges, C; Ye, L; Hou, M. Advances in fusion bonding techniques for joining thermoplastic matrix composites: A review. Composites Part A: Appl. Sci. Manufact 2001, 32, 839–857. [Google Scholar]
- Rao, VV; Singh, R; Malhotra, SK. Residual strength and fatigue life assessment of composite patch repaired specimens. Composites Part B 1999, 30, 621–627. [Google Scholar]
- Muller, R; Fredell, R. Analysis of multiple bonded patch interaction simple design guidelines for multiple bonded repairs in close proximity. Appl. Comp. Mater 1999, 6, 217–237. [Google Scholar]
- Klug, JC; Sun, CT. Large deflection effects of cracked aluminum plates repaired with bonded composite patches. Comp. Struct 1998, 42, 291–296. [Google Scholar]
- Fredell, R; Guijt, C; Mazza, J. An integrated bonded repair system: A reliable means of giving new life to aging airframes. Appl. Comp. Mater 1999, 6, 269–277. [Google Scholar]
- Kumar, AM; Hakeem, SA. Optimum design of symmetric composite patch repair to centre cracked metallic sheet. Comp. Struct 2000, 49, 285–292. [Google Scholar]
- Wang, WC; Hsu, JS. Investigation of the size effect of composite patching repaired on edge-cracked plates. Comp. Struct 2000, 49, 415–423. [Google Scholar]
- Christian, TF, Jr; Hammond, DO; Cochran, JB. Composite material repairs to metallic airframe components. J. Aircraft 1992, 29, 470–476. [Google Scholar]
- Umamaheswar, TV; Singh, R. Modelling of a patch repair to a thin cracked sheet. Eng. Fract. Mech 1999, 62, 267–289. [Google Scholar]
- Denney, JJ; Mall, S. Characterization of disbond effects on fatigue crack growth behavior in aluminum plate with bonded composite patch. Eng. Frac. Mech 1997, 57, 507–509. [Google Scholar]
- Baker, AA; Rose, RLF; Walker, KF. Repair substantiation for a bonded composite repair to F111 lower wing skin. Appl. Comp. Mater 1999, 6, 251–267. [Google Scholar]
- Jones, R; Smith, WR. Continued airworthiness of composite repairs to primary structures for military aircraft. Comp. Struct 1995, 33, 17–26. [Google Scholar]
- Fernandes, PJL; Jones, DRH. The effects of loading variables on fatigue-crack growth in liquid-metal environments. Int. J. Fatigue 1995, 17, 501–505. [Google Scholar]
- Siddaramaiah, JVA; Gowri, ANS; Leena, B; Hemmige, VV; Deepa, S. Tensile behavior of composite patched cracked metallic structures. J. Appl. Polym. Sci 1998, 68, 2063–2068. [Google Scholar]
- Wang, CH; Rose, LRF; Baker, AA. Modelling of the fatigue growth behaviour of patched cracks. Int. J. Fract 1998, 88, L65–L70. [Google Scholar]
- Fredell, R; Vanbarneveld, W; Vlot, A. Analysis of composite crack patching of fuselage structures- high patch elastic modulus is not the whole story. Moving Forward with 50 Years of Leadership in Adv. Mater.-39th Int. SAMPE Sym. Exhibition, Anaheim, California, USA, April 11–14, 1994; 39, pp. 610–623.
