Non-Covalent Supported of l-Proline on Graphene Oxide/Fe3O4 Nanocomposite: A Novel, Highly Efficient and Superparamagnetically Separable Catalyst for the Synthesis of Bis-Pyrazole Derivatives
Abstract
:1. Introduction
2. Results and Discussion
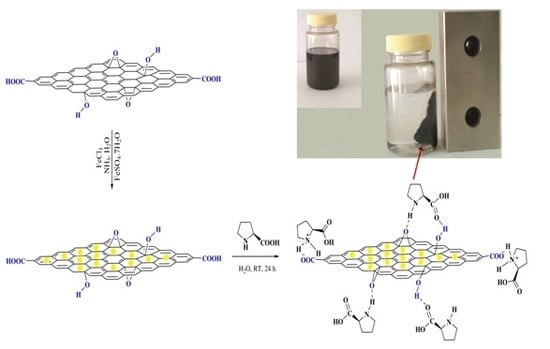
2.1. Catalyst Preparation
2.2. Characterization of the Catalyst
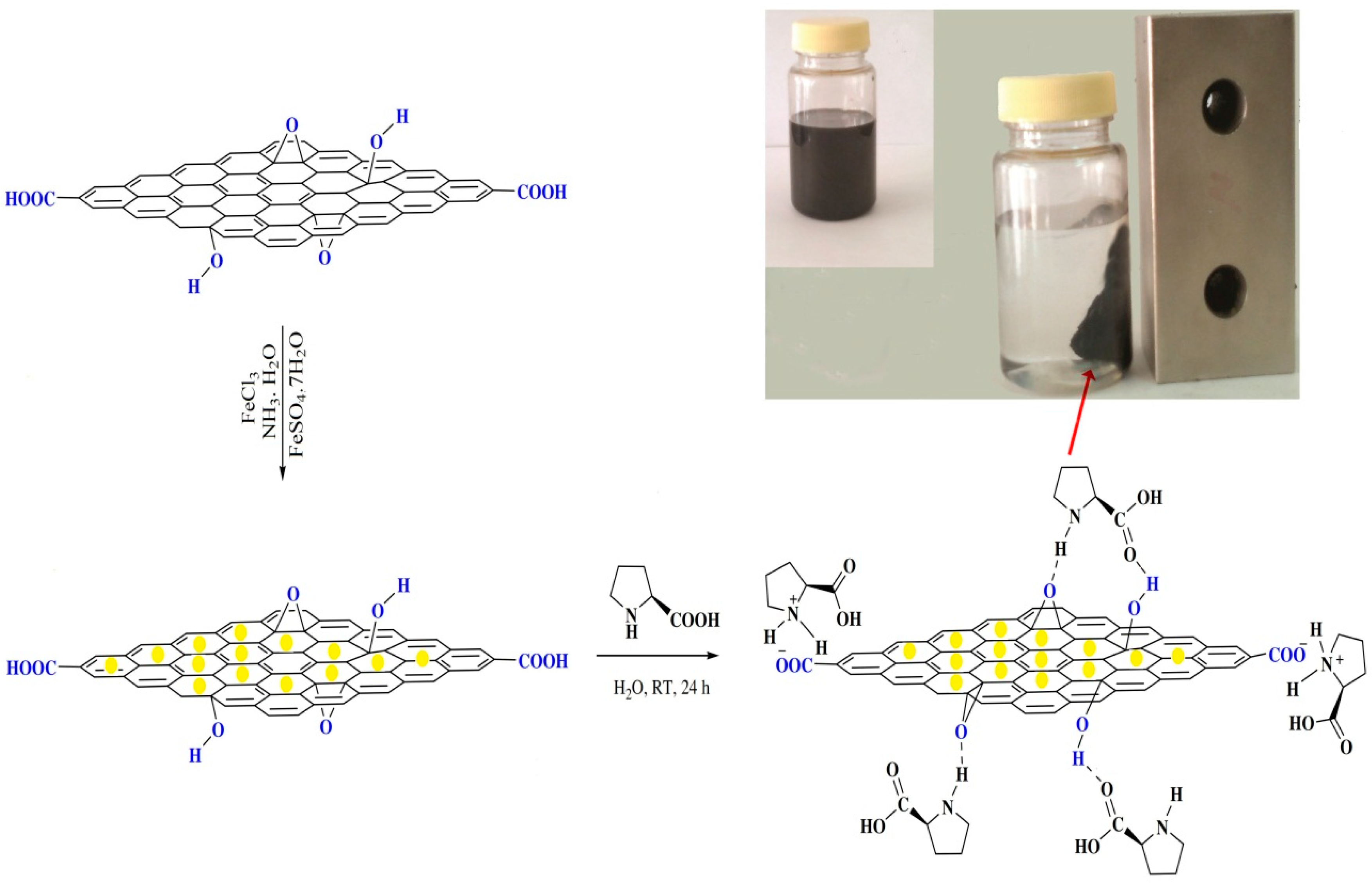
2.2.1. IR Spectra
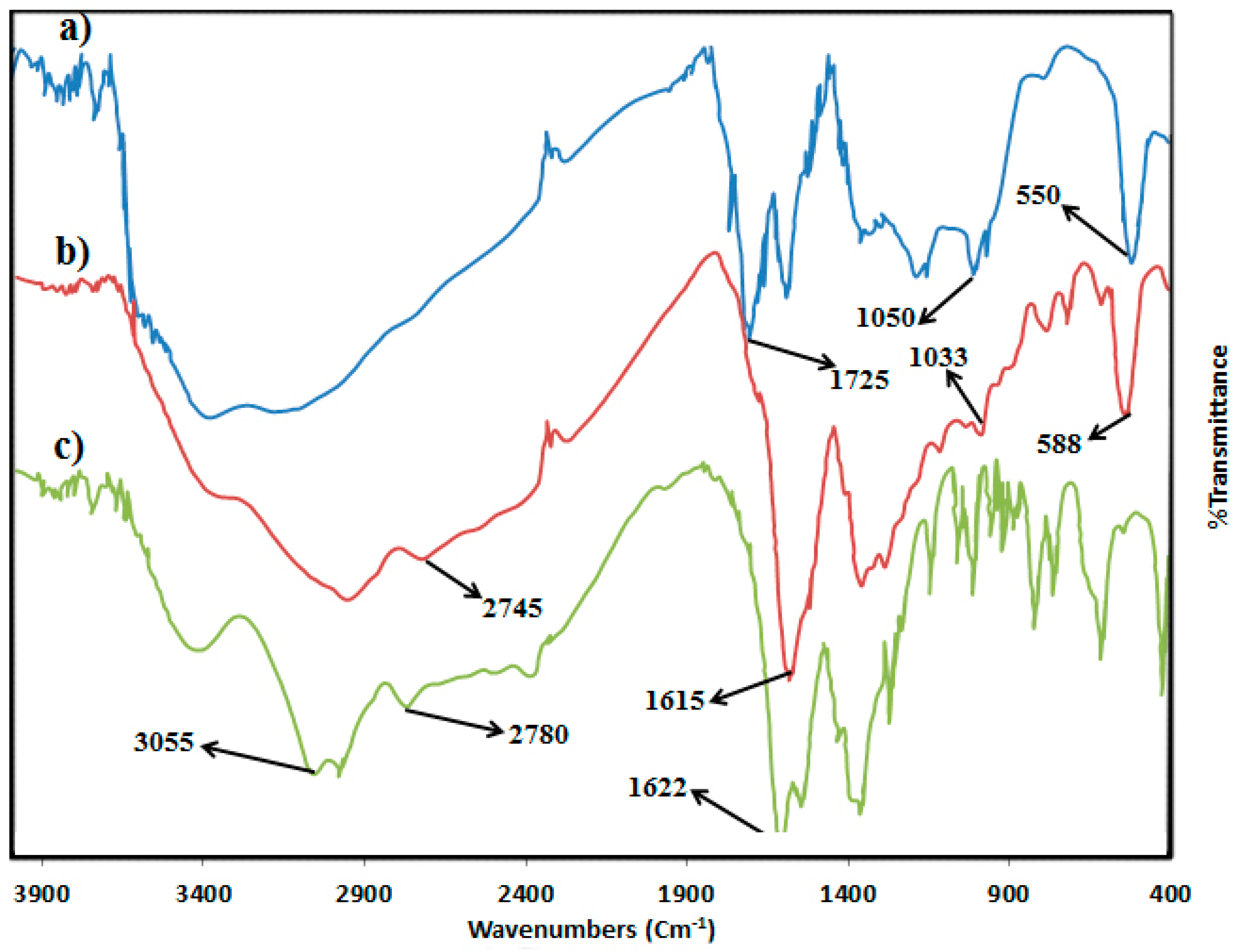
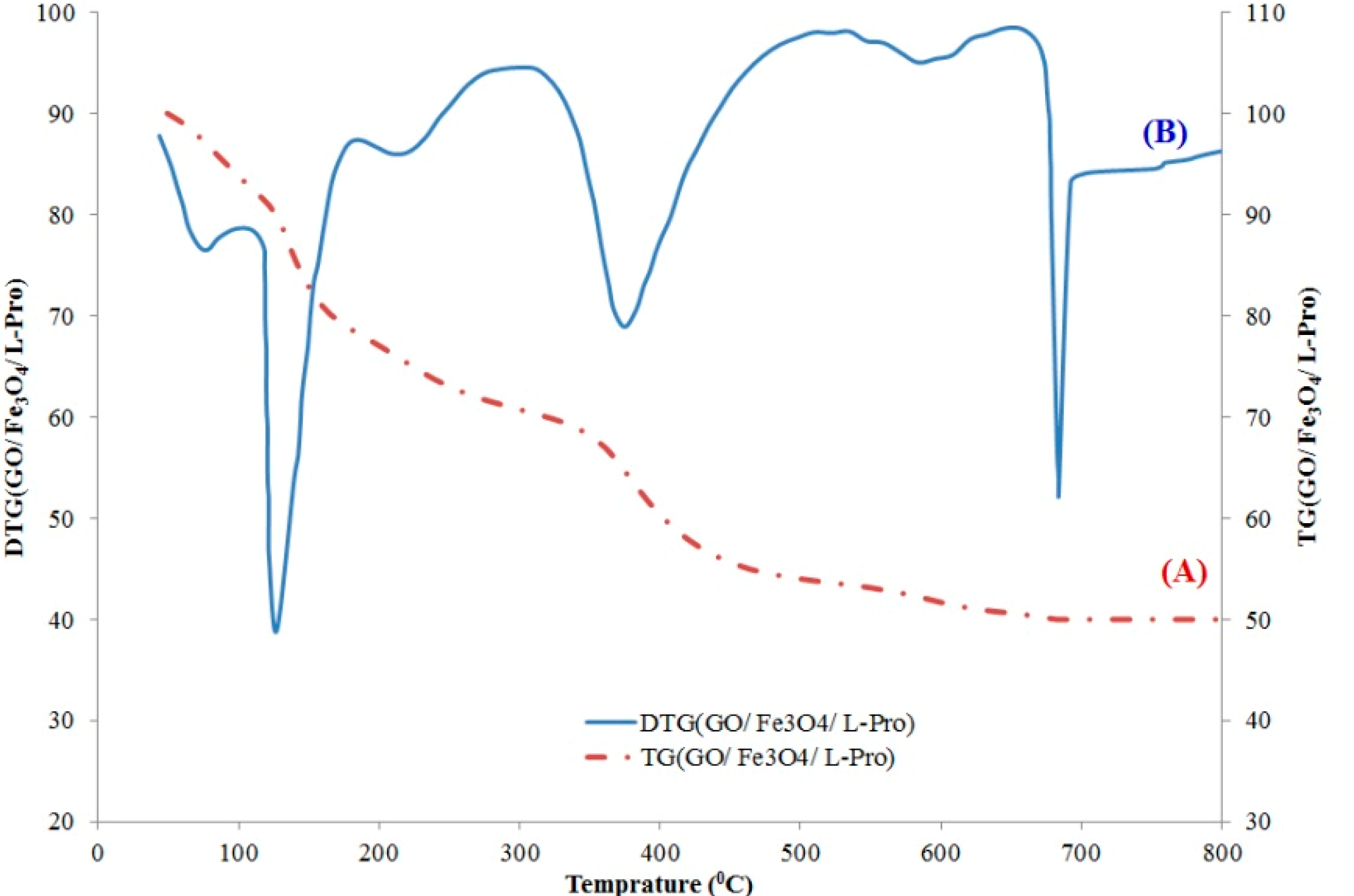
2.2.2. TGA and DTG Analysis
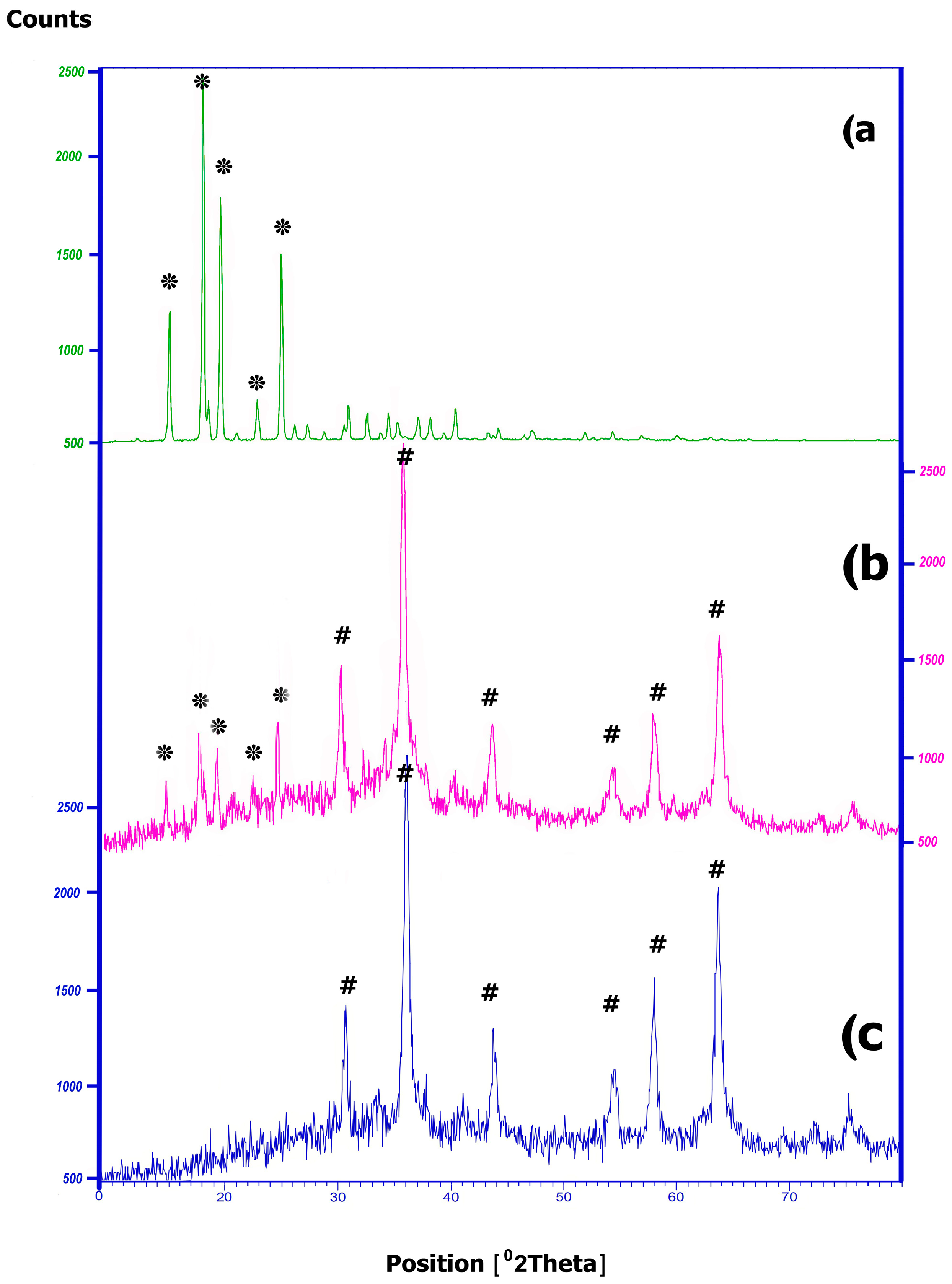
2.2.3. XRD
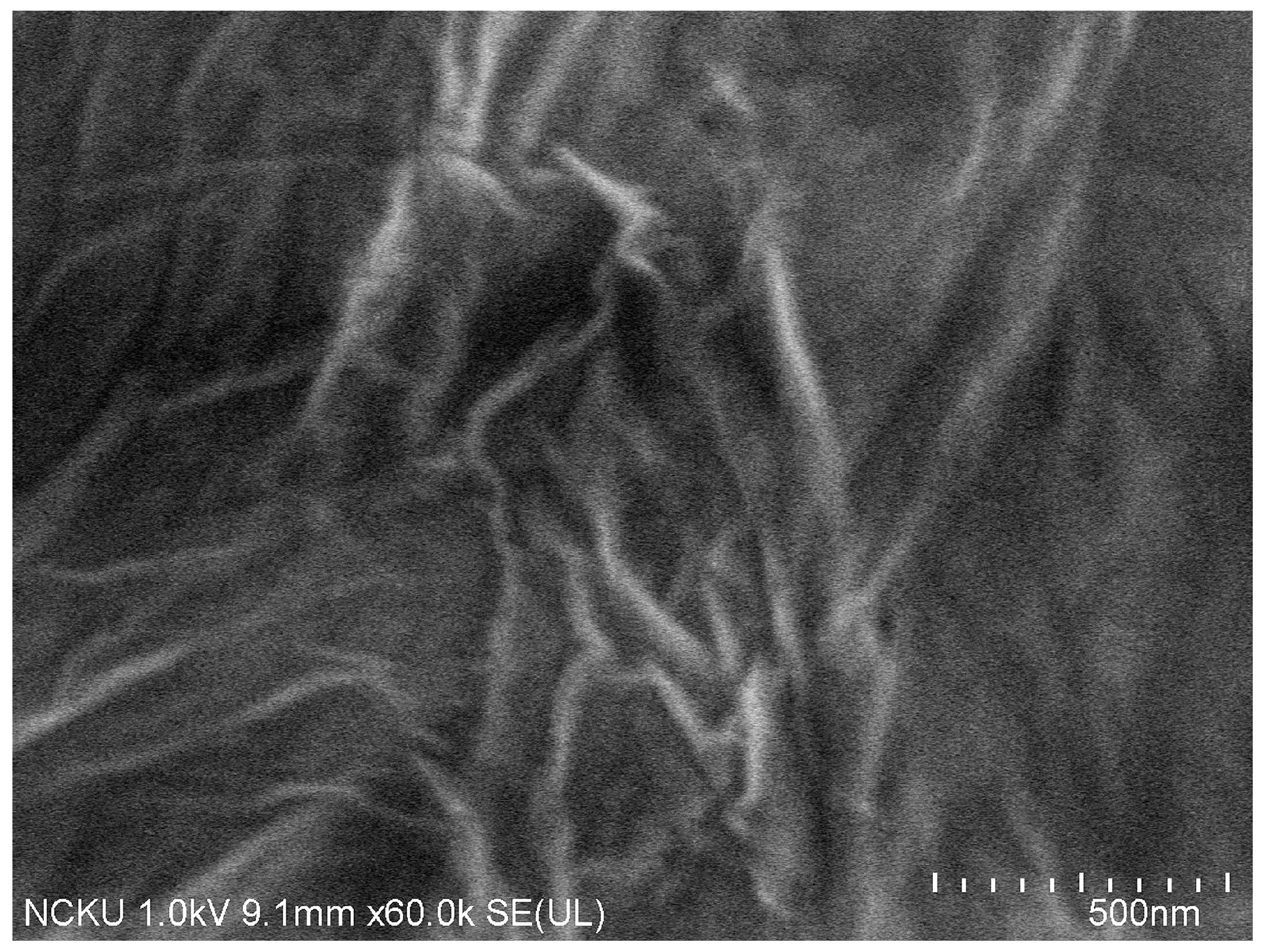
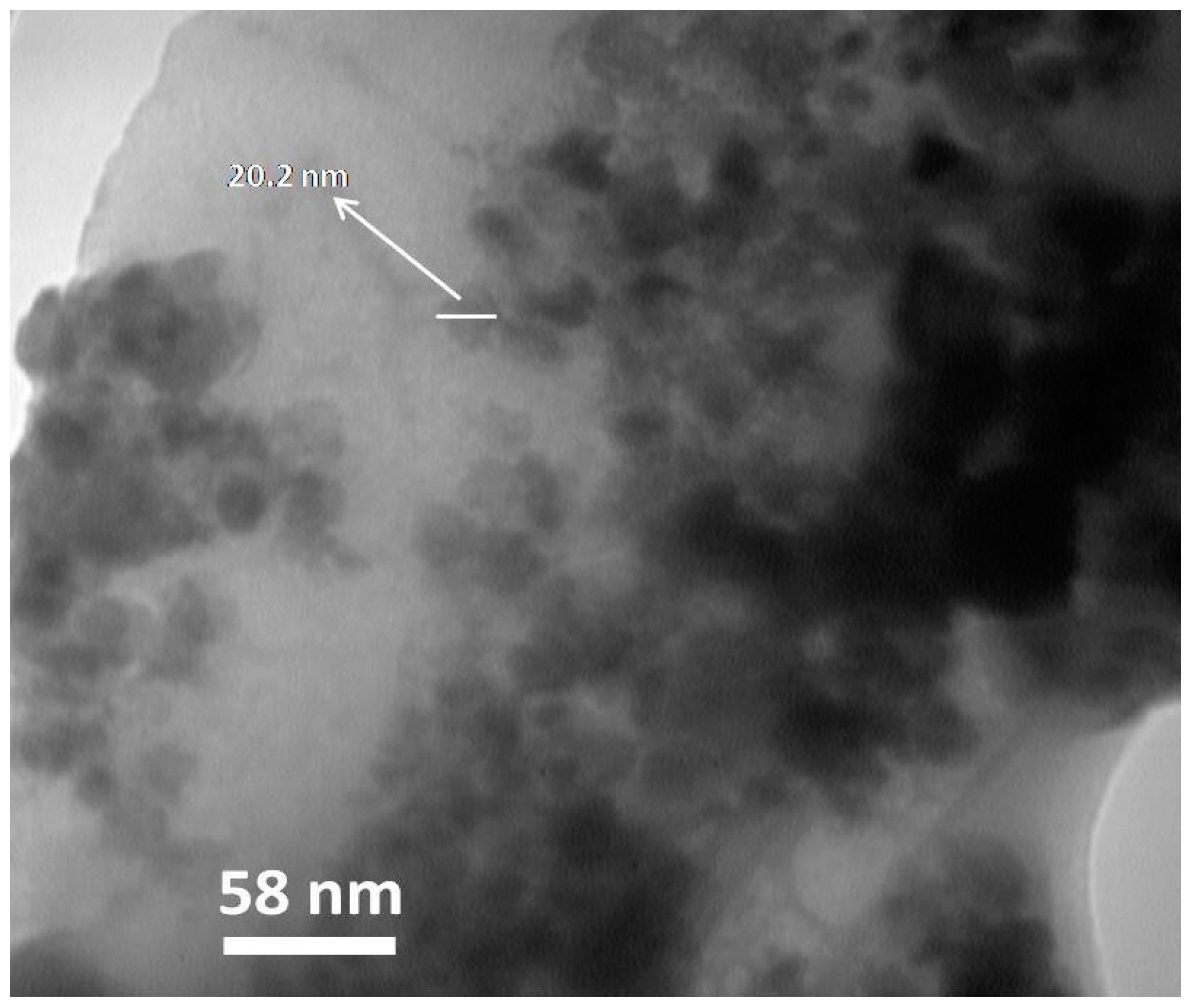
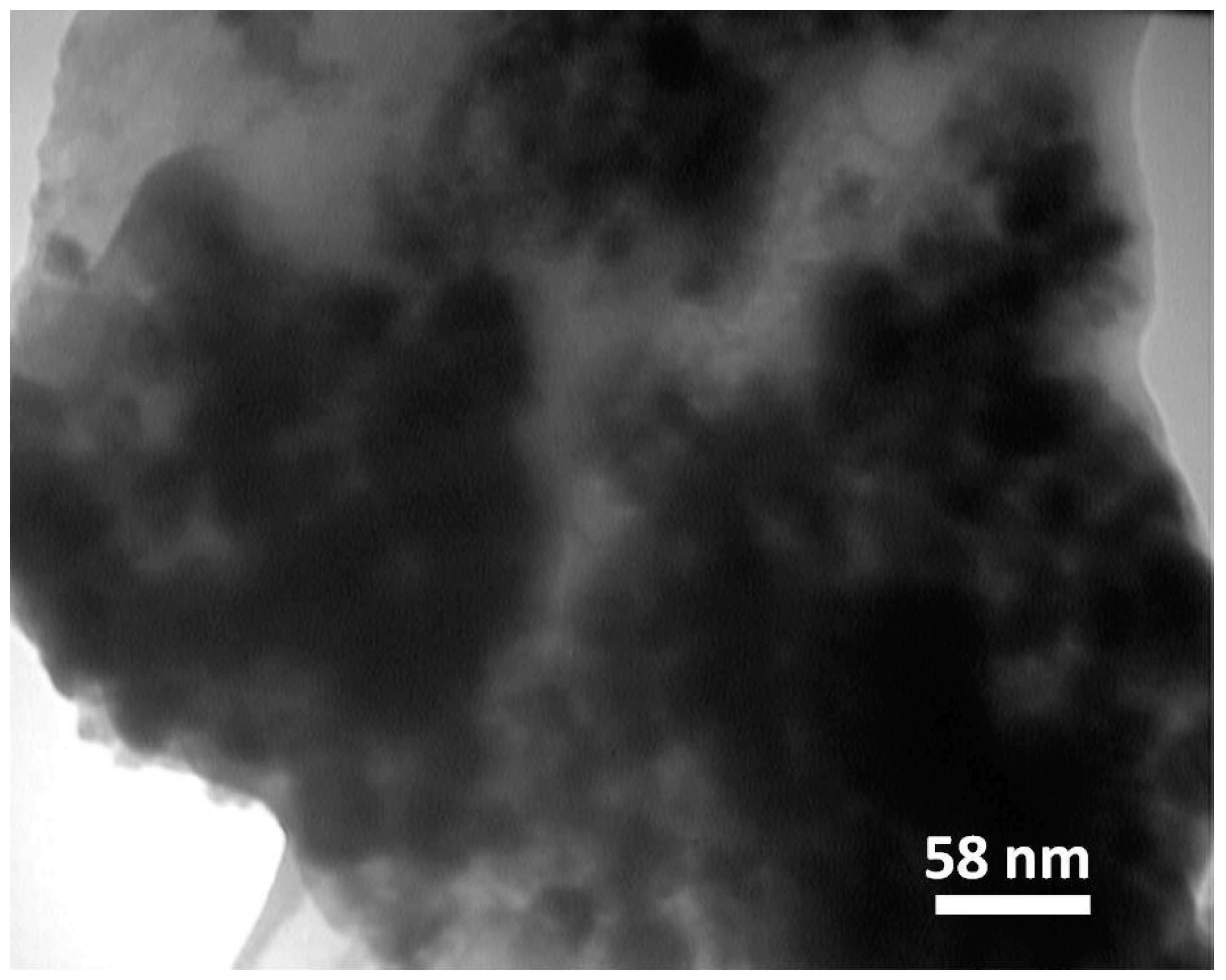
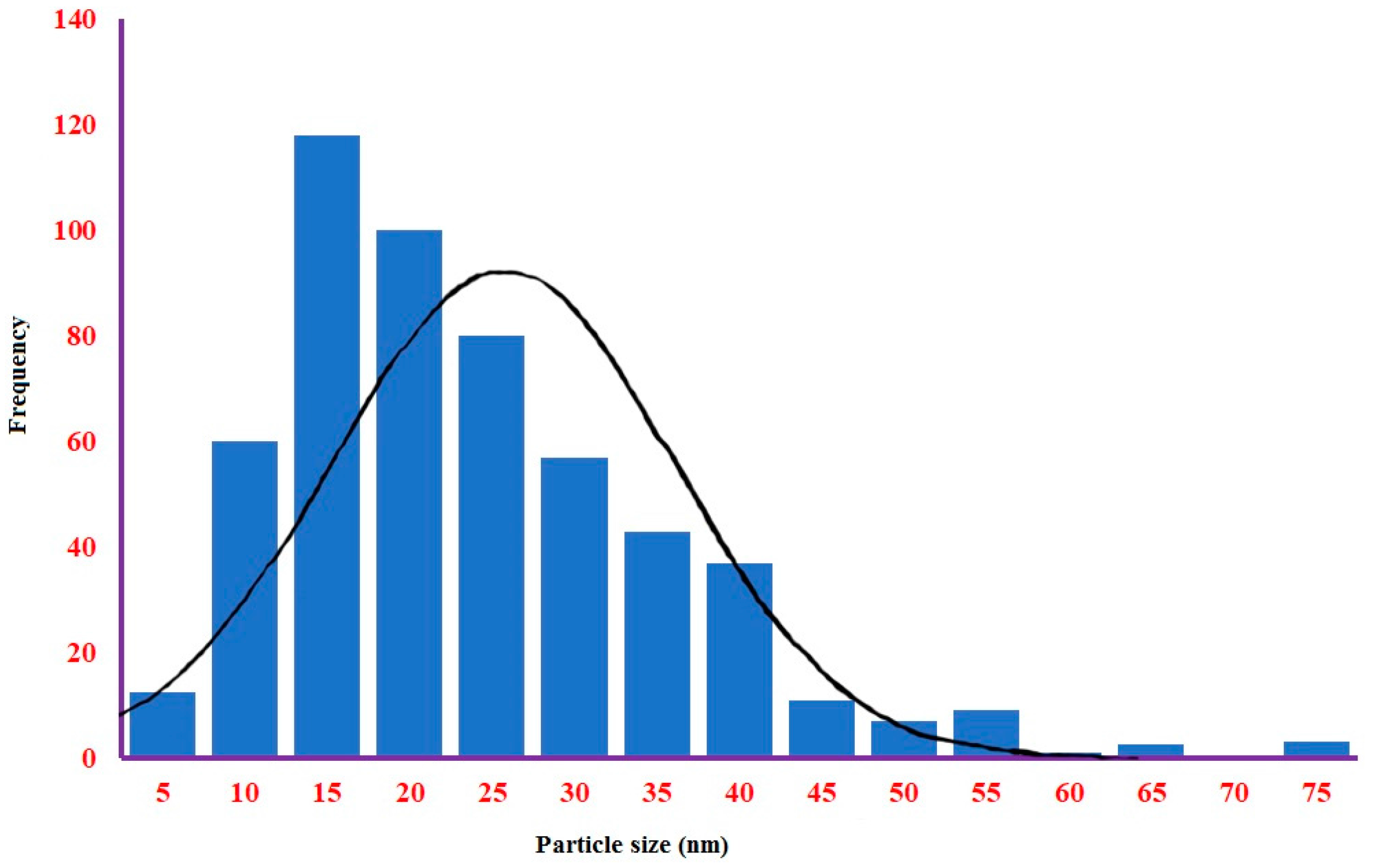
2.2.4. TEM and SEM
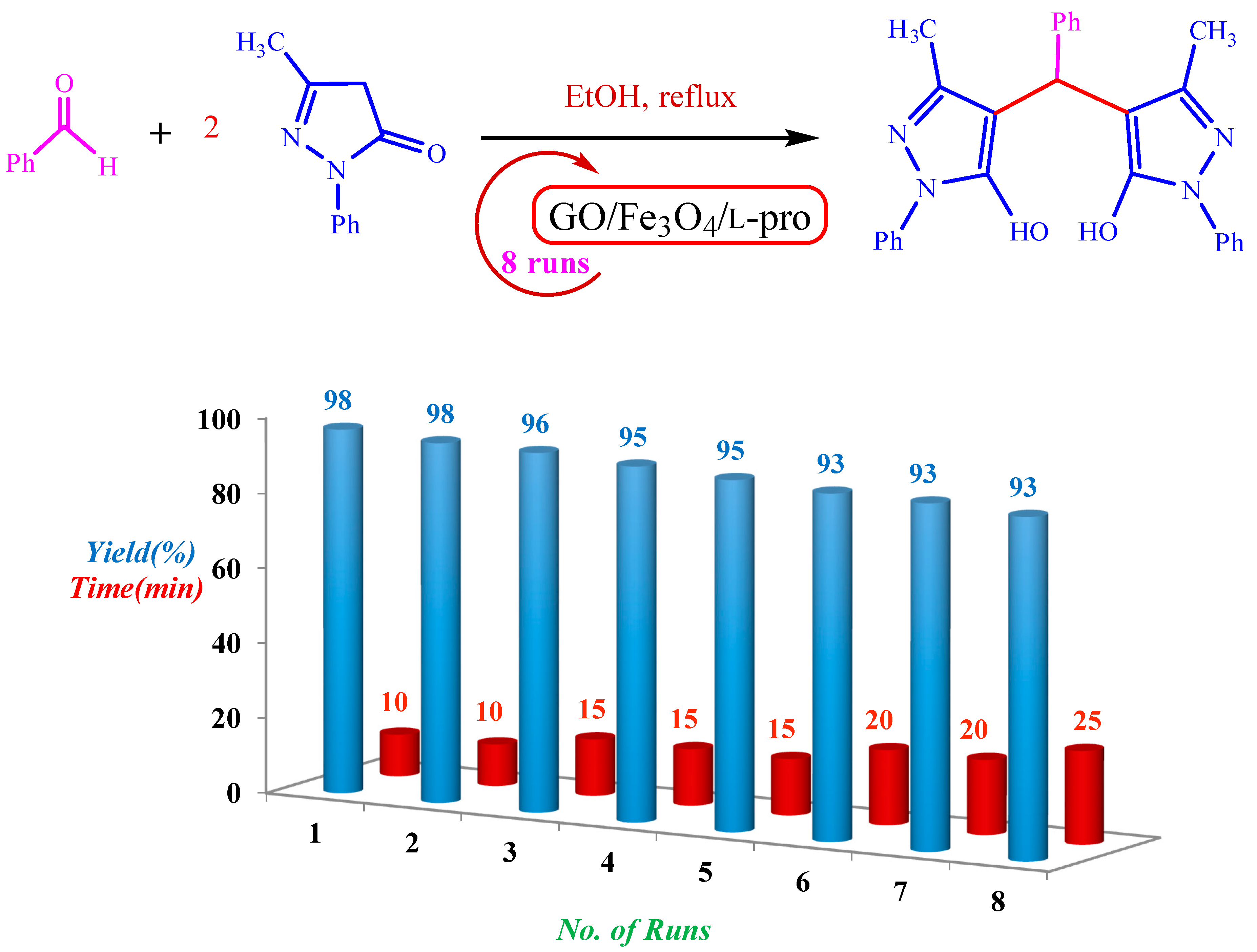
2.3. Optimization of the Reaction Conditions
3. Experimental
3.1. General Procedure for the Preparation of GO
3.2. Preparation of GO/Fe3O4 Nanocomposite
3.3. Preparation of GO/Fe3O4/l-Proline Nano Hybrid
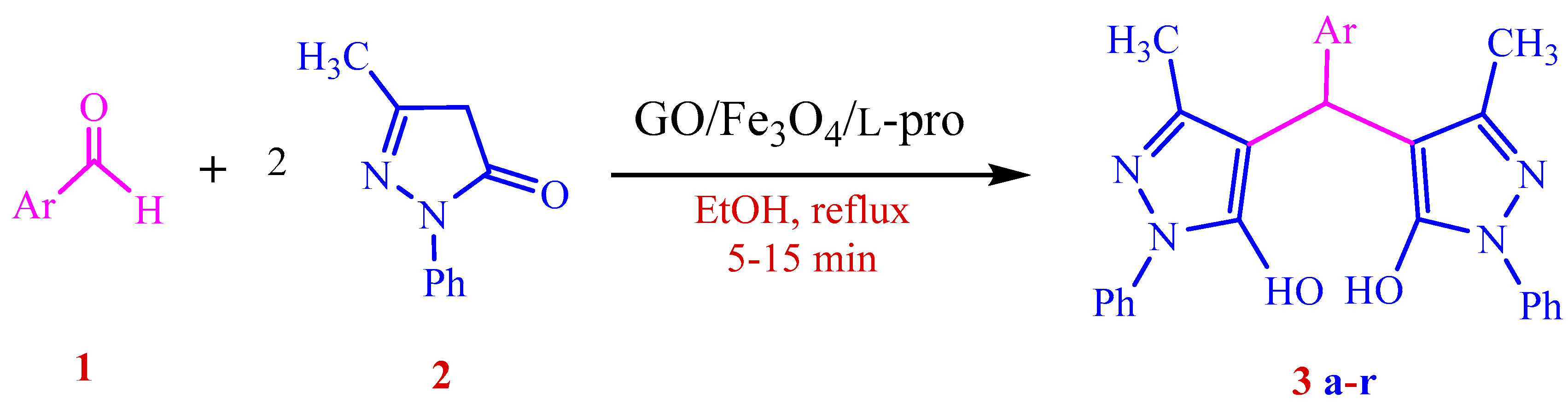
3.4. General Procedure for the GO/Fe3O4/l-Proline Nanohybrid Catalyzed Synthesis of 4,4′-(Arylmethylene)bis(1H-pyrazol-5-ol) Derivatives
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sharghi, H.; Beyzavi, M.H.; Safavi, A.; Doroodmand, M.M.; Khalifeh, R. Immobilization of porphyrinatocopper nanoparticles onto activated multi-walled carbon nanotubes and a study of its catalytic activity as an efficient heterogeneous catalyst for a click approach to the three-component synthesis of 1,2,3-triazoles in water. Adv. Synth. Catal. 2009, 351, 2391–2410. [Google Scholar] [CrossRef]
