Toxicity Assessment of Atrazine and Related Triazine Compounds in the Microtox Assay, and Computational Modeling for Their Structure-Activity Relationship
Abstract
:Introduction
Materials and methods
Reagents and Chemicals
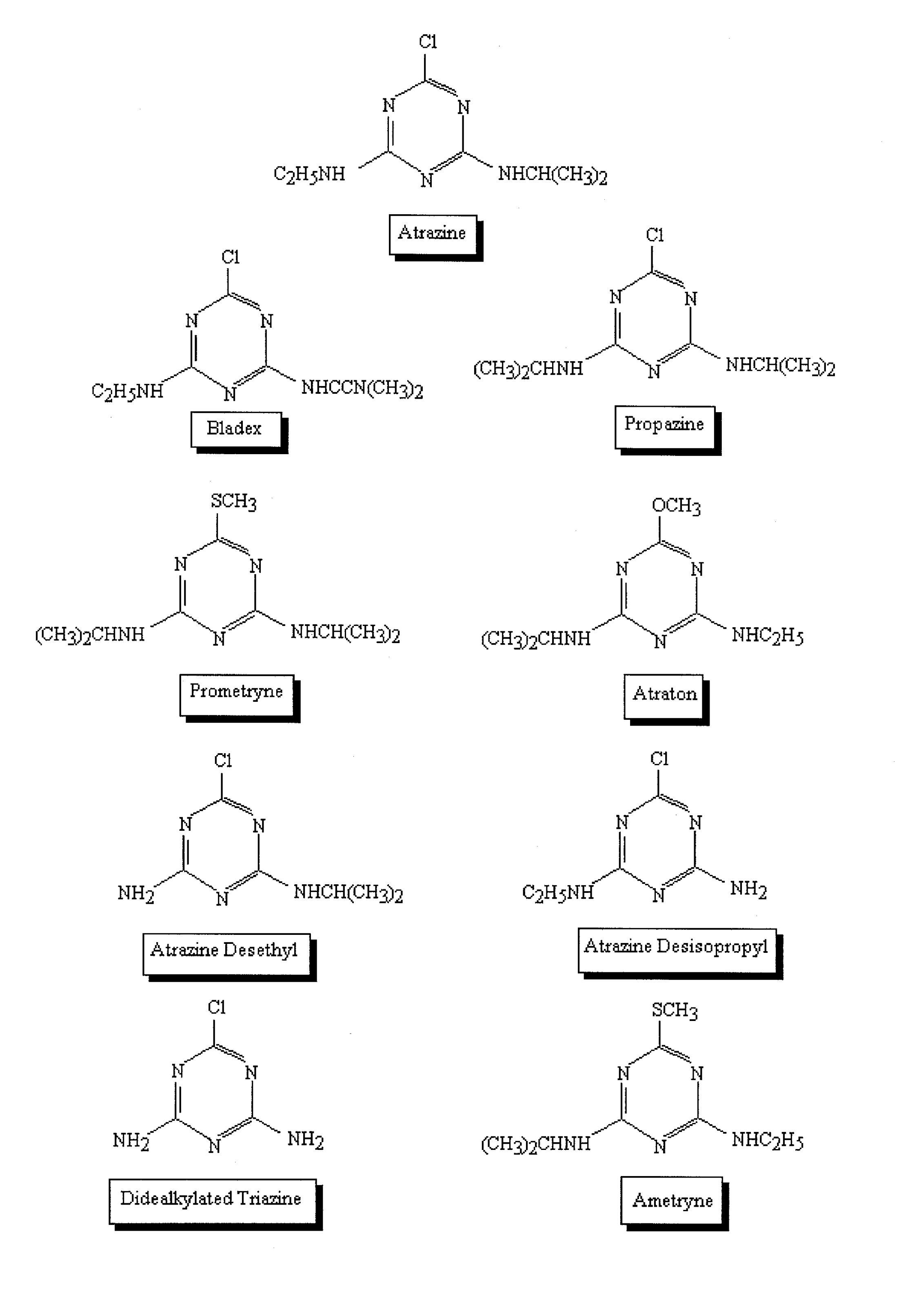
Microtox System
Instrument
Microtox Acute Toxicity Assay
Quality Control
Data Analysis
Quantitative Structure-Activity Relationship (QSAR)
Results and Discussion
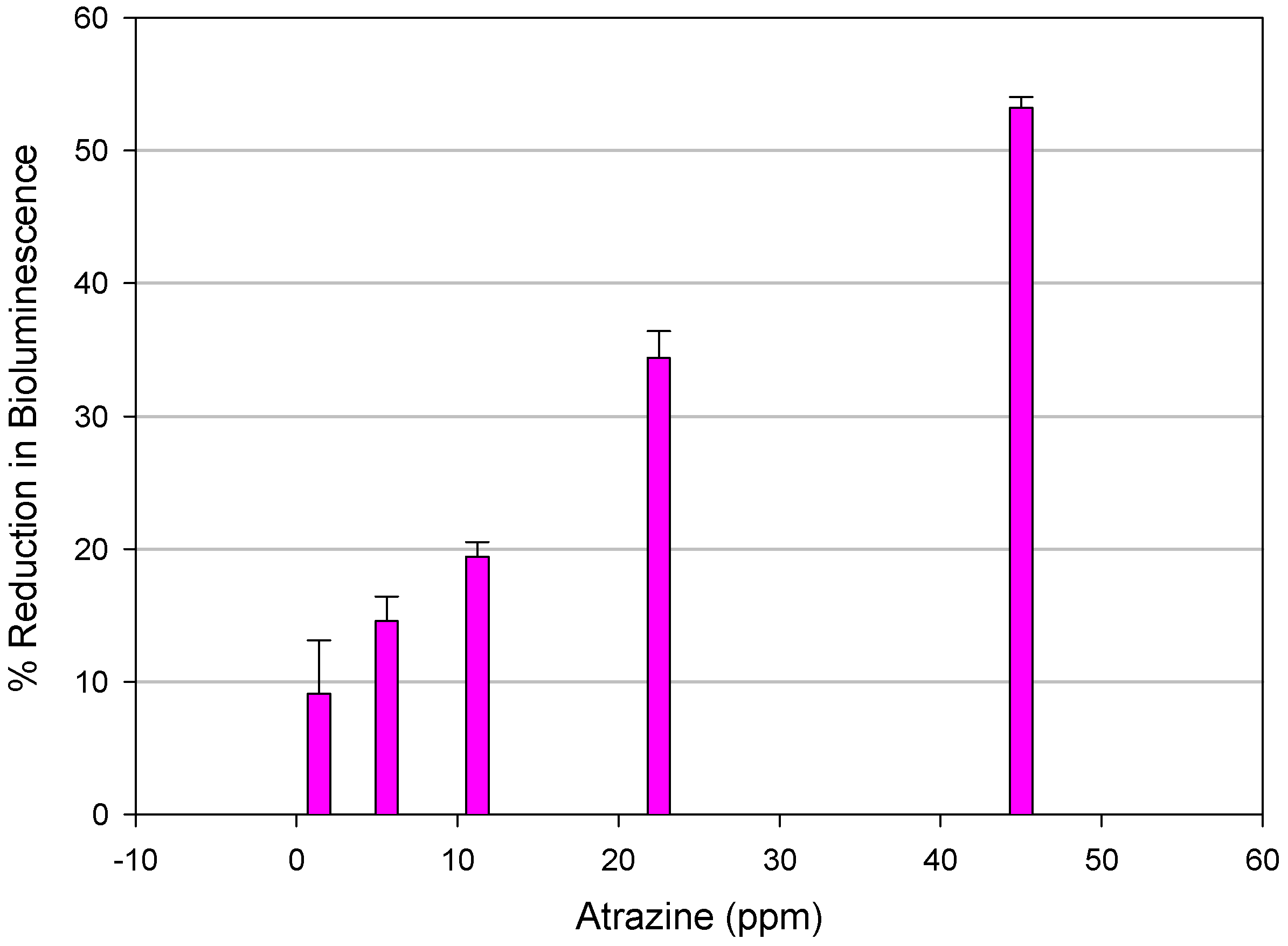
Microtox Assay
| Chemical | EC50* | 95% Confidence Limits (mg/L) |
|---|---|---|
| Ametryne | 11.80 | (11.45 - 12.10) |
| Atraton | 36.96 | (35.08 - 38.94) |
| Atrazine (reference triazine) | 39.87 | (35.37 - 44.93) |
| Atrazine Desethyl | 81.86 | (77.26 - 86.74) |
| Atrazine Desethyl Desisopropyl | 12.74 | (12.17 - 13.34) |
| Atrazine Desisopropyl | 82.68 | (76.10 - 89.80) |
| Bladex | 78.50 | (76.60 - 80.32) |
| Prometryne | 226.80 | (193.40 - 266.2) |
| Propazine | 273.20 | (154.20 - 484.10) |
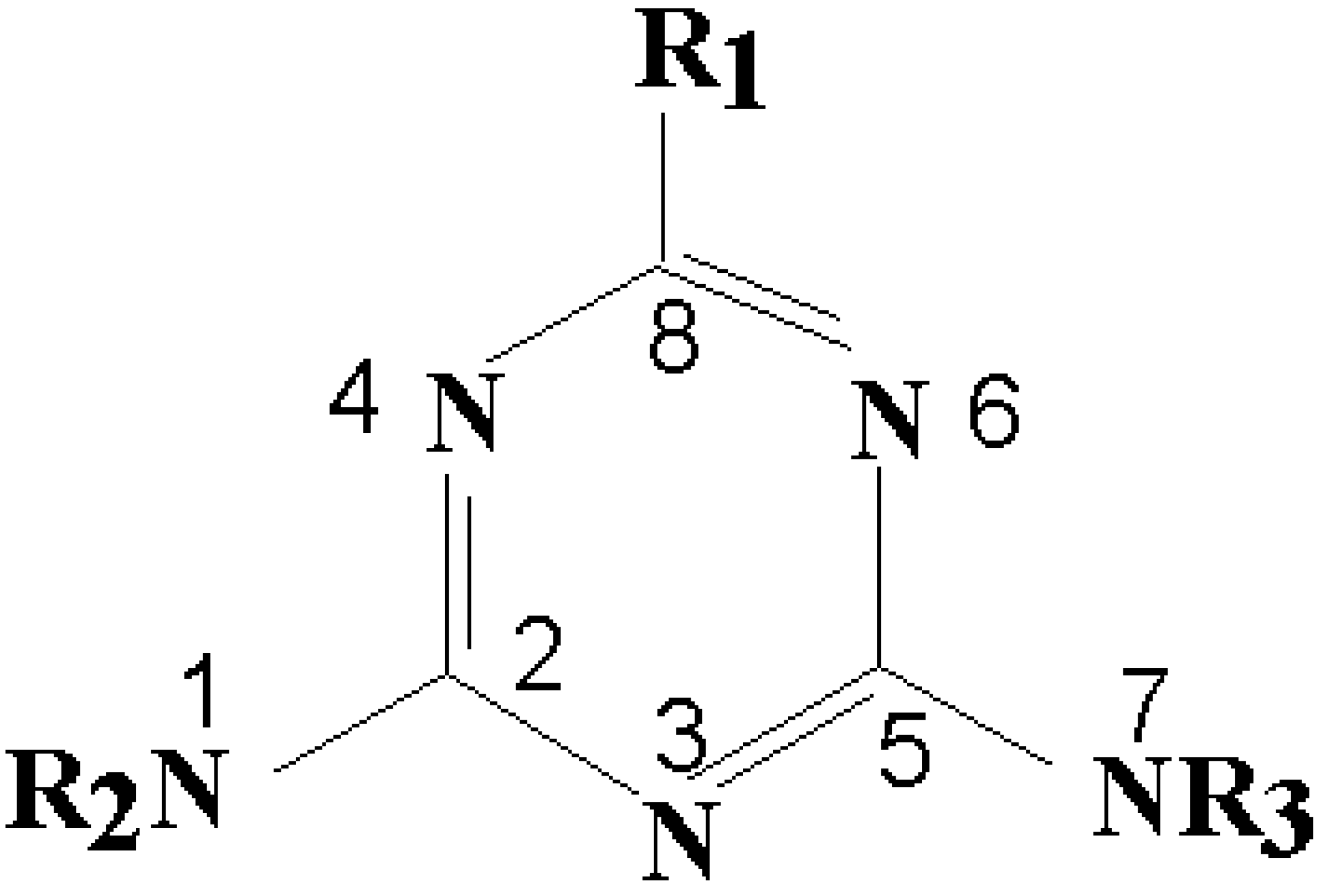
Quantitative Structure-Activity Relationship (QSAR) Analysis
| Chemical | Log EC50 | LogW | Charge Distributions | ||
|---|---|---|---|---|---|
| Experimental | Calculated | (Calculated) | N6 | N7 | |
| Ametryne | 1.072 | 1.192 | -7.580 | -0.2734 | -0.2644 |
| Atraton | 1.568 | 1.687 | -6.055 | -0.2867 | -0.2750 |
| Atrazine | 1.601 | 1.845 | -6.974 | -0.2807 | -0.2758 |
| Atrazine | 1.913 | 1.594 | -6.053 | -0.2856 | -0.2780 |
| Desethyl | |||||