- Chow, WT; Atluri, SN. Composite patch repairs of metal structures: Adhesive nonlinearity, thermal cycling, and debonding. AIAA J 1997, 35, 1528–1535. [Google Scholar]
- Naboulsi, S; Mall, S. Fatigue crack growth analysis of adhesively repaired panel using perfectly and imperfectly composite patches. Theor. Appl. Fract. Mech 1997, 28, 13–28. [Google Scholar]
- Schubbe, JJ; Mall, S. Investigation of a cracked thick aluminum panel repaired with a bonded composite patch. Eng. Fract. Mech 1999, 63, 305–323. [Google Scholar]
- Ong, CL; Shen, SB. Some results on metal and composite patch Reinforcement of aluminum honeycomb pannel. Theor. Appl. Fract. Mech 1991, 16, 145–153. [Google Scholar]
- Chalkley, P; Baker, A. Development of a generic repair joint for certification of bonded composite repairs. Int. J Adhesives 1999, 19, 121–132. [Google Scholar]
- Dong, CS. Modeling the process-induced dimensional variations of general curved composite components and assemblies. Composites Part A: Appl. Sci. Manuf 2009, 40, 1210–1216. [Google Scholar]
- Aglan, H; Gan, YX; Wang, QY; Kehoe, M. Design guidelines for composite patches bonded to cracked aluminum substrates. J. Adhesion Sci. Technol 2002, 16, 197–211. [Google Scholar]
- Salehi-Khojin, A; Zhamu, A; Zhong, W; Gan, Y. Effects of patch layer and loading frequency on fatigue fracture behavior of aluminum plate repaired with a boron/epoxy composite patch. J. Adhesion Sci. Technol 2006, 20, 107–123. [Google Scholar]
- Nassar, SA; Virupaksha, VL. Effect of adhesive thickness and properties on the biaxial interfacial shear stresses in bonded joints using a continuum mixture model. J. Eng. Mater. Technol 2009, 131, 021015. [Google Scholar]
- Rose, LRF. A cracked plate repaired by bonded reinforcements. Int. J Fract 1982, 18, 135–144. [Google Scholar]
- Rose, LRF. An application of the inclusion analogy for bonded Reinforcements. Int. J. Solids Struct 1981, 17, 827–838. [Google Scholar]
- Lam, YC; Zhu, CS. Analytical techniques for bonded repairs. Fract Stregth Solids Part 1–2 1998, 145–149, 543–552. [Google Scholar]
- Xiong, Y. An analytical model for stress analysis and failure prediction of bonded joints with tapered edges. Trans. Can. Soc. Mech. Eng 1998, 22, 143–155. [Google Scholar]
- Renton, WJ; Vinson, JR. Analysis of adhesively bonded joints between panels of composite-materials. J. Appl. Mech 1977, 44, 101–106. [Google Scholar]
- Muller, R; Fredell, R; Guijt, C. Experimental verification of Rose’s constant K solution in bonded crack patching. Appl. Comp. Mater 1999, 6, 205–216. [Google Scholar]
- Lena, MR; Klug, JC; Sun, CT. Composite patches as reinforcements and crack arrestors in aircraft structures. J. Aircraft 1998, 35, 318–323. [Google Scholar]
- Sun, CT; Klug, J; Arendt, C. Analysis of cracked aluminum plates repaired with bonded composite patches. AIAA J 1996, 34, 369–374. [Google Scholar]
- Monaco, A; Sinke, J; Benedictus, R. Experimental and numerical analysis of a beam made of adhesively bonded tailor-made blanks. Int. J. Adv. Manuf. Technol 2009, 44, 766–780. [Google Scholar]
- Kim, WS; Lee, JJ. Fracture characterization of interfacial cracks with frictional contact of the crack surfaces to predict failures in adhesive-bonded joints. Eng. Fract. Mech 2009, 76, 1785–1799. [Google Scholar]
- Lin, CC; Chu, RC; Lin, YS. A finite-element model for single-sided crack patching. Eng. Fract. Mech 1993, 46, 1005–1021. [Google Scholar]
- Kam, TY; Chu, KH; Tsai, YC. Fatigue of cracked plates repaired with single-sided composite patches. AIAA J 1998, 36, 645–650. [Google Scholar]
