Blood Pressure Lowering Effect of Cuban Policosanol is Accompanied by Improvement of Hepatic Inflammation, Lipoprotein Profile, and HDL Quality in Spontaneously Hypertensive Rats
Abstract
:1. Introduction
2. Results
2.1. Blood Pressure and Heart Rate
2.2. Serum Lipid Profile
2.3. Serum CETP Activity
2.4. Antioxidative Activity of Lipoproteins
2.5. Protein, TC, and TG Compositions of Lipoproteins
2.6. Electrophoretic Profiles of LDL and HDL
2.7. Electromobility of LDL
2.8. Aldosterone Levels in the Different Study Groups
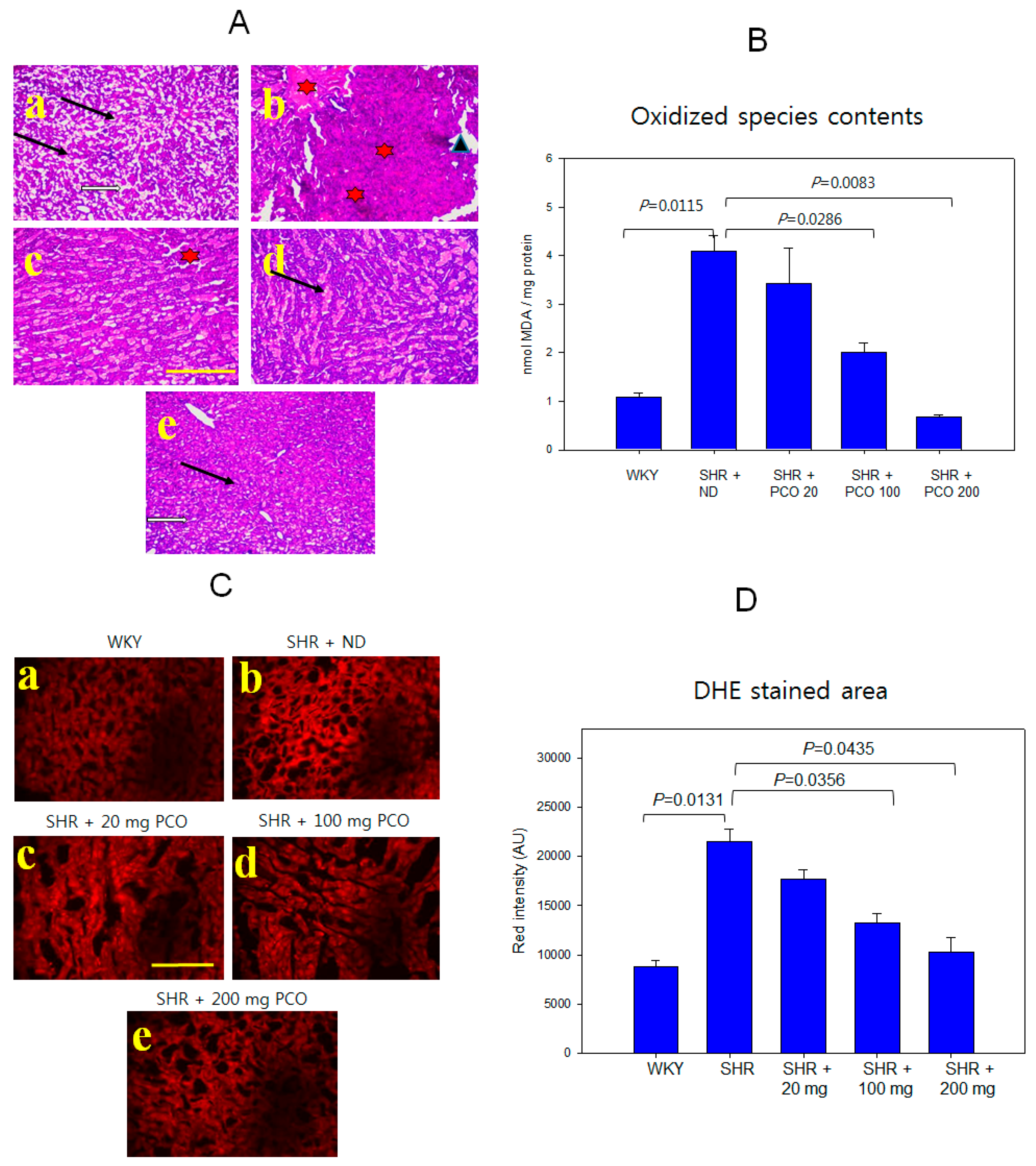
2.9. Histologic Analysis of Hepatic Tissue
2.10. Correlation Analysis
3. Discussion
4. Materials and methods
4.1. Animals and Diets
4.2. Measurement of Blood Pressure
4.3. Plasma Analysis
4.4. Purification and Analysis of Lipoproteins
4.5. Oxidation of LDL by Cupric Ion
4.6. Assay for Cholesteryl Ester Transfer Protein
4.7. Paraoxonase Assay
4.8. ELISA and Western Blotting
4.9. Histologic Analysis
4.10. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Laaksonen, D.E.; Niskanen, L.; Nyyssönen, K.; Lakka, T.A.; Laukkanen, J.A.; Salonen, J.T. Dyslipidaemia as a predictor of hypertension in middle-aged men. Eur. Heart J. 2008, 29, 2561–2568. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Íñigo, L.; Navarro-González, D.; Pastrana-Delgado, J.; Fernández-Montero, A.; Martínez, J.A. Association of triglycerides and new lipid markers with the incidence of hypertension in a spanish cohort. J. Hypertens. 2016, 34, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- González, J.; Valls, N.; Brito, R.; Rodrigo, R. Essential hypertension and oxidative stress: New insights. World J. Cardiol. 2014, 6, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Boyce, G.; Button, E.; Soo, S.; Wellington, C. The pleiotropic vasoprotective functions of high density lipoproteins (HDL). J. Biomed. Res. 2017. [Google Scholar] [CrossRef]