- Khodabakhshi, S.; Karami, B.; Eskandari, K.; Hoseini, S.J.; Rashidi, A. Graphene oxide nanosheets promoted regioselective and green synthesis of new dicoumarols. RSC Adv. 2014, 4, 17891–17895. [Google Scholar] [CrossRef]
- Su, C.; Loh, K.P. Carbocatalysts: Graphene oxide and its derivatives. Acc. Chem. Res. 2012, 46, 2275–2285. [Google Scholar] [CrossRef] [PubMed]
- Machado, B.F.; Serp, P. Graphene-based materials for catalysis. Catal. Sci. Technol. 2012, 2, 54–75. [Google Scholar] [CrossRef]
- Xu, Y.; Bai, H.; Lu, G.; Li, C.; Shi, G. Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets. J. Am. Chem. Soc. 2008, 130, 5856–5857. [Google Scholar] [CrossRef] [PubMed]
- Karousis, N.; Economopoulos, S.P.; Sarantopoulou, E.; Tagmatarchis, N. Porphyrin counter anion in imidazolium-modified graphene-oxide. Carbon 2010, 48, 854–860. [Google Scholar] [CrossRef]
- Petit, C.; Bandosz, T.J. Enhanced adsorption of ammonia on metal-organic framework/graphite oxide composites: Analysis of surface interactions. Adv. Funct. Mater. 2010, 20, 111–118. [Google Scholar] [CrossRef]
- Tan, R.; Li, C.; Luo, J.; Kong, Y.; Zheng, W.; Yin, D. An effective heterogeneous l-proline catalyst for the direct asymmetric aldol reaction using graphene oxide as support. J. Catal. 2013, 298, 138–147. [Google Scholar] [CrossRef]
- Nie, R.; Wang, J.; Wang, L.; Qin, Y.; Chen, P.; Hou, Z. Platinum supported on reduced graphene oxide as a catalyst for hydrogenation of nitroarenes. Carbon 2012, 50, 586–596. [Google Scholar] [CrossRef]