- Kam, TY; Tsai, YC; Chu, KH; Wu, JH. Fatigue analysis of cracked aluminum plates repaired with bonded composite patches. AIAA J 1998, 36, 115–118. [Google Scholar]
- Naboulsi, S; Mall, S. Characterization of fatigue crack growth in aluminium panels with a bonded composite patch. Comp. Struct 1997, 37, 321–334. [Google Scholar]
- Naboulsi, S; Mall, S. Methodology to analyse aerospace structures repaired with a bonded composite patch. J. Strain Analy. Eng. Design 1999, 34, 395–412. [Google Scholar]
- Naboulsi, S; Mall, S. Nonlinear analysis of bonded composite patch repair of cracked aluminum panels. Comp. Struct 1998, 41, 303–313. [Google Scholar]
- Ratwani, MM. Analyis of cracked, adhesively bonded laminated structures. AIAA J 1979, 17, 988–994. [Google Scholar]
- Chau, RWT; Lee, SWR. Computational modeling and analysis of a center-cracked panel repaired by bonded composite patch. Fract. Strength Solids, Part 1–2 1998, 145–149, 601–606. [Google Scholar]
- Paul, J; Bartholomeusz, RA; Jones, R; Ekstrom, M. Bonded composite repair of cracked load-bearing holes. Eng. Fract. Mech 1994, 48, 455–461. [Google Scholar]
- Chue, C; Liu, TJ. The effects of laminated composite patch with different stacking sequences on bonded repair. Comp. Eng 1995, 5, 223–230. [Google Scholar]
- Callinan, RJ; Galea, SC; Sanderson, S. Finite element analysis of bonded repairs to edge cracks in panels subjected to acoustic excitation. Comp. Struct 1997, 38, 649–660. [Google Scholar]
- Chue, CH; Chou, WC; Liu, TJC. The effects of size and stacking sequence of composite laminated patch on bonded repair for cracked hole. Appl. Comp. Mater 1996, 3, 355–367. [Google Scholar]
- Tarn, JQ; Shek, KL. Analusis of cracked plates with a bonded patch. Eng. Fract. Mech 1991, 40, 1055–1065. [Google Scholar]
- Young, A; Rooke, DP; Cartwright, DJ. Analysis of patched and stiffened cracked panels using the boundary element method. Int. J. Solids Struct 1992, 29, 2201–2216. [Google Scholar]
- Salgado, NK; Aliabadi, MH. The boundary element analysis of cracked stiffened sheets, reinforced by adhesively bonded patches. Int. J. Numer. Method Eng 1998, 42, 195–217. [Google Scholar]
- Zadpoor, AA; Sinke, J; Benedictus, R. The mechanical behavior of adhesively bonded tailor-made blanks. Int. J. Adhesion Adhesives 2009, 29, 558–571. [Google Scholar]
- Atluri, SN; Chow, WT; Wang, L. Computational mechanics of interfacial fracture for composite debonding; And of elastoplastic fracture with multi-site-damage. Adv Fract Res 1997, 1–6, 1885–1897. [Google Scholar]
- Nahas, MN. Experimental investigation of fatigue of cracked aluminum specimens repaired with fiber composite patches. J. Reinf. Plast. Comp 1992, 11, 932–938. [Google Scholar]
- Ong, CL; Chu, RC; Ko, TC; Shen, SB. Composite patch reinforcement of cracked aircraft upper longeron-analysis and specimen simulation. Theor. Appl. Fract. Mech 1990, 14, 13–26. [Google Scholar]
- Ong, CL; Shen, SB. The reinforced effect of composite patch repairs on metalic aircraft structures. Int. J. Adhesion and Adhesives 1992, 12, 19–26. [Google Scholar]
- Sabelkin, V; Mall, S; Hansen, MA; Vandawaker, RM; Derris, M. Investigation into cracked aluminum plate repaired with bonded composite patch. Comp. Struct 2007, 79, 55–66. [Google Scholar]
- Chester, RJ; Walker, KF; Chalkley, PD. Adhesively bonded repairs to primary aircraft structure. Int. J. Adhesion Adhesives 1999, 19, 1–8. [Google Scholar]
- Clark, RJ; Romilly, DP. Bending of bonded composite repairs for aluminum aircraft structures: A design study. J. Aircraft 2007, 44, 2012–2025. [Google Scholar]