- Gong, J.; Qin, X.; Yuan, F.; Hu, M.; Chen, G.; Fang, K.; Wang, D.; Jiang, S.; Li, J.; Zhao, Y. Efficacy and safety of sugarcane policosanol on dyslipidemia: A meta-analysis of randomized controlled trials. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.-M.; Yoo, J.-A.; Lee, E.-Y.; Cho, K.-H. Enhancement of high-density lipoprotein cholesterol functions by encapsulation of policosanol exerts anti-senescence and tissue regeneration effects via improvement of anti-glycation, anti-apoptosis, and cholesteryl ester transfer inhibition. Rejuvenation Res. 2016, 19, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-Y.; Yoo, J.-A.; Lim, S.-M.; Cho, K.-H. Anti-aging and tissue regeneration ability of policosanol along with lipid-lowering effect in hyperlipidemic zebrafish via enhancement of high-density lipoprotein functionality. Rejuvenation Res. 2016, 19, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Li, L.; Porter, T.D. Policosanol inhibits cholesterol synthesis in hepatoma cells by activation of amp-kinase. J. Pharmacol. Exp. Ther. 2006, 318, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Kim, S.-M.; Kim, S.-J.; Lee, E.-Y.; Kim, J.-R.; Cho, K.-H. Consumption of policosanol enhances HDL functionality via CETP inhibition and reduces blood pressure and visceral fat in young and middle-aged subjects. Int. J. Mol. Med. 2017, 39, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Yadav, D.; Park, H.-J.; Kim, J.-R.; Cho, K.-H. Long-term consumption of cuban policosanol lowers central and brachial blood pressure and improves lipid profile with enhancement of lipoprotein properties in healthy korean participants. Front. Physiol. 2018, 9, 412. [Google Scholar] [CrossRef]
