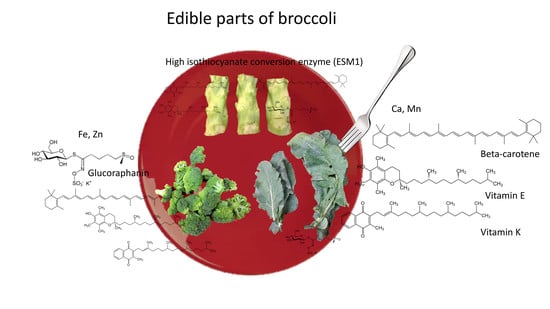
Comparative Phytonutrient Analysis of Broccoli By-Products: The Potentials for Broccoli By-Product Utilization
Abstract
:1. Introduction
2. Results and Discussion
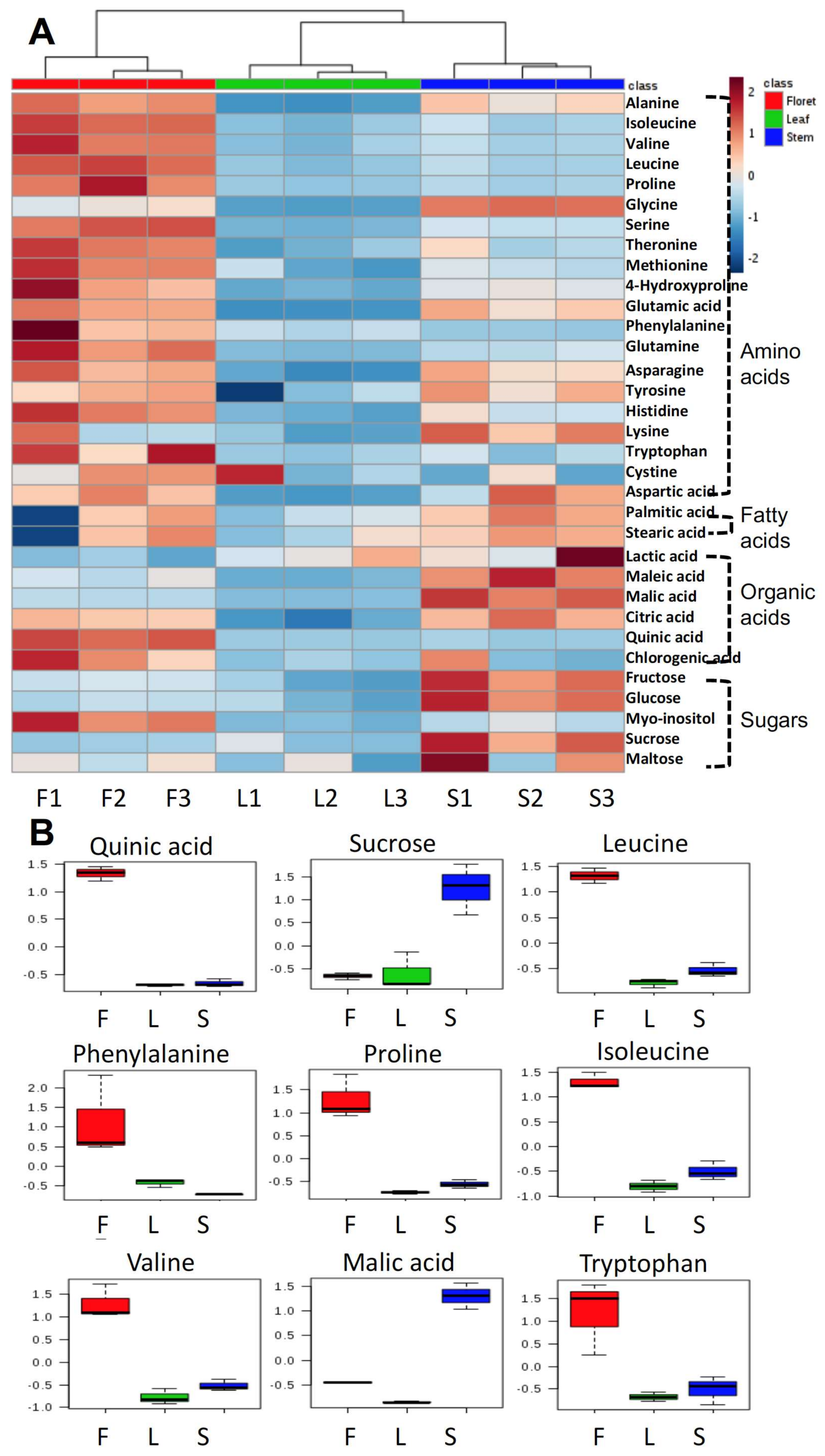
2.1. Broccoli Primary Metabolites
2.2. Glucosinolates
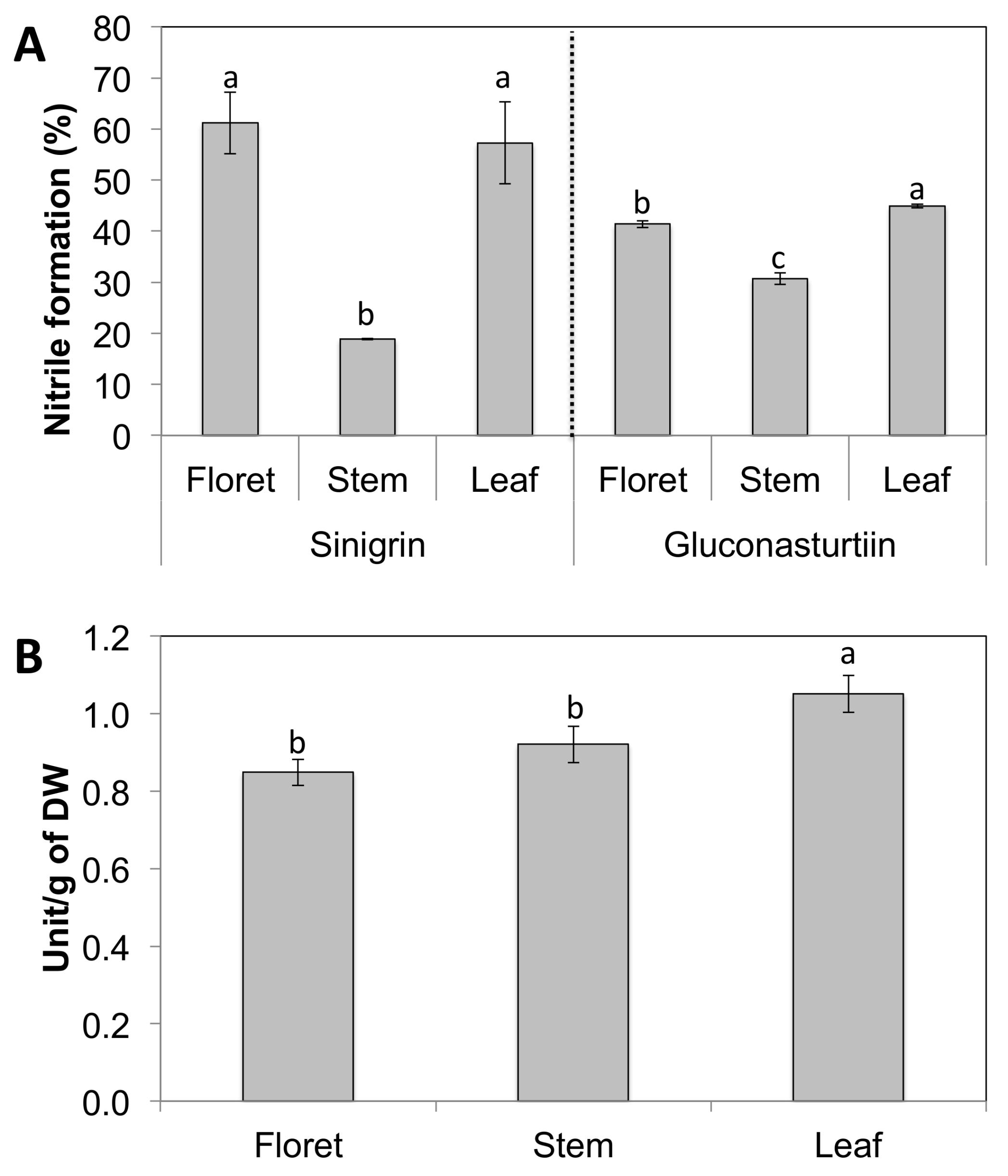
2.3. Nitrile Formation Percentage to Total Glucosinolate Hydrolysis Products and Myrosinase Activity
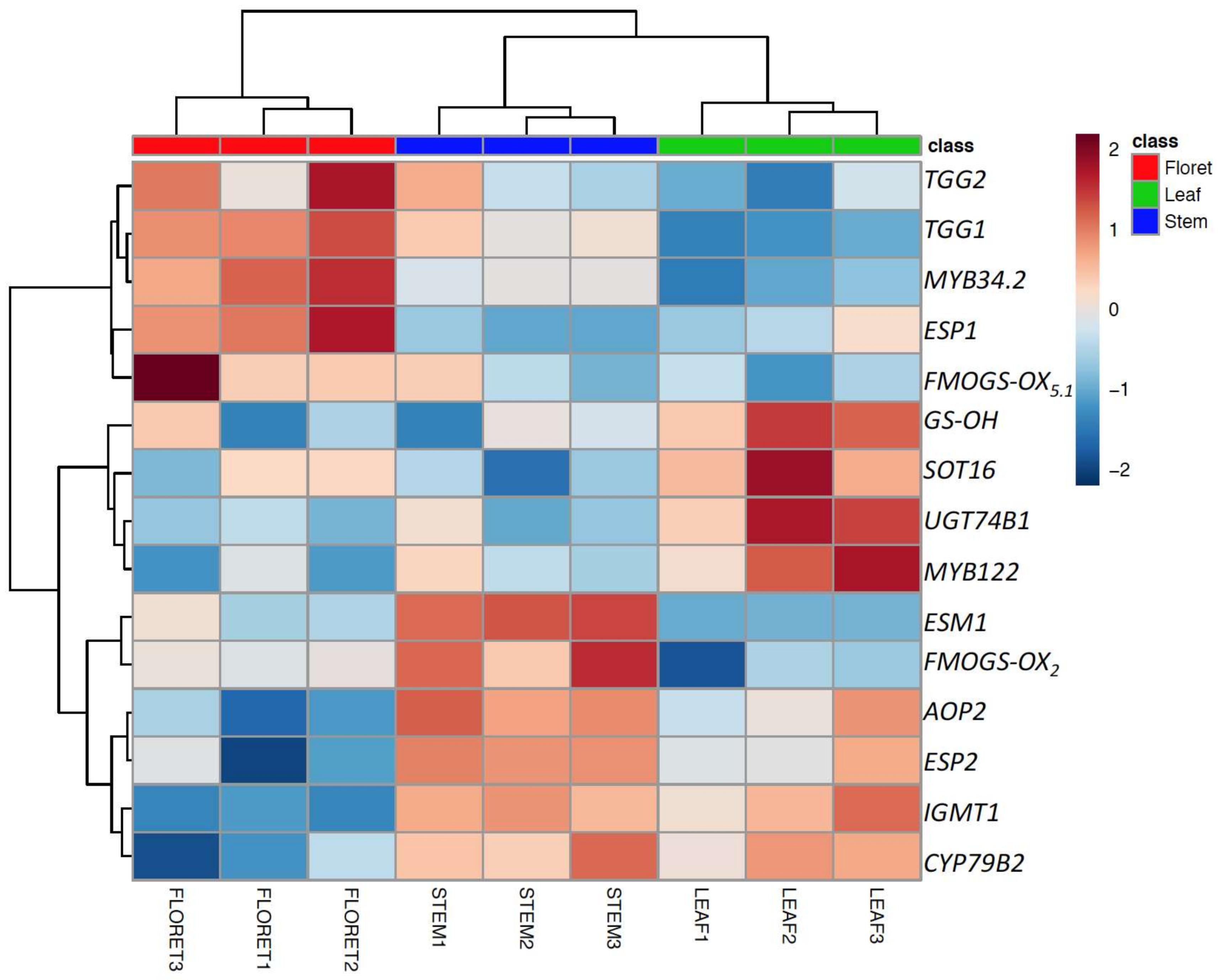
2.4. Gene Expression of Glucosinolate Biosynthesis and Myrosinase-Related Genes
2.5. Carotenoids and Chlorophylls
2.6. Essential Mineral Nutrients
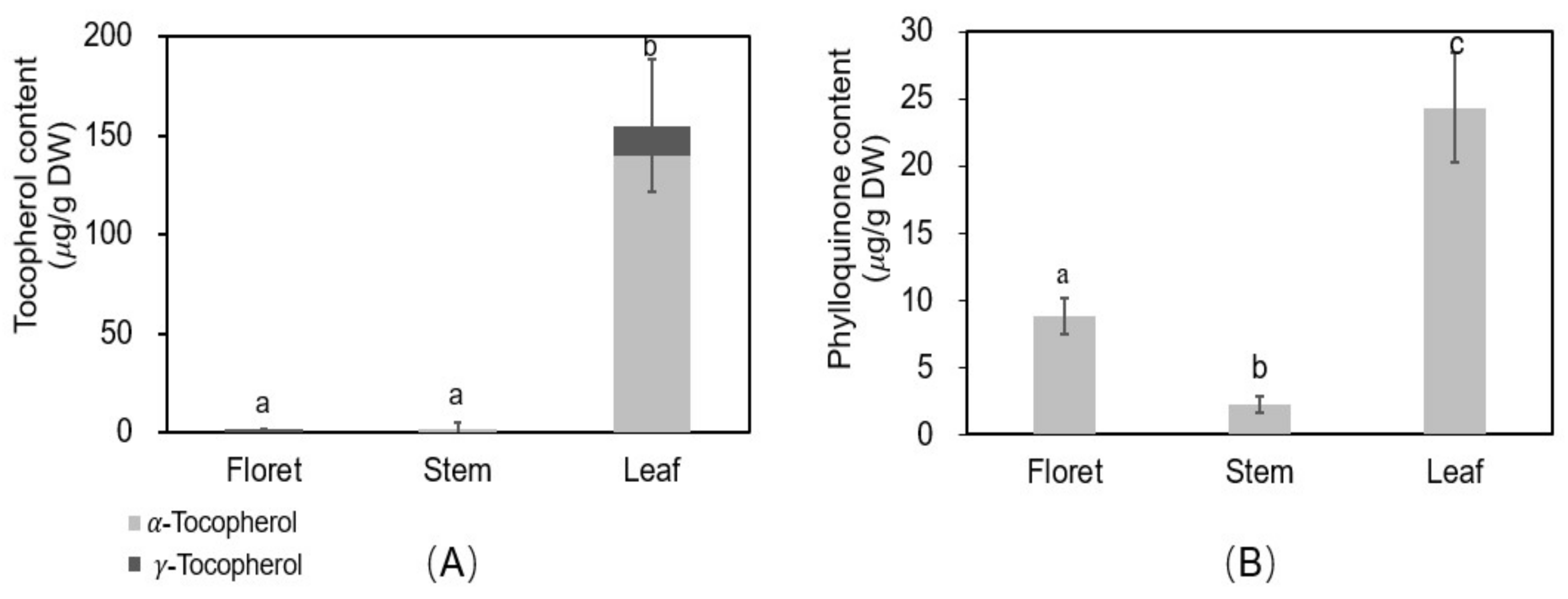
2.7. Vitamins E and K
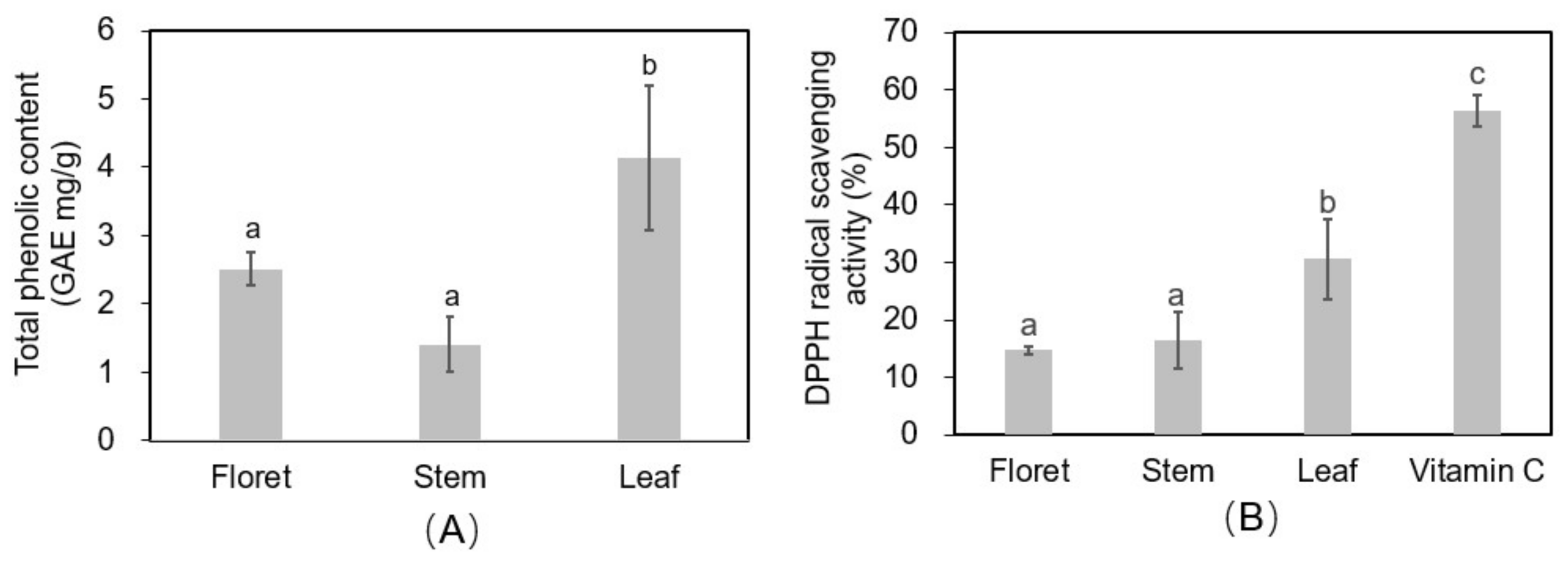
2.8. Total Phenolic Concentrations and Free Radical Scavenging Activity
2.9. Potential of Broccoli By-Products for Human Consumption and Nutraceutical Industries
3. Materials and Methods
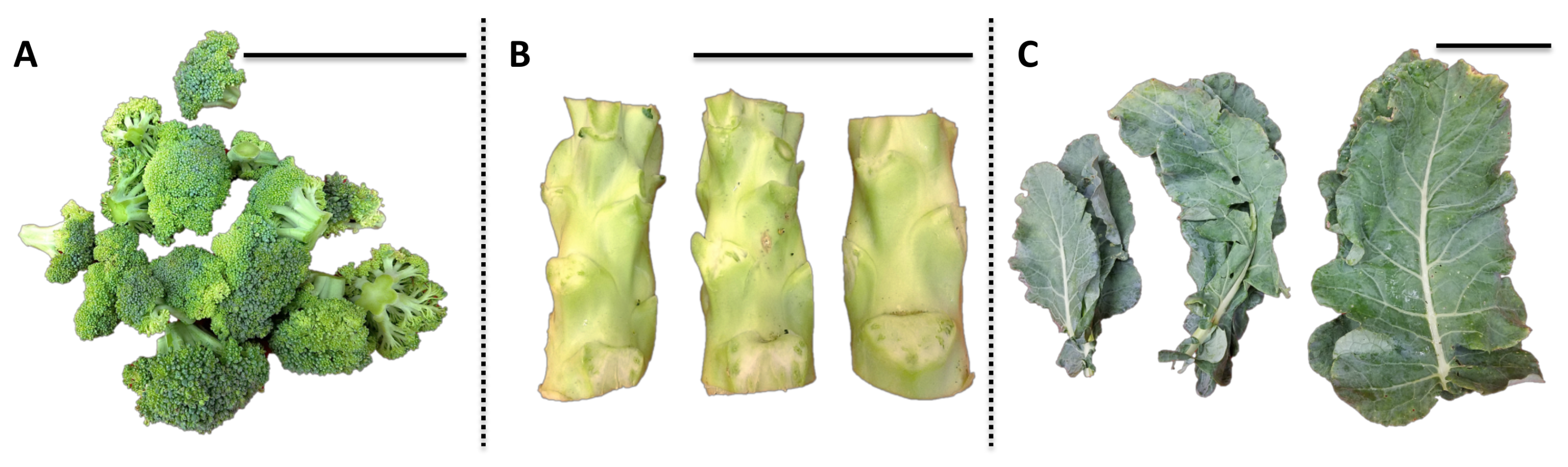
3.1. Broccoli Production
3.2. Primary Metabolite Extraction and Analysis
3.3. Quantitation of Glucosinolate
3.4. Quantitation of Myrosinase Activity and Nitrile Formation from Glucosinolate
3.5. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