- Hu, X.; Mu, L.; Wen, J.; Zhou, Q. Covalently synthesized graphene oxide-aptamer nanosheets for efficient visible-light photocatalysis of nucleic acids and proteins of viruses. Carbon 2012, 50, 2772–2781. [Google Scholar] [CrossRef]
- Siamaki, A.R.; Abd El Rahman, S.K.; Abdelsayed, V.; El-Shall, M.S.; Gupton, B.F. Microwave-assisted synthesis of palladium nanoparticles supported on graphene: A highly active and recyclable catalyst for carbon–carbon cross-coupling reactions. J. Catal. 2011, 279, 1–11. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Liu, Z.; Ma, Y.; Huang, Y.; Chen, Y. High-efficiency loading and controlled release of doxorubicin hydrochloride on graphene oxide. J. Phys. Chem. C 2008, 112, 17554–17558. [Google Scholar] [CrossRef]
- Gupta, S.; Uhlmann, P.; Agrawal, M.; Chapuis, S.; Oertel, U.; Stamm, M. Immobilization of silver nanoparticles on responsive polymer brushes. Macromolecules 2008, 41, 2874–2879. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Wu, H.; Li, C. Silver nanocomposite layer-by-layer films based on assembled polyelectrolyte/dendrimer. J. Colloid Interface Sci. 2005, 287, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Caceres, R.; Dawson, C.; Formanek, P.; Fischer, D.; Simon, F.; Janke, A.; Uhlmann, P.; Stamm, M. Polymers as templates for Au and Au@Ag bimetallic nanorods: UV–vis and surface enhanced raman spectroscopy. Chem. Mater. 2013, 25, 158–169. [Google Scholar] [CrossRef]