- Castano, G.; Mas, R.; Fernandez, J.C.; Fernandez, L.; Illnait, J.; Lopez, E. Effects of policosanol on older patients with hypertension and type ii hypercholesterolaemia. Drugs R D 2002, 3, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Trippodo, N.C.; Frohlich, E.D. Similarities of genetic (spontaneous) hypertension. Man and rat. Circ. Res. 1981, 48, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Lemmer, B.; Mattes, A.; Böhm, M.; Ganten, D. Circadian blood pressure variation in transgenic hypertensive rats. Hypertension 1993, 22, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Leong, X.-F.; Ng, C.-Y.; Jaarin, K. Animal Models in Cardiovascular Research: Hypertension and Atherosclerosis. Biomed. Res. Int. 2015, 2015, 528757. [Google Scholar] [CrossRef] [PubMed]
- Badyal, D.; Dadhich, A.; Lata, H. Animal models of hypertension and effect of drugs. Indian J. Pharmacol. 2003, 35, 349–362. [Google Scholar]
- Andrzejczak, D.; Górska, D. The effects of celiprolol on serum concentrations of proinflammatory cytokines in hypertensive (SHR) and normotensive (WKY) rats. Pharmacol. Rep. 2014, 66, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Peng, Z.; Zu, C.; Ma, J.; Lu, S.; Zhong, J.; Zhang, S. Losartan attenuates myocardial endothelial-to-mesenchymal transition in spontaneous hypertensive rats via inhibiting TGF-β/Smad Signaling. PLoS ONE 2016, 11, e0155730. [Google Scholar] [CrossRef] [PubMed]
- Simic, B.; Hermann, M.; Shaw, S.G.; Bigler, L.; Stalder, U.; Dorries, C.; Besler, C.; Luscher, T.F.; Ruschitzka, F. Torcetrapib impairs endothelial function in hypertension. Eur. Heart J. 2012, 33, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chen, X.Y.; Xu, X.P. The Relationship Between Lipoprotein-Associated Phospholipase A(2), Cholesteryl Ester Transfer Protein and Lipid Profile and Risk of Atherosclerosis in Women with Iron Deficiency Anaemia. Clin. Lab. 2015, 61, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Shin, D.G.; Baek, S.H.; Kim, J.R. Myocardial infarction patients show altered lipoprotein properties and functions when compared with stable angina pectoris patients. Exp. Mol. Med. 2009, 41, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Lee, S.M.; Kim, S.J.; Kim, B.J.; Shin, S.; Kim, J.R.; Cho, K.H. Cord and maternal sera from small neonates share dysfunctional lipoproteins with proatherogenic properties: Evidence for Barker’s hypothesis. J. Clin. Lipidol. 