| Atrazine | 1.105 | 1.165 | -4.317 | -0.2853 | -0.3444 |
| Desethyl | |||||
| Desisopropyl | |||||
| Atrazine | 1.917 | 1.919 | -5.765 | -0.2799 | -0.3421 |
| Desisopropyl | |||||
| Bladex | 1.895 | 1.673 | -7.210 | -0.2760 | -0.2867 |
| Prometryne | 2.356 | 2.266 | -7.780 | -0.2779 | -0.2733 |
| Propazine | 2.436 | 2.521 | -7.326 | -0.2825 | -0.2771 |
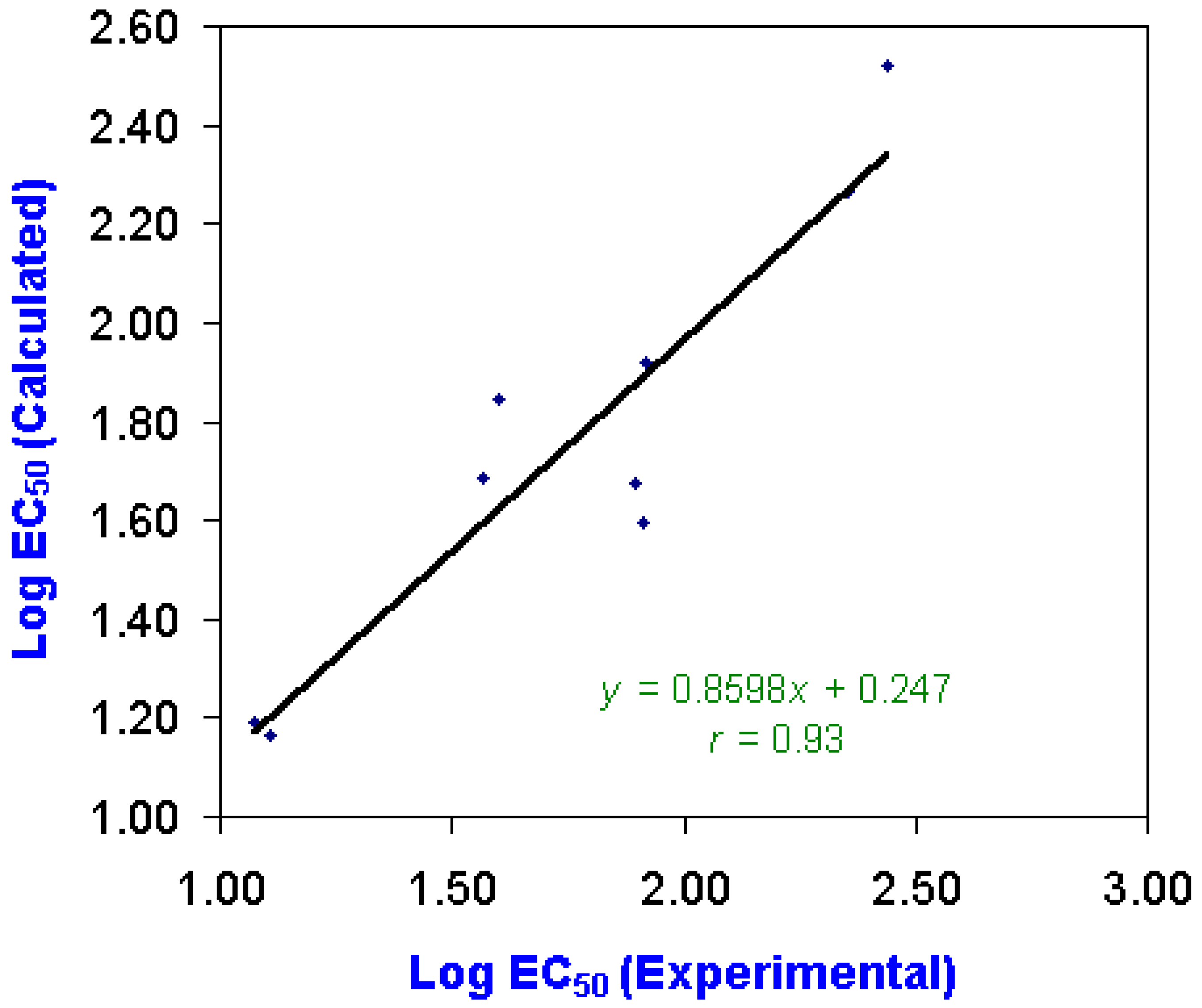
Conclusions
Acknowledgments
References
- Azur Environmental. Microtox - Acute Toxicity Basic Test Procedures. Carlsbad, CA, 1995. [Google Scholar]
- U.S. EPA. Drinking Water and Health: Technical Fact Sheet on Atrazine. United States Environmental Protection Agency: Washington DC, 1999. on line at: http://www.epa.gov/ OGWDW/dwh/t-soc/atrazine.html. [Google Scholar]
- Thurman, E. M.; Goolsby, D. A.; Meye, M. T.; Kolpin, D. W. Herbicides in surface waters of the Midwestern United States: The effects of spring flush. Environ. Sci.Technol. 1991, 25, 1794–1796. [Google Scholar] [CrossRef]
- Thurman, E. M.; Goolsby, D. A.; Meyer, M. T.; Mills, M. S.; Pomes, M. L.; Kolpin, D. W. A reconnaissance study of herbicides and their metabolites in surface waters of the Midwestern United States using immunoassay and gas chromatography/mass spectrometry. Environ. Sci. Technol. 1992, 26, 2440–2447. [Google Scholar] [CrossRef]
- Grover, R.A. Environmental Chemistry of Herbicides. CRC Press: Boca Raton, Florida, 1988; pp. 45–88. [Google Scholar]