- Li, J.; Liu, C.-Y.; Liu, Y. Au/graphene hydrogel: Synthesis, characterization and its use for catalytic reduction of 4-nitrophenol. J. Mater. Chem. 2012, 22, 8426–8430. [Google Scholar] [CrossRef]
- Shushizadeh, M.R.; Kiany, M. Solvent-free alkylation of dimethyl malonate using benzyl alcohols catalyzed by FeCl3/SiO2. Chin. Chem. Lett. 2009, 20, 1068–1072. [Google Scholar] [CrossRef]
- Shushizadeh, M.R.; Mostoufi, A.; Fakhrian, M. Marine sponge/cuo nanocrystal: A natural and efficient catalyst for sulfonamides synthesis. Jundishapur J. Nat. Pharm. Prod. 2012, 7, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Ahmady, A.Z.; Keshavarz, M.; Kardani, M.; Mohtasham, N. Cuo nanoparticles as an efficient catalyst for the synthesis of flavanones. Orient. J. Chem. 2015, 31, 1841–1846. [Google Scholar]
- Igder, S.; Kiasat, A.R.; Shushizadeh, M.R. Melamine supported on hydroxyapatite-encapsulated-γ-Fe2O3: A novel superparamagnetic recyclable basic nanocatalyst for the synthesis of 1,4-dihydropyridines and polyhydroquinolines. Res. Chem. Intermed. 2015, 41, 7227–7244. [Google Scholar] [CrossRef]
- Charisiou, N.; Siakavelas, G.; Papageridis, K.; Baklavaridis, A.; Tzounis, L.; Avraam, D.; Goula, M. Syngas production via the biogas dry reforming reaction over nickel supported on modified with CeO2 and/or La2O3 alumina catalysts. J. Nat. Gas Sci. Eng. 2016, 31, 164–183. [Google Scholar] [CrossRef]
- Beattie, C.; North, M.; Villuendas, P. Proline-catalysed amination reactions in cyclic carbonate solvents. Molecules 2011, 16, 3420–3432. [Google Scholar] [CrossRef] [PubMed]