2017, 11, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.C.; Barter, P.J. Differences in plasma cholesteryl ester transfer activity in sixteen vertebrate species. Comp. Biochem. Physiol. Part B Comp. Biochem. 1982, 71, 265–269. [Google Scholar] [CrossRef]
- Davidson, M.H. Update on CETP inhibition. J. Clin. Lipidol. 2010, 4, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Zachariah, J.P.; Pencina, M.J.; Lyass, A.; Kaur, G.; D’agostino, R.B.; Ordovas, J.M.; Vasan, R.S. Circulating plasma cholesteryl ester transfer protein activity and blood pressure tracking in the community. J. Hypertens. 2011, 29, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Suico, J.G.; Wang, M.D.; Friedrich, S.; Cannady, E.A.; Konkoy, C.S.; Ruotolo, G.; Krueger, K.A. Effects of the cholesteryl ester transfer protein inhibitor evacetrapib on lipoproteins, apolipoproteins and 24-h ambulatory blood pressure in healthy adults. J. Pharm. Pharmacol. 2014, 66, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Shah, S.; Dansky, H.M.; Davidson, M.; Brinton, E.A.; Gotto, A.M., Jr.; Stepanavage, M.; Liu, S.X.; Gibbons, P.; Ashraf, T.B. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N. Engl. J. Med. 2010, 363, 2406–2415. [Google Scholar] [CrossRef] [PubMed]
- Mayer, O.; Seidlerova, J.; Filipovsky, J.; Vagovicova, P.; Wohlfahrt, P.; Cifkova, R.; Windrichova, J.; Topolcan, O. Soluble receptor for advanced glycation end products and increased aortic stiffness in the general population. Hypertens. Res. 2016, 39, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Mandai, N.; Akazawa, K.; Hara, N.; Ide, Y.; Ide, K.; Dazai, U.; Chishaki, A.; Chishaki, H. Body