3.6. Carotenoid and Chlorophyll Analysis
3.7. Mineral Analysis
3.8. Analysis of Vitamins E and K
3.9. Total Phenolic Content and Antioxidant Capacity
3.10. Statistical Analyses
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Maggioni, L. Domestication of Brassica oleracea L. Ph.D. Thesis, Swedish University of Agricultural Sciences, Alnarp, Sweden, 2015. [Google Scholar]
- Domínguez-Perles, R.; Martínez-Ballesta, M.C.; Carvajal, M.; García-Viguera, C.; Moreno, D.A. Broccoli-derived by-products—A promising source of bioactive ingredients. J. Food Sci. 2010, 75, C383–C392. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Ku, K.M.; Becker, T.M.; Juvik, J.A. Chemopreventive glucosinolate accumulation in various broccoli and collard tissues: Microfluidic-based targeted transcriptomics for by-product valorization. PLoS ONE 2017, 12, e0185112. [Google Scholar] [CrossRef] [PubMed]
- Shiva, R.B.; Jung-Ho, K. Seasonal variation in phytochemicals and antioxidant activities in different tissues of various broccoli cultivars. Afr. J. Biotechnol. 2014, 13, 604–615. [Google Scholar] [CrossRef]
- Hwang, J.H.; Lim, S.B. Antioxidant and anticancer activities of broccoli by-products from different cultivars and maturity stages at harvest. Prev. Nutr. Food Sci. 2015, 20, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Drabińska, N.; Ciska, E.; Szmatowicz, B.; Krupa-Kozak, U. Broccoli by-products improve the nutraceutical potential of gluten-free mini sponge cakes. Food Chem. 2017. [Google Scholar] [CrossRef]
- Hu, C.H.; Wang, D.G.; Pan, H.Y.; Zheng, W.B.; Zuo, A.Y.; Liu, J.X. Effects of broccoli stem and leaf meal on broiler performance, skin pigmentation, antioxidant function, and meat quality. Poult. Sci. 2012, 91, 2229–2234. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.W.; Yang, F.; Liu, J.X.; Wang, J.K. Effects of replacement of concentrate mixture by broccoli byproducts on lactating performance in dairy cows. Asian Australas. J. Anim.Sci. 2015, 28, 1449–1453. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, E.H.; Brown, A.F.; Kurilich, A.C.; Keck, A.S.; Matusheski, N.; Klein, B.P.; Juvik, J.A. Variation in content of bioactive components in broccoli. J. Food Compos. Anal. 2003, 16, 323–330. [Google Scholar] [CrossRef]
- Moreno, D.A.; Carvajal, M.; Lopez-Berenguer, C.; Garcia-Viguera, C. Chemical and biological characterisation of nutraceutical compounds of broccoli. J. Pharm. Biomed. Anal. 2006, 41, 1508–1522. [Google Scholar] [CrossRef] [PubMed]