- Kotrusz, P.; Toma, S. L-proline catalyzed michael additions of thiophenols to α, β-unsaturated compounds, particularly α-enones, in the ionic liquid [bmim] PF6. Molecules 2006, 11, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Verdía, P.; Santamarta, F.; Tojo, E. Knoevenagel reaction in [MMIm][MSO4]: Synthesis of coumarins. Molecules 2011, 16, 4379–4388. [Google Scholar]
- Martín-Rapún, R.; Fan, X.; Sayalero, S.; Bahramnejad, M.; Cuevas, F.; Pericàs, M.A. Highly active organocatalysts for asymmetric anti-mannich reactions. Chem.-A Eur. J. 2011, 17, 8780–8783. [Google Scholar] [CrossRef] [PubMed]
- Jimeno, C.; Sayalero, S.; Pericàs, M.A. Covalent heterogenization of asymmetric catalysts on polymers and nanoparticles. In Heterogenized Homogeneous Catalysts for Fine Chemicals Production; Springer: Dordrecht, The Netherlands, 2010; pp. 123–170. [Google Scholar]
- Keshavarz, M.; Iravani, N.; Azqhandi, M.H.A.; Nazari, S. Ion-pair immobilization of l-prolinate anion onto cationic polymer support and a study of its catalytic activity as an efficient heterogeneous catalyst for the synthesis of 2-amino-4H-chromene derivatives. Res. Chem. Intermed. 2016, 42, 4591–4604. [Google Scholar] [CrossRef]
- Keshavarz, M. Ion-pair immobilization of l-prolinate anion onto cationic polymer support and a study of its catalytic activity for one-pot synthesis of spiroindolones. J. Iran. Chem. Soc. 2016, 13, 553–561. [Google Scholar] [CrossRef]
- Keshavarz, M.; Iravani, N.; Ahmady, A.Z.; Vafaei-Nezhad, M. Efficient synthesis of 3,3-diaryloxindoles catalyzed by l-prolinate anion immobilized onto amberlite as a novel heterogeneous organocatalyst. J. Chin. Chem. Soc. 2015, 62, 1079–1086. [Google Scholar] [CrossRef]