weight reduction results in favorable changes in blood pressure, serum lipids, and blood sugar in middle-aged japanese persons: A 5-year interval observational study of 26,824 cases. Glob. J. Health Sci. 2015, 7, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.J.; Rye, K.-A. Cholesteryl ester transfer protein inhibition as a strategy to reduce cardiovascular risk. J. Lipid Res. 2012, 53, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- Tomaschitz, A.; Maerz, W.; Pilz, S.; Ritz, E.; Scharnagl, H.; Renner, W.; Boehm, B.O.; Fahrleitner-Pammer, A.; Weihrauch, G.; Dobnig, H. Aldosterone/renin ratio determines peripheral and central blood pressure values over a broad range. J. Am. Coll. Cardiol. 2010, 55, 2171–2180. [Google Scholar] [CrossRef] [PubMed]
- Vasan, R.S.; Evans, J.C.; Larson, M.G.; Wilson, P.W.; Meigs, J.B.; Rifai, N.; Benjamin, E.J.; Levy, D. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N. Engl. J. Med. 2004, 351, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Manrique, C.; Lastra, G.; Gardner, M.; Sowers, J.R. The Renin Angiotensin Aldosterone System in Hypertension: Roles of Insulin Resistance and Oxidative Stress. Med. Clin. N. Am. 2009, 93, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Streeten, D.P.; Clift, G.V.; Schletter, F.E.; Dalakos, T.S. The renin-angiotensin-aldosterone system in hypertension. Ann. Intern. Med. 1968, 68, 1164. [Google Scholar] [CrossRef]
- Malkoff, J. Non-invasive blood pressure for mice and rats. Anim. Lab News Kent Sci. Corp. 2005, 1–12. [Google Scholar]
- Fouad-Tarazi, F.; Calcatti, J.; Christian, R.; Armstrong, R.; Depaul, M. Blood volume measurement as a tool in diagnosing syncope. Am. J. Med. Sci. 2007, 334, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Yoshikawa, N.; Kagota, S.; Nakamura, K.; Haginaka, J.; Kunitomo, M. Elevated circulating levels of markers of oxidative-nitrative stress