- Coupe, R. H.; Thurman, E. M.; Zimmerman, L. R. Relation of usage to the occurrence of cotton and rice herbicides in three streams of the Mississippi Delta. Environ. Sci.Technol. 1998, 32, 3673–3680. [Google Scholar] [CrossRef]
- Kolpin, D. W.; Kalkoff, S. J.; Goolsby, D. A.; Sneck-Fahrer, D. A.; Thurman, E. M. Occurrence of selected herbicides and herbicide degradation products in Iowa’s ground water 1995. Ground water 1997, 35(4), 679–688. [Google Scholar] [CrossRef]
- Kolpin, D. W.; Thurman, E. M.; Linhart, S. M. The environmental occurrence of herbicides: The importance of degradates in ground water. Arch. Environ. Contam. Toxicol. 1998, 1–6. [Google Scholar] [CrossRef]
- Squillace, P. J.; Thurman, E. M.; Furlong, E. T. Ground water as non point-source for atrazine and deethyl atrazine in a river during base flow conditions. Water Resources Res. 1993, 29(6), 1719–1729. [Google Scholar] [CrossRef]
- Squillace, P. J.; Thurman, E. M. Herbicide transport in rivers: Importance of hydrology and geochemistry of non point-source contamination. Environ. Sci.Technol. 1992, 26, 538–545. [Google Scholar] [CrossRef]
- U.S. EPA. Pesticides and Groundwater SMP Rule Technical support Document: Summary of Evidence of Adverse Human Health Effects for 5 SMP Candidates. Office of Pesticide Programs. United States Environmental Protection Agency: Washington DC, 1996; pp. 1–30. [Google Scholar]
- U.S. EPA. The Triazine Pesticides. United States Environmental Protection Agency: Washington DC, 1997. on line at: http://www.epa.gov/opp00001/citizens/triazine.html. [Google Scholar]