- Rao, VV; Singh, R; Malhotra, SK. Residual strength and fatigue life assessment of composite patch repaired specimens. Composites Part B: Eng 1999, 30, 621–627. [Google Scholar]
- Alawi, H; Saleh, IE. Fatigue crack-growth retardation by bonding patches. Eng. Fract. Mech 1992, 42, 861–868. [Google Scholar]
- Hastie, RL; Fredell, R; Dally, JW. A photoelastic study of crack repair. Exp. Mech 1998, 38, 29–36. [Google Scholar]
- Jones, R; Bartholomeusz, R; Kayer, R; Roberts, J. Bonded-composite repair of representative multisite damage in a full-scale fatigue-test. Theor. Appl. Fract Mech 1994, 21, 41–49. [Google Scholar]
- Tay, TE; Chau, FS; Er, CJ. Bonded boron-epoxy composite repair and reinforcement of cracked aluminium structures. Comp. Struct 1996, 34, 339–347. [Google Scholar]
- Butkus, J. Considering environmental conditions in the design of bonded structures: A fracture mechanics approach. Fatig. Fract. Eng. Mater. Sruct 1998, 21, 465–478. [Google Scholar]
- Yang, Z; Huang, Y; Dong, B; Li, H; Shi, S. Densely packed single-crystal Bi2Fe4O9 nanowires fabricated from a template-induced solgel route. J. Solid State Chem 2006, 179, 3324–3329. [Google Scholar]
- Zhao, Y; Chen, M; Zhang, Y; Xu, Z; Liu, W. A facile approach to formation of through-hole porous anodic aluminum oxide film. Mater. Lett 2005, 59, 40–43. [Google Scholar]
- Zhong, WH; Li, J; Xu, LR; Lukehart, CM. Graphitic carbon nanofiber (GCNF)/polymer materials, II. GCNF/epoxy monoliths using active oxydianiline linker molecules and the effect of nanofiber reinforcement on curing conditions. Polym. Compos 2005, 26, 128–135. [Google Scholar]
- Corral, EL; Cesarano, J; Shyam, A; Lara-Curzio, E; Bell, N; Stuecker, J; Perry, N; Di Prima, M; Munir, Z; Garay, J; Barrera, EV. Engineered nanostructures for multifunctional single-Walled carbon nanotube reinforced silicon nitride nanocomposites. J. Am. Ceram. Soc 2008, 91, 3129–3137. [Google Scholar]
- Chipara, M; Lozano, K; Wilkins, R; Barrera, EV; Pulikkathara, MX; Penia-Para, L. ESR investigations on polyethylene-single wall carbon nanotube composites. J. Mater. Sci 2008, 43, 1228–1233. [Google Scholar]
- Zhu, J; Imam, A; Crane, R; Lozano, K; Khabashesku, VN; Barrera, EV. Processing a glass fiber reinforced vinyl ester composite with nanotube enhancement of interlaminar shear strength. Comp. Sci. Technol 2007, 67, 1509–1517. [Google Scholar]
- McIntosh, D; Khabashesku, VN; Barrera, EV. Benzoyl peroxide initiated in situ functionalization, processing, and mechanical properties of single-walled carbon nanotube-polypropylene composite fibers. J. Phys. Chem. C 2007, 111, 1592–1600. [Google Scholar]
- McIntosh, D; Khabashesku, VN; Barrera, EV. Nanocomposite fiber systems processed from fluorinated single-walled carbon nanotubes and a polypropylene matrix. Chem. Mater 2006, 18, 4561–4569. [Google Scholar]
- Shofner, ML; Khabashesku, VN; Barrera, EV. Processing and mechanical properties of fluorinated single-wall carbon nanotube-polyethylene composites. Chem. Mater 2006, 18, 906–913. [Google Scholar]
- Wilkins, R; Pulikkathara, MX; Khabashesku, VN; Barrera, EV; Vaidyanathan, RK; Thibeault, SA. Ground-based space radiation effects studies on single-walled carbon nanotube materials. Mater. Space Appl 2005, 851, 267–278. [Google Scholar]
- Zeng, Q; Bayazitoglu, Y; Zhu, J; Wilson, K; Imam, MA; Barrera, EV. Coating of SWNTs with nickel by electroless plating method. The 5th Pacific Rim Int. Conf. Adv. Mater. Processing Proceedings, Beijing, China, November 2–5, 2005; 475–479, pp. 1013–1018.