and inflammation in a genetic rat model of metabolic syndrome. Nitric Oxide 2006, 15, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Pravenec, M.; Kajiya, T.; Zídek, V.; Landa, V.; Mlejnek, P.; Šimáková, M.; Šilhavý, J.; Malínská, H.; Oliyarnyk, O.; et al. Effects of Human C-Reactive Protein on Pathogenesis of Features of the Metabolic Syndrome. Hypertension 2011, 57. [Google Scholar] [CrossRef] [PubMed]
- Havel, R.J.; Eder, H.A.; Bragdon, J.H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Investig. 1955, 34, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Markwell, M.A.; Haas, S.M.; Bieber, L.L.; Tolbert, N.E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 1978, 87, 206–210. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Esterbauer, H.; Striegl, G.; Puhl, H.; Rotheneder, M. Continuous monitoring of in vitro oxidation of human low density lipoprotein. Free Radic. Res. Commun. 1989, 6, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Noble, R.P. Electrophoretic separation of plasma lipoproteins in agarose gel. J. Lipid Res. 1968, 9, 693–700. [Google Scholar] [PubMed]
- Cho, K.-H. Synthesis of reconstituted high density lipoprotein (rHDL) containing apoA-I and apoC-III: The functional role of apoC-III in rHDL. Mol. Cells 2009, 27, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Eckerson, H.W.; Wyte, C.M.; la Du, B. The human serum paraoxonase/arylesterase polymorphism. Am. J. Hum. Genet. 1983, 35, 1126–1138. [Google Scholar] [PubMed]
- Park, K.-H.; Shin, D.-G.; Kim, J.-R.; Hong, J.-H.; Cho, K.-H. The functional and compositional properties of lipoproteins are altered in patients with metabolic syndrome with increased cholesteryl ester transfer protein activity. Int. J. Mol. Med. 2010, 25, 129–136. [Google Scholar] [PubMed]
- Owusu-Ansah, E.; Yavari, A.; Mandal, S.; Banerjee, U. Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nat. Genet. 2008, 40, 356–361. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