- IRIS. Atrazine-CARSN 1912-29-9. Integrated Risk Information System. Environmental Protection Agency: Washington DC, 1987. on line at: http://www.epa.gov/ ngispgm3/iris/subst/0209.html. [Google Scholar]
- Thurman, E. M.; Meyer, M. T.; Mills, M. S.; Zimmerman, L R.; Perry, C. A. Formation and transport of deethyl atrazine and deisopropyl atrazine in surface water. Environ. Sci.Technol. 1994, 28(3), 2267–2277. [Google Scholar] [CrossRef] [PubMed]
- Bodor, N.; Gabanyi, Z.; Wong, C.-K. A new method for the estimation of partition coefficient. J. Am. Chem. Soc. 1989, 111, 3783–3786. [Google Scholar] [CrossRef]
- Bodor, N.; Huang, M.-J. An extended version of a novel method for the estimation of partition coefficients. J. Pharm. Sci. 1992, 81, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Bodor, N.; Huang, M.-J. A new method for the estimation of the aqueous solubility of organic compounds. J. Pharm. Sci. 1992, 81, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Creuzet, S.; Langlet, J. Theoretical determination of structural parameters for s-triazine and some derivatives. Comparison between ab initio and semi-empirical calculations. Chem. Phys. Lett. 1993, 208, 511–516. [Google Scholar] [CrossRef]
- Pankratov, A. N. Semi-empirical quantum chemical methods: testing of thermodynamic and molecular properties of cyclic non-aromatic hydrocarbons and unsaturated heterocycles. J. Mol.Struc. (Theochem) 1998, 453, 7–15. [Google Scholar] [CrossRef]