- Keshavarz, M.; Vafaei-Nezhad, M. Design and characterization of l-prolinate-amberlite as a novel heterogeneous organocatalyst and its catalytic application in the synthesis of pyrazole derivatives. Catal. Lett. 2016, 146, 353–363. [Google Scholar] [CrossRef]
- Nazari, S.; Keshavarz, M. Amberlite-supported l-prolinate: A novel heterogeneous organocatalyst for the three-component synthesis of 4H-pyrano[2,3-c]pyrazole derivatives. Russ. J. Gen. Chem. 2017, 87, 539–545. [Google Scholar] [CrossRef]
- Amin, M.; Rakhisi, Z.; Ahmady, A.Z. Isolation and identification of bacillus species from soil and evaluation of their antibacterial properties. Avicenna J. Clin. Microbiol. Infect. 2015, 2, e23233. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; He, Z.; Hou, H.; Wang, X.; Wang, L. Architecture of Fe3O4–graphene oxide nanocomposite and its application as a platform for amino acid biosensing. Electrochim. Acta 2012, 71, 58–65. [Google Scholar] [CrossRef]
- An, Z.; Zhang, W.; Shi, H.; He, J. An effective heterogeneous l-proline catalyst for the asymmetric aldol reaction using anionic clays as intercalated support. J. Catal. 2006, 241, 319–327. [Google Scholar] [CrossRef]
- Maleki, B.; Eshghi, H.; Barghamadi, M.; Nasiri, N.; Khojastehnezhad, A.; Ashrafi, S.S.; Pourshiani, O. Silica-coated magnetic NiFe2O4 nanoparticles-supported H3PW12O40; synthesis, preparation, and application as an efficient, magnetic, green catalyst for one-pot synthesis of tetrahydrobenzo[b]pyran and pyrano[2,3-c]pyrazole derivatives. Res. Chem. Intermed. 2016, 42, 3071–3093. [Google Scholar] [CrossRef]