- Ares, A.M.; Nozal, M.J.; Bernal, J. Extraction, chemical characterization and biological activity determination of broccoli health promoting compounds. J. Chromatogr. A 2013, 1313, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Ferruzzi, M.G.; Blakeslee, J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr. Res. 2007, 27, 1–12. [Google Scholar] [CrossRef]
- Lanfer-Marquez, U.M.; Barros, R.M.C.; Sinnecker, P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
- Sakdarat, S.; Shuyprom, A.; Pientong, C.; Ekalaksananan, T.; Thongchai, S. Bioactive constituents from the leaves of Clinacanthus nutans Lindau. Bioorg. Med. Chem. 2009, 17, 1857–1860. [Google Scholar] [CrossRef] [PubMed]
- Simonich, M.T.; Egner, P.A.; Roebuck, B.D.; Orner, G.A.; Jubert, C.; Pereira, C.; Groopman, J.D.; Kensler, T.W.; Dashwood, R.H.; Williams, D.E.; et al. Natural chlorophyll inhibits aflatoxin B1-induced multi-organ carcinogenesis in the rat. Carcinogenesis 2007, 28, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Carotenoids: Potential allies of cardiovascular health? Food Nutr. Res. 2015, 59, 26762. [Google Scholar] [PubMed]
- Saldeen, K.; Saldeen, T. Importance of tocopherols beyond α-tocopherol: Evidence from animal and human studies. Nutr. Res. 2005, 25, 877–889. [Google Scholar] [CrossRef]
- Damon, M.; Zhang, N.Z.; Haytowitz, D.B.; Booth, S.L. Phylloquinone (vitamin K1) content of vegetables. J. Food Compos. Anal. 2005, 18, 751–758. [Google Scholar] [CrossRef]
- Said, A.; Abuotabl, E.A.; Raoof, G.F.A.; Huefner, A.; Nada, S.A. Phenolic contents and bioactivities of pericarp and seeds of Pleiogynium solandri(Benth.) Engl. (Anacardiaceae). Iran. J. Basic Med. Sci. 2014, 18, 164. [Google Scholar]
- Tusevski, O.; Kostovska, A.; Iloska, A.; Trajkovska, L.; Simic, S. Phenolic production and antioxidant properties of some Macedonian medicinal plants. Open Life Sci. 2014, 9, 888–900. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Dueñas, M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Characterization of phenolic compounds and antioxidant properties of Glycyrrhiza glabra L. rhizomes and roots. RSC Adv. 2015, 5, 26991–26997. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. USDA National Nutrient Database for Standard Reference. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/nutrient-data-laboratory/docs/usda-national-nutrient-database-for-standard-reference/ (accessed on 1 April 2018).