- Shofner, ML; Peng, HQ; Gu, ZN; Khabashesku, VN; Margrave, JL; Barrera, EV. Mechanical properties of polyethylene containing defunctionalized single wall carbon nanotubes. Mech. Prop. Nanostruct. Mater. Nanocomp 2004, 791, 373–378. [Google Scholar]
- Zhu, J; Peng, HQ; Rodriguez-Macias, F; Margrave, JL; Khabashesku, VN; Imam, AM; Lozano, K; Barrera, EV. Reinforcing epoxy polymer composites through covalent integration of functionalized nanotubes. Adv. Funct. Mater 2004, 14, 643–648. [Google Scholar]
- Yang, SY; Castilleja, JR; Barrera, EV; Lozano, K. Thermal analysis of an acrylonitrile-butadiene-styrene/SWNT composite. Polym. Degrad. Stability 2004, 83, 383–388. [Google Scholar]
- Shofner, ML; Rodriguez-Macias, FJ; Vaidyanathan, R; Barrera, EV. Single wall nanotube and vapor grown carbon fiber reinforced polymers processed by extrusion freeform fabrication. Composites Part A Appl. Sci. Manuf 2003, 34, 1207–1217. [Google Scholar]
- Pulikkathara, MX; Shofner, ML; Wilkins, RT; Vera, JG; Barrera, EV; Rodriguez-Macias, FJ; Vaidyanathan, RK; Green, CE; Condon, CG. Fluorinated single wall nanotube/polyethylene composites for multifunctional radiation protection. Nanomater. Struct. Appl 2003, 740, 365–370. [Google Scholar]
- Barrera, EV. Key methods for developing single-wall nanotube composites. JOM-J. Min. Met. Mater. Soc 2000, 52, A38–A42. [Google Scholar]
- Saito, R; Dresselhaus, G; Dresselhaus, MS. Physical Properties of Carbon Nanotubes; Imperial College Press: London, UK, 1998; pp. 37–52. [Google Scholar]
- Meyyappan, M. Carbon Nanotubes Science and Applications; CRC Press: Boca Raton, 2005; pp. 21–74. [Google Scholar]
- Liao, YH; Marietta-Tondin, O; Liang, ZY; Zhang, C; Wang, B. Investigation of the dispersion process of SWNTs/SC-15 epoxy resin nanocomposites. Mater. Sci. Eng. A 2004, 385, 175–181. [Google Scholar]
- Xie, J; Wong, P. Nanofluids containing multiwalled carbon nanotubes and their enhanced thermal conductivities. J. Appl. Phys 2003, 94, 4967–4968. [Google Scholar]
- Sillikas, N; Al-Kheraif, A; Watts, DC. Influence of P/L ratio and peroxide/amine concentrations on shrinkage-strain kinetics during setting of PMMA/MMA biomaterial formulations. Biomaterials 2005, 26, 197–204. [Google Scholar]
- White, SR; Sottos, NR; Geubelle, PH; Moore, JS; Kessler, MR; Sriram, SR; Brown, EN; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–796. [Google Scholar]
- Liu, X; Sheng, X; Lee, JK; Kessler, MR; Kim, JS. Rheokinetic evaluation of self-healing agents polymerized by Grubbs catalyst embedded in various thermosetting systems. Comp. Sci. Technol 2009, 69, 2102–2107. [Google Scholar]
- Urban, MW. Stratification, stimuli-responsiveness, self-healing, and signaling in polymer networks. Progr. Polym. Sci 2009, 34, 679–687. [Google Scholar]
- Kavitha, AA; Singha, NK. ”Click Chemistry” in tailor-made polymethacrylates bearing reactive furfuryl functionality: A new class of self-healing polymeric material. ACS Appl. Mater. Interf 2009, 1, 1427–1436. [Google Scholar]
- Yun, J; Choi, Y; Lee, H. Crack-healing capability and high temperature oxidation resistance of multilayer coatings for carbon-carbon composites. J. Ceram. Proc. Res 2009, 10, 340–343. [Google Scholar]
- Iyer, AS; Lyon, LA. Self-healing colloidal crystals. Angewandte Chemie-Int. Ed 2009, 48, 4562–4566. [Google Scholar]