- Sadeghi, B.; Ghorbani Rad, M. Synthesis of 4,4′-(arylmethylene) bis (1H-pyrazol-5-ols) catalyzed by nanosilica supported perchloric acid in water. Iran. J. Catal. 2014, 4, 67–70. [Google Scholar]
- Niknam, K.; Habibabad, M.S.; Deris, A.; Aeinjamshid, N. Preparation of silica-bonded n-propyltriethylenetetramine as a recyclable solid base catalyst for the synthesis of 4,4′-(arylmethylene) bis (1H-pyrazol-5-ols). Monatshefte Chem.-Chem. Mon. 2013, 144, 987–992. [Google Scholar] [CrossRef]
- Moosavi-Zare, A.R.; Zolfigol, M.A.; Zarei, M.; Zare, A.; Khakyzadeh, V.; Hasaninejad, A. Design, characterization and application of new ionic liquid 1-sulfopyridinium chloride as an efficient catalyst for tandem knoevenagel–michael reaction of 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one with aldehydes. Appl. Catal. A Gen. 2013, 467, 61–68. [Google Scholar] [CrossRef]
- Phatangare, K.R.; Padalkar, V.S.; Gupta, V.D.; Patil, V.S.; Umape, P.G.; Sekar, N. Phosphomolybdic acid: An efficient and recyclable solid acid catalyst for the synthesis of 4,4′-(arylmethylene) bis (1H-pyrazol-5-ols). Synth. Commun. 2012, 42, 1349–1358. [Google Scholar] [CrossRef]
- Sobhani, S.; Hasaninejad, A.-R.; Maleki, M.F.; Parizi, Z.P. Tandem knoevenagel–michael reaction of 1-phenyl-3-methyl-5-pyrazolone with aldehydes using 3-aminopropylated silica gel as an efficient and reusable heterogeneous catalyst. Synth. Commun. 2012, 42, 2245–2255. [Google Scholar] [CrossRef]