- Ku, K.M.; Choi, J.H.; Kim, H.S.; Kushad, M.M.; Jeffery, E.H.; Juvik, J.A. Methyl jasmonate and 1-methylcyclopropene treatment effects on quinone reductase inducing activity and post-harvest quality of broccoli. PLoS ONE 2013, 8, e77127. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.M.; Jeffery, E.H.; Juvik, J.A. Influence of seasonal variation and methyl jasmonate mediated induction of glucosinolate biosynthesis on quinone reductase activity in broccoli florets. J. Agric. Food Chem. 2013, 61, 9623–9631. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.M.; Becker, T.M.; Juvik, J.A. Transcriptome and metabolome analyses of glucosinolates in two broccoli cultivars following jasmonate treatment for the induction of glucosinolate defense to Trichoplusia ni (Hübner). Int. J. Mol. Sci. 2016, 17, 1135. [Google Scholar] [CrossRef] [PubMed]
- Neave, A.S.; Sarup, S.M.; Seidelin, M.; Duus, F.; Vang, O. Characterization of the N-methoxyindole-3-carbinol (NI3C)–induced cell cycle arrest in human colon cancer cell lines. Toxicol. Sci. 2005, 83, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Wittstock, U.; Burow, M. Glucosinolate breakdown in arabidopsis: Mechanism, regulation and biological significance. Arabidopsis Book/A. Soc. Plant Biol. 2010, 8, e0134. [Google Scholar] [CrossRef] [PubMed]
- Matusheski, N.V.; Swarup, R.; Juvik, J.A.; Mithen, R.; Bennett, M.; Jeffery, E.H. Epithiospecifier protein from broccoli (Brassica oleracea L. ssp. Italica) inhibits formation of the anticancer agent sulforaphan. J. Agric. Food Chem. 2006, 54, 2069–2076. [Google Scholar] [CrossRef] [PubMed]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Vitamin A. Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional/ (accessed on 7 February 2018).
- Guzman, I.; Yousef, G.G.; Brown, A.F. Simultaneous extraction and quantitation of carotenoids, chlorophylls, and tocopherols in brassica vegetables. J. Agric. Food Chem. 2012, 60, 7238–7244. [Google Scholar] [CrossRef] [PubMed]
- Sevik, H.; Guney, D.; Karakas, H.; Aktar, G. Change to amount of chlorophyll on leaves depend on insolation in some landscape plants. Int. J. Environ. Sci. 2012, 3, 1057. [Google Scholar]
- Parnikoza, I.Y.; Loro, P.; Miryuta, N.Y.; Kunakh, V.A.; Kozeretska, I.A. The influence of some environmental factors on cytological and biometric parameters and chlorophyll content of Deschampsia antarctica Desv. in the maritime Antarctic. Cytol. Genet. 2011, 45, 170–176. [Google Scholar] [CrossRef]
- Page, V.; Feller, U. Heavy metals in crop plants: Transport and redistribution processes on the whole plant level. Agronomy 2015, 5, 447–463. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: San Diego, CA, USA, 1995. [Google Scholar]
- Grilo, E.C.; Costa, P.N.; Gurgel, C.S.S.; Beserra, A.F.d.L.; Almeida, F.N.d.S.; Dimenstein, R. Alpha-tocopherol and gamma-tocopherol concentration in vegetable oils. Food Sci. Technol. (Camp.) 2014, 34, 379–385. [Google Scholar] [CrossRef]
- Knecht, K.; Sandfuchs, K.; Kulling, S.E.; Bunzel, D. Tocopherol and tocotrienol analysis in raw and cooked vegetables: A validated method with emphasis on sample preparation. Food Chem. 2015, 169, 20–27. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Vitamin E. Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional/ (accessed on 7 February 2018).