- Park, JS; Kim, HS; Hahn, HT. Healing behavior of a matrix crack on a carbon fiber/mendomer composite. Comp. Sci. Technol 2009, 69, 1082–1087. [Google Scholar]
- Ensslin, S; Moll, KP; Haefele-Racin, T; Maeder, K. Safety and robustness of coated pellets: self-healing film properties and storage stability. Pharm. Res 2009, 26, 1534–1543. [Google Scholar]
- Wouters, M; Craenmehr, E; Tempelaars, K; Fisher, H; Stroeks, N; van Zanten, J. Preparation and properties of a novel remendable coating concept. Progr. Org. Coat 2009, 64, 156–162. [Google Scholar]
- Chipara, MD; Chipara, M; Shansky, E; Zaleski, JM. Self-healing of high elasticity block copolymers. Polym. Adv. Technol 2009, 20, 427–431. [Google Scholar]
- Pegoretti, A. The way to autonomic self-healing polymers and composites. Expr. Polym. Lett 2009, 3, 62–62. [Google Scholar]
- Dubey, R; Shami, TC; Rao, KUB. Microencapsulation technology and applications. Defence Sci. J 2009, 59, 82–95. [Google Scholar]
- Zhang, Y; Broekhuis, AA; Picchioni, F. Thermally self-healing polymeric materials: the next step to recycling thermoset polymers? Macromolecules 2009, 42, 1906–1912. [Google Scholar]
- Neema, S; Salehi-Khojin, A; Zhamu, A; Zhong, WH; Jana, S; Gan, YX. Wettability of nano-epoxies to UHMWPE fibers. J. Colloid Interf. Sci 2006, 299, 332–341. [Google Scholar]
- Jana, S; Zhamu, A; Zhong, WH; Gan, YX. Experimental evaluation of adhesion property of UHMWPE fibers/nano-epoxy by a pullout test. J. Adhesion 2006, 82, 1157–1175. [Google Scholar]
- Jana, S; Zhamu, A; Zhong, WH; Gan, YX; Stone, JJ. Effect of reactive graphitic nanofibers (r-GNFs) on tensile behavior of UHMWPE fiber/nano-epoxy bundle composites. Mater. Manuf. Proc 2008, 23, 102–110. [Google Scholar]
- Pervin, F; Zhou, Y; Rangari, VK; Jeelani, S. Testing and evaluation on the thermal and mechanical properties of carbon nano fiber reinforced SC-15 epoxy. Mater. Sci. Eng. A 2005, 405, 246–253. [Google Scholar]
- Jana, S; Zhong, W; Gan, YX. Characterization of the flexural behavior of a reactive graphitic nanofibers reinforced epoxy using a non-linear damage model. Mater Sci Eng A 2007, 445–446, 106–112. [Google Scholar]
- Zhong, WH; Zhamu, A; Aglan, A; Stone, J; Gan, YX. J. Adhesive Sci. Technol 2005, 19, 1113–1128.
| Nanocomposite material type | Epoxy | r-GNF/E-1 | r-GNF/E-2 | r-GNF/E-3 |
|---|---|---|---|---|
| The r-GNF content (wt%) | 0 | 0.20 | 0.30 | 0.50 |
| Young’s modulus, E (MPa) | 2770 | 3004 | 3337 | 3269 |
| Flexural strength, σu (MPa) | 132 | 152 | 166 | 160 |
| Weibull shape parameter, m | 26.4 | 8.3 | 3.4 | 4.1 |
| Weibull scale parameter, σo(MPa) | 150 | 211 | 299 | 278 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gan, Y.X. Effect of Interface Structure on Mechanical Properties of Advanced Composite Materials. Int. J. Mol. Sci. 2009, 10, 5115-5134. https://doi.org/10.3390/ijms10125115
Gan YX. Effect of Interface Structure on Mechanical Properties of Advanced Composite Materials. International Journal of Molecular Sciences. 2009; 10(12):5115-5134. https://doi.org/10.3390/ijms10125115
Chicago/Turabian StyleGan, Yong X. 2009. "Effect of Interface Structure on Mechanical Properties of Advanced Composite Materials" International Journal of Molecular Sciences 10, no. 12: 5115-5134. https://doi.org/10.3390/ijms10125115