- Johnson, B. T.; Long, E. R. Rapid toxicity assessment of sediments from estuarine ecosystems: A new tandem in vitro testing approach. Environ. Toxicol. Chem. 1998, 17(6), 1099–1106. [Google Scholar] [CrossRef]
- Tchounwou, P. B.; Reed, L. Assessment of lead toxicity to the marine bacterium, Vibrio fischeri, and to a heterogenous population of microorganisms derived from the Pearl river in Jackson, Mississippi, USA. Rev. Environ. Hth. 1999, 14(2), 51–61. [Google Scholar]
- Al-Muzaini, S.; Beg, M. V.; Ali, L. N. Microtox assay for assessment of marine pollution in industrial discharge zone of Kuwait. Environ. Toxicol. Water Quality 1997, 12, 109–115. [Google Scholar] [CrossRef]
- Qureshi, A. A.; Coleman, R. N.; Paran, J. H. Evaluation and refinement of Microtox test for use in toxicity screening. Dutka, BJ, Liu, D, Eds.; Toxicity Screening Procedures using Bacterial Systems. Toxicology Series; Vol. 1, Marcel Dekker: New York, 1984; pp. 1–22. [Google Scholar]
- Nealson, K. H.; Hastings, J. W. Bacterial luminescence: Its control and ecological significance. Microbiol. Rev. 1979, 43, 496–518. [Google Scholar] [PubMed]
- Hastings, J. W. Bacterial bioluminescence: An overview. Deluca, M, Ed.; Methods in Enzymology; Vol. 57, Academic Press: New York, 1978; pp. 125–153. [Google Scholar]