- Okano, T.; Shimomura, Y.; Yamane, M.; Suhara, Y.; Kamao, M.; Sugiura, M.; Nakagawa, K. Conversion of phylloquinone (Vitamin K1) into menaquinone-4 (Vitamin K2) in mice: Two possible routes for menaquinone-4 accumulation in cerebra of mice. J. Biol. Chem. 2008, 283, 11270–11279. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Vitamin K. Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/VitaminK-HealthProfessional/ (accessed on 7 February 2018).
- Beulens, J.W.; Booth, S.L.; van den Heuvel, E.G.; Stoecklin, E.; Baka, A.; Vermeer, C. The role of menaquinones (vitamin K2) in human health. Br. J. Nutr. 2013, 110, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, H.; Czajkowska, K.; Cichowska, J.; Lenart, A. What's new in biopotential of fruit and vegetable by-products applied in the food processing industry. Trends Food Sci. Technol. 2017, 67, 150–159. [Google Scholar] [CrossRef]
- Gómez, M.; Martinez, M.M. Fruit and vegetable by-products as novel ingredients to improve the nutritional quality of baked goods. Crit. Rev. Food Sci. Nutr. 2017, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, M. Valorization of Food Processing by-Products; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Megias, M.; Marcos, M.; Madrid, J.; Hernandez, F.; Martinez-Teruel, A.; A. Cano, J. Nutritive, Fermentative and Environmental Characteristics of Silage of Two Industrial Broccoli (Brassica Oleracea var. Italica) by-Products for Ruminant Feed; Friends Science Publishers: Faisalabad, Pakistan, 2014; Volume 16, pp. 307–313. [Google Scholar]
- Sanz-Puig, M.; Pina-Perez, M.C.; Criado, M.N.; Rodrigo, D.; Martinez-Lopez, A. Antimicrobial potential of cauliflower, broccoli, and okara byproducts against foodborne bacteria. Foodborne Pathog. Dis. 2015, 12, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, K.V.; Hubbard, J.C.; Koike, S.T. Evaluation of broccoli residue incorporation into field soil for Verticillium wilt control in cauliflower. Plant Dis. 1999, 83, 124–129. [Google Scholar] [CrossRef]
- Dominguez-Perles, R.; Moreno, D.A.; Carvajal, M.; Garcia-Viguera, C. Composition and antioxidant capacity of a novel beverage produced with green tea and minimally-processed byproducts of broccoli. Innov. Food Sci. Emerg. Technol. 2011, 12, 361–368. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Cardoso, S.M.; Wessel, D.F.; Coimbra, M.A. Microwave assisted dehydration of broccoli by-products and simultaneous extraction of bioactive compounds. Food Chem. 2018, 246, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.M.; Kim, H.-S.; Kim, B.-S.; Kang, Y.-H. Antioxidant activities and antioxidant constituents of pepper leaves from various cultivars and correlation between antioxidant activities and antioxidant constituents. J. Appl. Biol. Chem. 2009, 52, 70–76. [Google Scholar] [CrossRef]
- Ku, K.M.; Kang, Y.H. Quinone reductase inductive activity of capsicum annuum leaves and isolation of the active compounds. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 709–715. [Google Scholar] [CrossRef]
- Martin Aregheore, E. Nutritive value and inherent anti-nutritive factors in four indigenous edible leafy vegetables in human nutrition in nigeria: A review. J. Food Res. Sci. 2012, 1, 1–14. [Google Scholar]
- Ryu, R.; Jeong, T.-S.; Kim, Y.J.; Choi, J.-Y.; Cho, S.-J.; Kwon, E.-Y.; Jung, U.J.; Ji, H.-S.; Shin, D.-H.; Choi, M.-S. Beneficial effects of pterocarpan-high soybean leaf extract on metabolic syndrome in overweight and obese korean subjects: Randomized controlled trial. Nutrients 2016, 8, 734. [Google Scholar] [CrossRef] [PubMed]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.M.; Jeffery, E.H.; Juvik, J.A.; Kushad, M.M. Correlation of quinone reductase activity and allyl isothiocyanate formation among different genotypes and grades of horseradish roots. J. Agric. Food Chem. 2015, 63, 2947–2955. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Chiu, Y.-C.; Ku, K.-M. Glucosinolates, carotenoids, and vitamins E and K variation from selected kale and collard cultivars. J. Food Qual. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, Y.; Kopsell, D.A.; Park, S.; Tou, J.C.; Waterland, N.L. Nutritional value of crisphead ‘iceberg’ and romaine lettuces (Lactuca sativa L.). J. Agric. Sci. 2016, 8, 734. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of vitamin and carotenoid concentrations of emerging food products: Edible microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.M.; Choi, J.N.; Kim, J.; Kim, J.K.; Yoo, L.G.; Lee, S.J.; Hong, Y.S.; Lee, C.H. Metabolomics analysis reveals the compositional differences of shade grown tea (Camellia sinensis L.). J. Agric. Food Chem. 2010, 58, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. Using metaboanalyst 3.0 for comprehensive metabolomics data analysis. In Current Protocols in Bioinformatics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002. [Google Scholar]
Sample Availability: Samples of broccoli powder are available from the authors. |

| Tissue | GIB | PRO | GRA | GNA | GER | GNS | GBC | 4-OH-GBC | 4-Methoxy-GBC | Neo-GBC | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Floret | 2.26 ± 0.33a (6.52) | 1.01 ± 0.11a (2.90) | 11.25 ± 1.82a (32.45) | 0.11 ± 0.02a (0.31) | 0.08 ± 0.05b (0.23) | 0.89 ± 0.29a (2.58) | 1.54 ± 0.30a (4.45) | 0.02 ± 0.01a (0.07) | 0.58 ± 0.02a (1.66) | 16.67 ± 1.64a (48.11) | 34.66 |
| Stem | 0.97 ± 0.16b (13.04) | 0.24 ± 0.04b (3.22) | 3.79 ± 0.78b (50.88) | 0.03 ± 0.00b (0.47) | 0.89 ± 0.19a (11.97) | 0.02 ± 0.00b (0.27) | 0.10 ± 0.02b (1.40) | 0.07 ± 0.02a (0.91) | 0.16 ± 0.01b (2.17) | 1.11 ± 0.47c (14.85) | 7.45 |
| Leaf | 0.65 ± 0.10b (6.43) | 0.02 ± 0.00c (0.18) | 2.77 ± 0.59b (27.45) | 0.04 ± 0.01b (0.36) | 0.04 ± 0.01b (0.40) | 0.11 ± 0.01b (1.08) | 0.24 ± 0.05b (2.41) | 0.26 ± 0.31a (2.56) | 0.17 ± 0.03b (1.66) | 5.78 ± 0.64b (57.35) | 10.08 |
| Tissue | Carotenoids | Chlorophylls | ||||||
|---|---|---|---|---|---|---|---|---|
| β-carotene | Violaxanthin | Neoxanthin | Lutein | Total | Chlorophyll a | Chlorophyll b | Total | |
| Floret | 30.6 ± 4.6b (16.9) | 34.7 ± 2.4b (19.1) | 30.2 ± 1.3b (16.7) | 85.5 ± 8.3b (47.2) | 181.0 | 852.1 ± 105.5b (86.4) | 134.6 ± 14.3b (13.6) | 986.7 |
| Stem | 0.0c | 0.0c | 4.8 ± 8.3c (30.8) | 10.8 ± 9.5c (69.2) | 15.6 | 143.7 ± 51.6c (86.6) | 22.2 ± 9.2c (13.4) | 165.8 |
| Leaf | 248.4 ± 28.9a (22.7) | 206.3 ± 20.3a (18.8) | 156.2 ± 10.6a (14.3) | 484.1 ± 33.2a (44.2) | 1095.0 | 4477.9 ± 408.6a (85.2) | 780.9 ± 56.3a (14.8) | 5258.8 |
| Tissue | Fe | Zn | Mn | Cu | Ca | Mg | P | Na | K |
|---|---|---|---|---|---|---|---|---|---|
| μg/g DW | mg/g DW | ||||||||
| Floret | 45.83 ± 0.83a (3.5) | 54.00 ± 3.01a (6.8) | 18.83 ± 0.17b (11.4) | 0.29 ± 0.08a (0.45) | 4.65 ± 0.10b (5.4) | 1.78 ± 0.03a (5.9) | 7.01 ± 0.12a (13.9) | 0.39 ± 0.03ab (0.4) | 145 ± 22a (3.1) |
| Stem | 15.83 ± 3.08b (0.9) | 22.67 ± 5.17b (2.2) | 7.00 ± 0.29c (3.3) | 0.24 ± 0.12a (0.29) | 7.10 ± 0.36b (5.3) | 1.67 ± 0.19a (4.3) | 5.07 ± 0.18b (7.7) | 6.43 ± 0.16a (0.5) | 182 ± 7a (3.9) |
| Leaf | 40.50 ± 1.26a (4.3) | 23.33 ± 2.42b (4.1) | 26.17 ± 1.69a (22.0) | 0.21 ± 0.11a (0.46) | 28.99 ± 1.97a (46.6) | 1.33 ± 0.13a (6.1) | 3.42 ± 0.08c (9.4) | 2.63 ± 0.03b (0.3) | 136 ± 72a (2.9) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Zhang, L.; Ser, S.L.; Cumming, J.R.; Ku, K.-M. Comparative Phytonutrient Analysis of Broccoli By-Products: The Potentials for Broccoli By-Product Utilization. Molecules 2018, 23, 900. https://doi.org/10.3390/molecules23040900
Liu M, Zhang L, Ser SL, Cumming JR, Ku K-M. Comparative Phytonutrient Analysis of Broccoli By-Products: The Potentials for Broccoli By-Product Utilization. Molecules. 2018; 23(4):900. https://doi.org/10.3390/molecules23040900
Chicago/Turabian StyleLiu, Mengpei, Lihua Zhang, Suk Lan Ser, Jonathan R. Cumming, and Kang-Mo Ku. 2018. "Comparative Phytonutrient Analysis of Broccoli By-Products: The Potentials for Broccoli By-Product Utilization" Molecules 23, no. 4: 900. https://doi.org/10.3390/molecules23040900