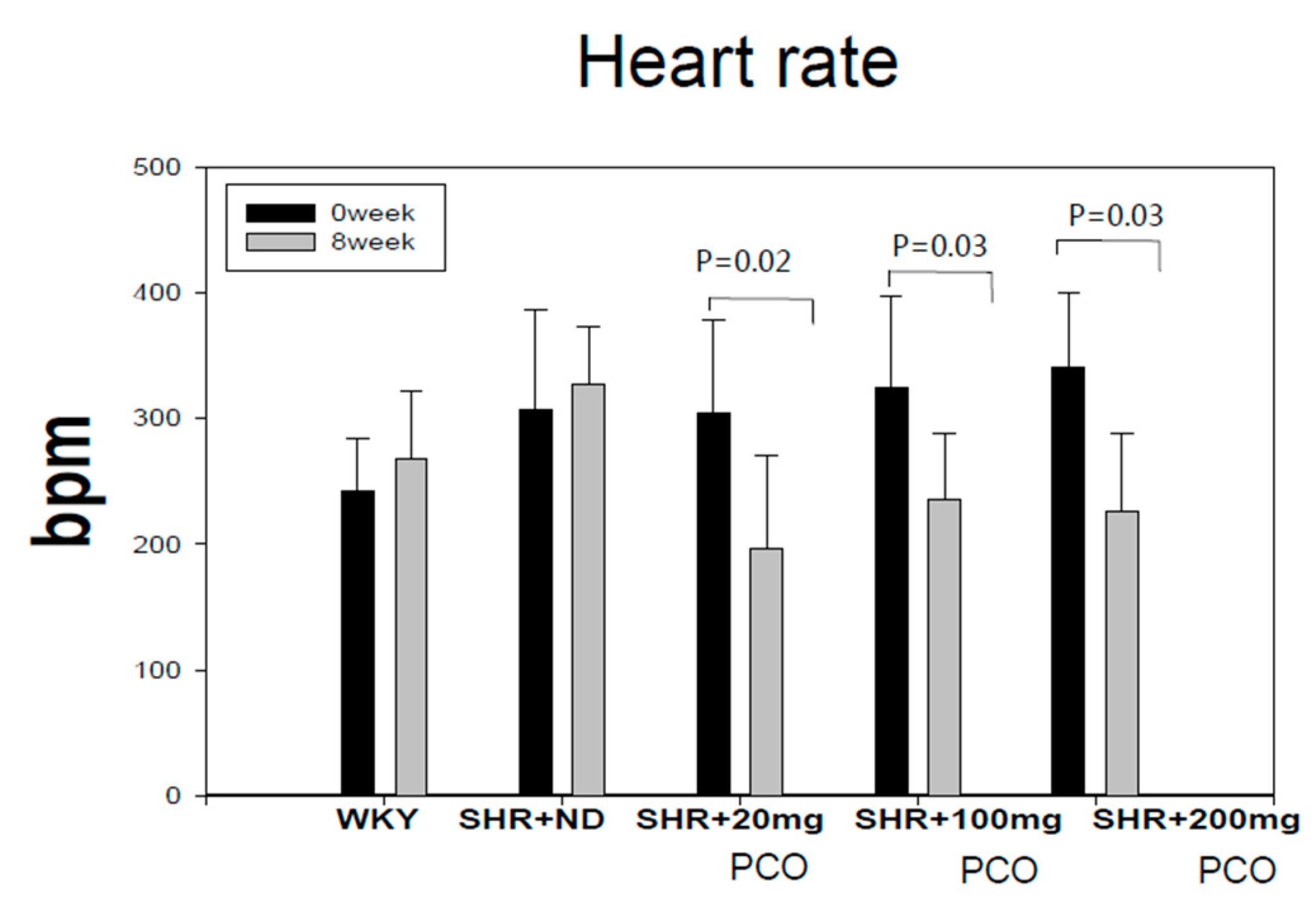
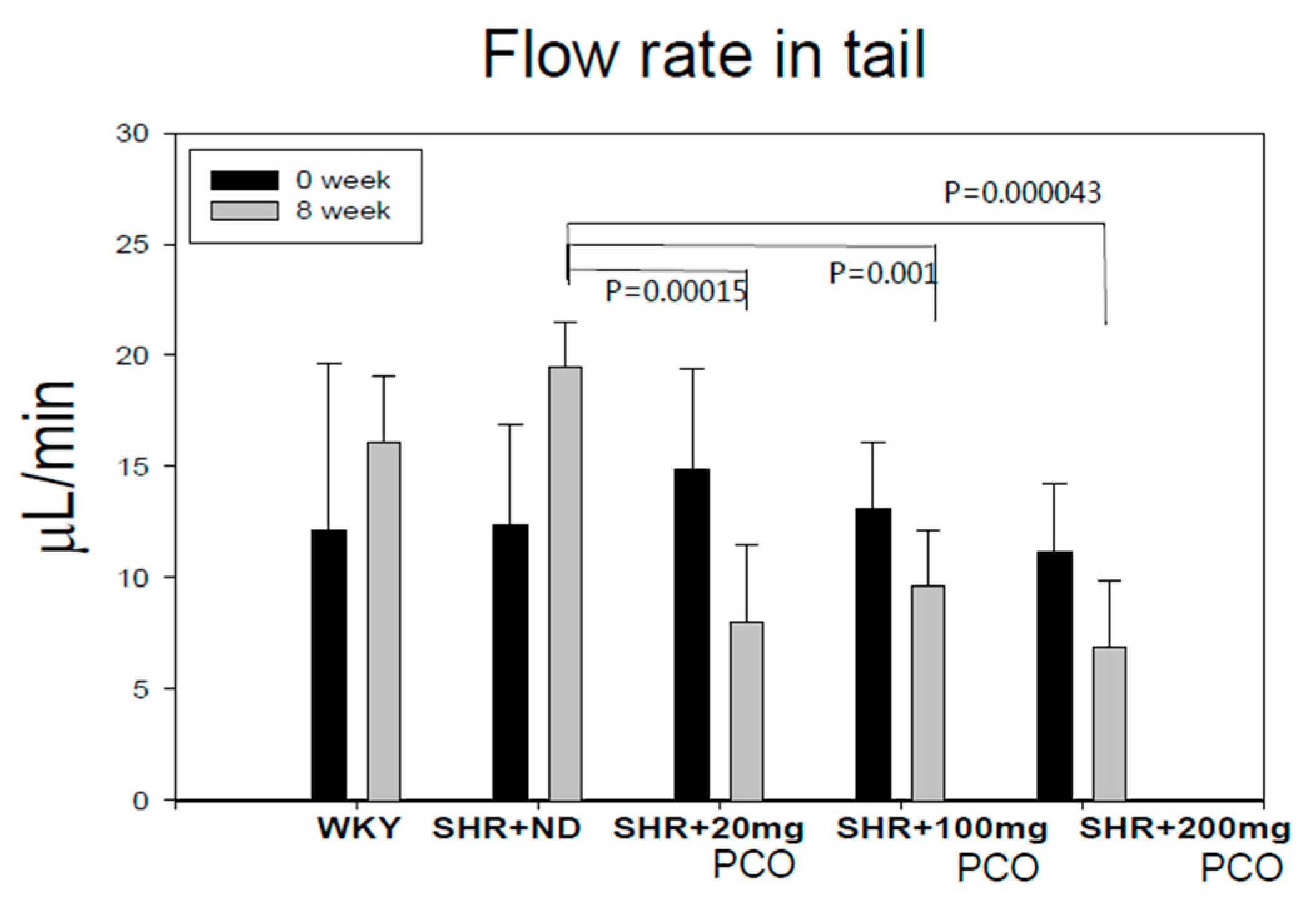
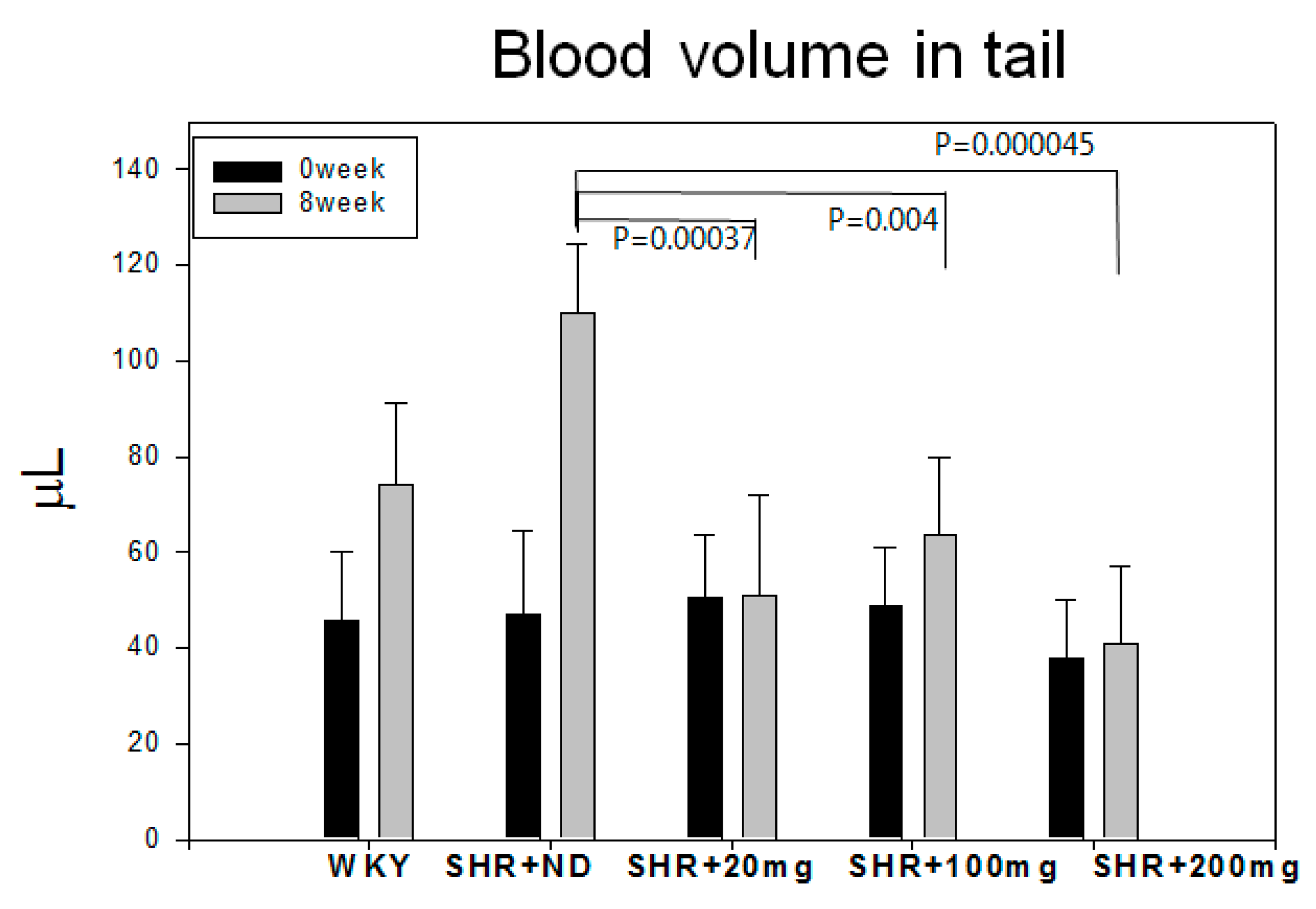


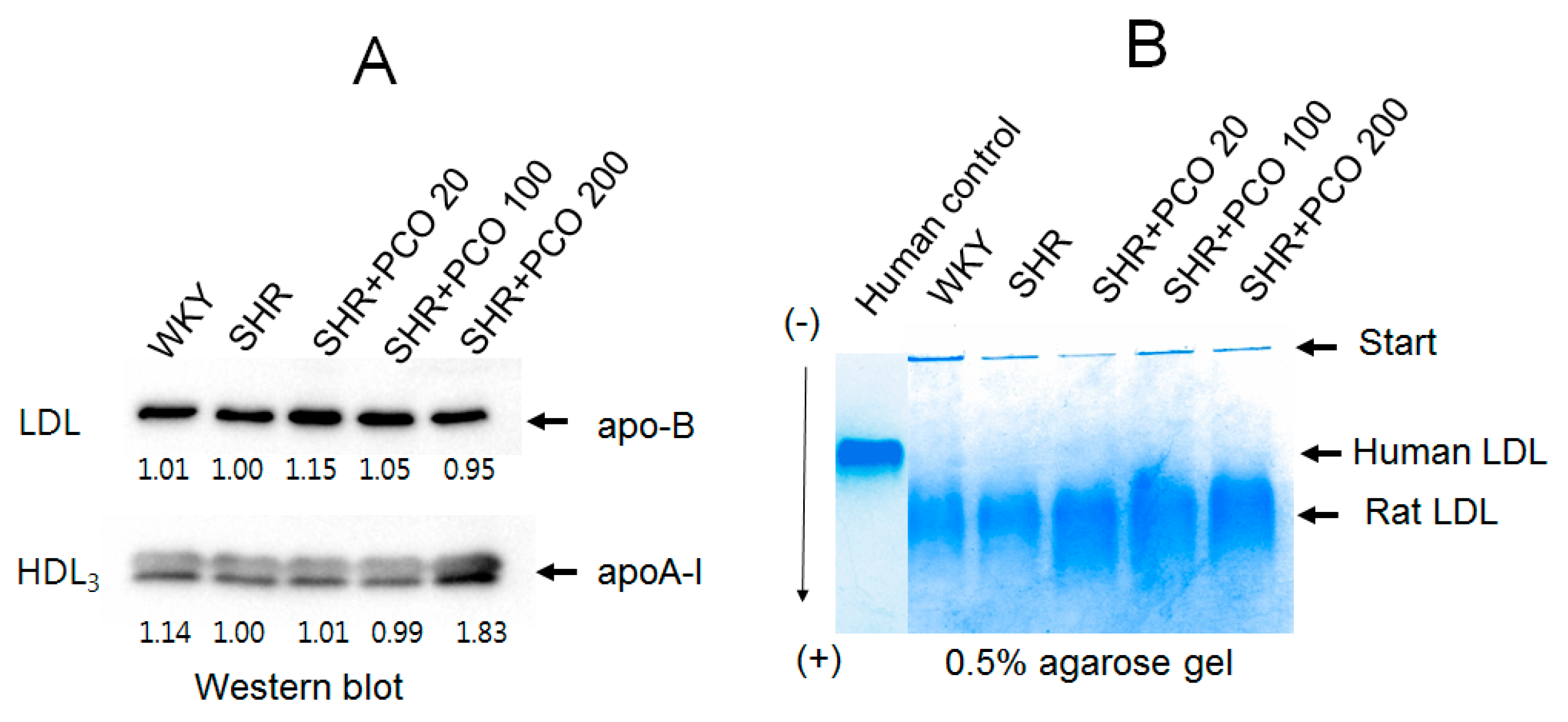


| Groups | SBP (Mean ± SD) Week 0 | SBP (Mean ± SD) Week 8 | p-Value | DBP (Mean ± SD) Week 0 | DBP (Mean ± SD) Week 8 | p-Value |
|---|---|---|---|---|---|---|
| WKY (n = 10) | 117.7 ± 11.1 | 127.6 ± 10.3 * | 0.048 | 89.1 ± 7.8 | 82.9 ± 7.3 * | 0.039 |
| SHR + ND (n = 10) | 187.6 ± 12.2 | 228.9 ± 5.3 ** | <0.001 | 151.6 ± 13.7 | 178.3 ± 12.4 ** | <0.001 |
| SHR + 20 mg (n = 10) | 188.7 ± 12.4 | 196.3 ± 12.8 | 0.08 | 144.7 ± 14.6 | 139.9 ± 21.2 | 0.331 |
| SHR + 100 mg (n = 10) | 189.0 ± 11.5 | 180.6 ± 17.5 | 0.293 | 145.0 ± 10.1 | 126.3 ± 19.1 | 0.06 |
| SHR + 200 mg (n = 6) | 190.3 ± 17.3 | 160.3 ± 20.3 * | 0.03 | 148.8 ± 8.3 | 124.5 ± 33.5 * | 0.01 |
| Group | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| WKY (n =10) | SHR + ND (n = 10) | SHR + 20mg (n = 10) | SHR + 100mg (n = 10) | SHR + 200mg (n = 10) | |
| Body weight | |||||
| week 0 | 313 ± 10 | 288 ± 8 | 294 ± 7 | 291 ± 7 | 293 ± 13 |
| week 8 | 409 ± 10 | 378 ± 8 | 384 ± 7 | 375 ± 6 | 374 ± 7 |
| TC (mg/dL) | 133 ± 3 ¶¶¶ | 75 ± 3 | 75 ± 4 | 73 ± 3 | 74 ± 5 |
| TG (mg/dL) | 61 ± 4 ¶ | 83 ± 7 | 89 ± 8 | 77 ± 9 | 68 ± 3 * |
| HDL-C (mg/dL) | 67 ± 6 ¶¶ | 44 ± 3 | 44 ± 4 | 52 ± 4 * | 52 ± 4 * |
| % HDL-C | 48 ± 4 | 58 ± 3 | 60 ± 5 | 69 ± 3 * | 70 ± 3 * |
| TG/HDL-C | 0.9 ± 0.1 ¶¶¶ | 1.9 ± 0.1 | 2.1 ± 0.2 | 1.6 ± 0.1 ** | 1.5 ± 0.1 * |
| LDL-C 2 | 58 ± 4 ¶¶¶ | 14 ± 2.3 | 12 ± 4.6 | 8 ± 3.2 * | 7 ± 1.8 * |
| Glucose (mg/dL) | 131 ± 15 | 161 ± 22 | 149 ± 25 | 153 ± 23 | 162 ± 14 |
| GOT (U/L) | 25 ± 3 | 28 ± 3 | 19 ± 2 | 30 ± 4 | 30 ± 4 |
| GPT (U/L) | 20 ± 0.3 | 20 ± 0.5 | 21 ± 0.4 | 20 ± 0.4 | 21 ± 0.4 |
| CRP (ng/L) | 476 ± 25 ¶¶ | 988 ± 48 | 670 ± 40 * | 660 ± 32 * | 546 ± 29 ** |