- Indorato, A. M.; Snyder, K. B.; Vsinowica, P. J. Toxicity screening using microtox analyzer. Liu, D, Dutka, BJ, Eds.; Toxicity screening using bacterial systems; Marcel Dekker, Inc.: New York. N.Y., 1984; pp. 37–53. [Google Scholar]
- Carder, J. P.; Hoagland, K. D. Combined effects of alachlor and atrazine on benthic algal communities in artificial streams. Environ. Toxicol. Chem. 1998, 17(7), 1415–1420. [Google Scholar] [CrossRef]
- Tang, J.; Hoaglang, K. D.; Siegfried, B. Uptake and bioconcentration of atrazine by selected freshwater algae. Environ. Toxicol. Chem. 1998, 17(6), 1085–1090. [Google Scholar] [CrossRef]
- Veber, K.; Zahradnik, J.; Breyl, I.; Kredl, F. Toxic effects of atrazine on algae. Bull. Environ. Contam. Toxicol. 1981, 27, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Hoberg, J. R. Atrazine-technical: Toxicity to duckweed (Lemna gibba). SLI Report 93-4-4755; Springborn Laboratories: Wareham, MA, 1993. [Google Scholar]
- Kirby, M. F.; Sheahan, D. A. Effects of atrazine, isoproturon, and mesoprop on the macrophyte Lemna minor and the alga Scenedesmus subspicatus. Bull. Environ. Contam. Toxicol. 1994, 53, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Lytle, J. S.; Lytle, T. F. Atrazine effects on estuarine macrophytes Spartina alterniflora and Juncus roemerianus. Environ. Toxicol. Chem. 1998, 17(10), 1972–178. [Google Scholar] [CrossRef]
- Macek, K. J.; Buxton, K. S.; Sauter, S.; Gnilka, S.; Dean, J. W. Chronic toxicity of atrazine to selected aquatic invertebrates and fishes. EPA-600/3-76-047; U.S. Environmental Protection Agency: Duluth, NM, 1976. [Google Scholar]