| Group | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|
| WKY (n = 10) | SHR + ND (n = 10) | SHR + 20 mg (n = 10) | SHR + 100 mg (n = 10) | SHR + 200 mg (n = 10) | ||
| LDL | TP (mg/mL) | 1.6 ± 0.1 | 1.3 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.1 * |
| TC (mg/mg) | 11.4 ± 2.1 | 9.3 ± 0.8 | 8.4 ± 0.3 | 7.5 ± 0.4 * | 7.0 ± 0.2 * | |
| TG (mg/mg) | 3.1 ± 0.1 | 4.8 ± 0.1 | 4.4 ± 0.1 * | 3.2 ± 0.0 ** | 3.0 ± 0.0 ** | |
| HDL2 | TP (mg/mL) | 2.8 ± 0.2 | 2.3 ± 0.2 | 2.2 ± 0.2 | 2.3 ± 0.2 | 3.2 ± 0.2 * |
| TC (mg/mg) | 12.0 ± 0.5 | 6.6 ± 0.2 | 10.2 ± 0.2 ** | 10.6 ± 0.5** | 11.0 ± 0.2 ** | |
| TG (mg/mg) | 1.4 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.2 | 1.5 ± 0.1 | 1.4 ± 0.1 * | |
| HDL3 | TP (mg/mL) | 2.9 ± 0.2 | 2.7 ± 0.2 | 2.4 ± 0.1 | 2.6 ± 0.1 | 3.3 ± 0.2 * |
| TC (mg/mg) | 4.2 ± 0.1 | 3.2 ± 0.1 | 4.1 ± 0.1 ** | 4.3 ± 0.2 ** | 4.5 ± 0.2 ** | |
| TG (mg/mg) | 0.9 ± 0.0 | 1.2 ± 0.1 | 1.1 ± 0.0 | 0.9 ± 0.0 * | 0.8 ± 0.0 ** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, K.-H.; Yadav, D.; Kim, S.-J.; Kim, J.-R. Blood Pressure Lowering Effect of Cuban Policosanol is Accompanied by Improvement of Hepatic Inflammation, Lipoprotein Profile, and HDL Quality in Spontaneously Hypertensive Rats. Molecules 2018, 23, 1080. https://doi.org/10.3390/molecules23051080
Cho K-H, Yadav D, Kim S-J, Kim J-R. Blood Pressure Lowering Effect of Cuban Policosanol is Accompanied by Improvement of Hepatic Inflammation, Lipoprotein Profile, and HDL Quality in Spontaneously Hypertensive Rats. Molecules. 2018; 23(5):1080. https://doi.org/10.3390/molecules23051080
Chicago/Turabian StyleCho, Kyung-Hyun, Dhananjay Yadav, Suk-Jeong Kim, and Jae-Ryong Kim. 2018. "Blood Pressure Lowering Effect of Cuban Policosanol is Accompanied by Improvement of Hepatic Inflammation, Lipoprotein Profile, and HDL Quality in Spontaneously Hypertensive Rats" Molecules 23, no. 5: 1080. https://doi.org/10.3390/molecules23051080