- Streit, B.; Peter, H. M. Long term effects of atrazine to selected freshwater invertebrates. Arch. Hydrobiol. Suppl. 1978, 55, 62–77. [Google Scholar]
- Oris, J. T.; Winner, R. W.; Moore, M. V. A four-day survival and reproduction toxicity test for Ceriodaphnia dublia. Environ. Toxicol. Chem. 1991, 10, 217–224. [Google Scholar] [CrossRef]
- Schober, U.; Lampert, W. Effects of sublethal concentrations of the herbicide atrazine on growth and reproduction of Daphnia pulex. Bull. Environ. Contam. Toxicol. 1977, 17, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Davies, P. E.; Cook, L. S. J.; Goenarso, D. Sublethal responses to pesticides of several species of Australian freshwater fish and crustaceans and rainbow trout. Environ. Toxicol. Chem. 1994, 13, 1341–1354. [Google Scholar] [CrossRef]
- Kopriva, J. The toxicity of triazine and diazine based herbicides to fishes. Agrochemica 1981, 21, 344–347. [Google Scholar]
- Solomon, K. R.; Baker, D. B.; Richards, R. P.; Dixon, K. R.; Klaine, S. J.; LaPoint, T. W.; Kenkdall, R. J.; Weisskopf, C. P.; Giddings, J. M.; Giesy, J. P.; Hall, L. W.; Williams, W. M. Ecological risk assessment of atrazine in north american surface waters. Environ. Toxicol. Chem. 1996, 15(1), 31–76. [Google Scholar] [CrossRef]
- Restek Corporation, Environmental Application Note #59586: Analysis of Triazine herbicides. 1999. on line at: http://www.restekcorp.com/appnotes/59586.htm.
- Biagi, G. L.; Guerra, M. C.; Barbaro, A. M.; Recanatini, M.; Borea, P. A.; Sapone, A. Lipophilicity indices of triazine herbicides. Sci. Total Environ.. 1991, 109-110, 33–40. [Google Scholar] [CrossRef]
- Oettmeier, W.; Hilp, U.; Draber, W.; Fedtke, C.; Schmidt, R. R. Structure-activity relationships of triazinone herbicides on resistant weeds and resistant Chlamydomonas reinhardtii. Pesticide Sci. 1991, 33, 399–409. [Google Scholar] [CrossRef]
- Eilers, R. J.; Crouse, G. D; Durst, G. L.; Manly, C. J.; Webster, J. D.; Streusand, V. J. Inhibition of photosystem II electron transport and structure-activity relationships among herbicidally active 3-butenanilides. Pesticide Biochem. Physiol. 1992, 43(2), 162–170. [Google Scholar] [CrossRef]
- Rhyu, K. B.; Patel, H. C.; Hopfinger, A. J. A 3D-QSAR study of anticoccidial triazines using molecular shape analysis. J. Chem. Inf. Comp. Sci. 1995, 35(4), 771–778. [Google Scholar] [CrossRef]
- Šoškić, M.; Plavšić, D.; Trinajstić, N. Inhibition of the Hill reaction by 2- methylthio-4,6-bis(monoalkylamino)-1,3,5-triazines. J. Mol. Struct. (Theochem) 1997, 394, 57–65. [Google Scholar] [CrossRef]
© 2000 by MDPI (http://www.mdpi.org).
Share and Cite
Tchounwou, P.B.; Wilson, B.; Ishaque, A.; Ransome, R.; Huang, M.-J.; Leszczynski, J. Toxicity Assessment of Atrazine and Related Triazine Compounds in the Microtox Assay, and Computational Modeling for Their Structure-Activity Relationship. Int. J. Mol. Sci. 2000, 1, 63-74. https://doi.org/10.3390/ijms1040063
Tchounwou PB, Wilson B, Ishaque A, Ransome R, Huang M-J, Leszczynski J. Toxicity Assessment of Atrazine and Related Triazine Compounds in the Microtox Assay, and Computational Modeling for Their Structure-Activity Relationship. International Journal of Molecular Sciences. 2000; 1(4):63-74. https://doi.org/10.3390/ijms1040063
Chicago/Turabian StyleTchounwou, P.B., B. Wilson, A. Ishaque, R. Ransome, Ming-Ju Huang, and Jerzy Leszczynski. 2000. "Toxicity Assessment of Atrazine and Related Triazine Compounds in the Microtox Assay, and Computational Modeling for Their Structure-Activity Relationship" International Journal of Molecular Sciences 1, no. 4: 63-74. https://doi.org/10.3390/ijms1040063