- Hasaninejad, A.; Shekouhy, M.; Zare, A.; Ghattali, S.H.; Golzar, N. PEG-SO3H as a new, highly efficient and homogeneous polymeric catalyst for the synthesis of bis (indolyl) methanes and 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)s in water. J. Iran. Chem. Soc. 2011, 8, 411–423. [Google Scholar] [CrossRef]
- Gouda, M.A.; Abu-Hashem, A.A. An eco-friendly procedure for the efficient synthesis of arylidinemalononitriles and 4,4′-(arylmethylene) bis (3-methyl-1-phenyl-1H-pyrazol-5-ols) in aqueous media. Green Chem. Lett. Rev. 2012, 5, 203–209. [Google Scholar] [CrossRef]
- Khazaei, A.; Zolfigol, M.A.; Moosavi-Zare, A.R.; Asgari, Z.; Shekouhy, M.; Zare, A.; Hasaninejad, A. Preparation of 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)s over 1,3-disulfonic acid imidazolium tetrachloroaluminate as a novel catalyst. RSC Adv. 2012, 2, 8010–8013. [Google Scholar] [CrossRef]
- Tayebi, S.; Baghernejad, M.; Saberi, D.; Niknam, K. Sulfuric acid ([3-(3-silicapropyl)sulfanyl]propyl) ester as a recyclable catalyst for the synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ols). Chin. J. Catal. 2011, 32, 1477–1483. [Google Scholar] [CrossRef]
- Niknam, K.; Saberi, D.; Sadegheyan, M.; Deris, A. Silica-bonded s-sulfonic acid: An efficient and recyclable solid acid catalyst for the synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ols). Tetrahedron Lett. 2010, 51, 692–694. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 3a–3r are available from the authors. |
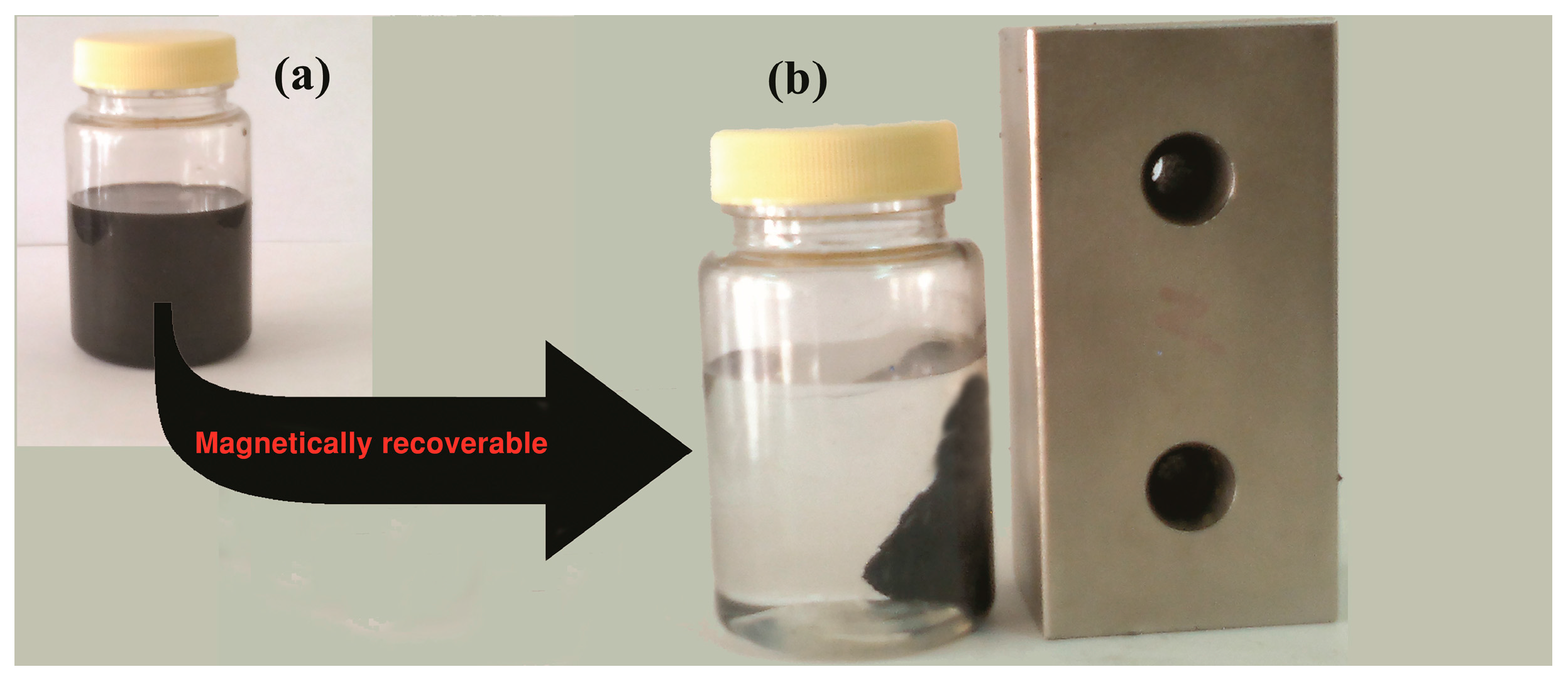
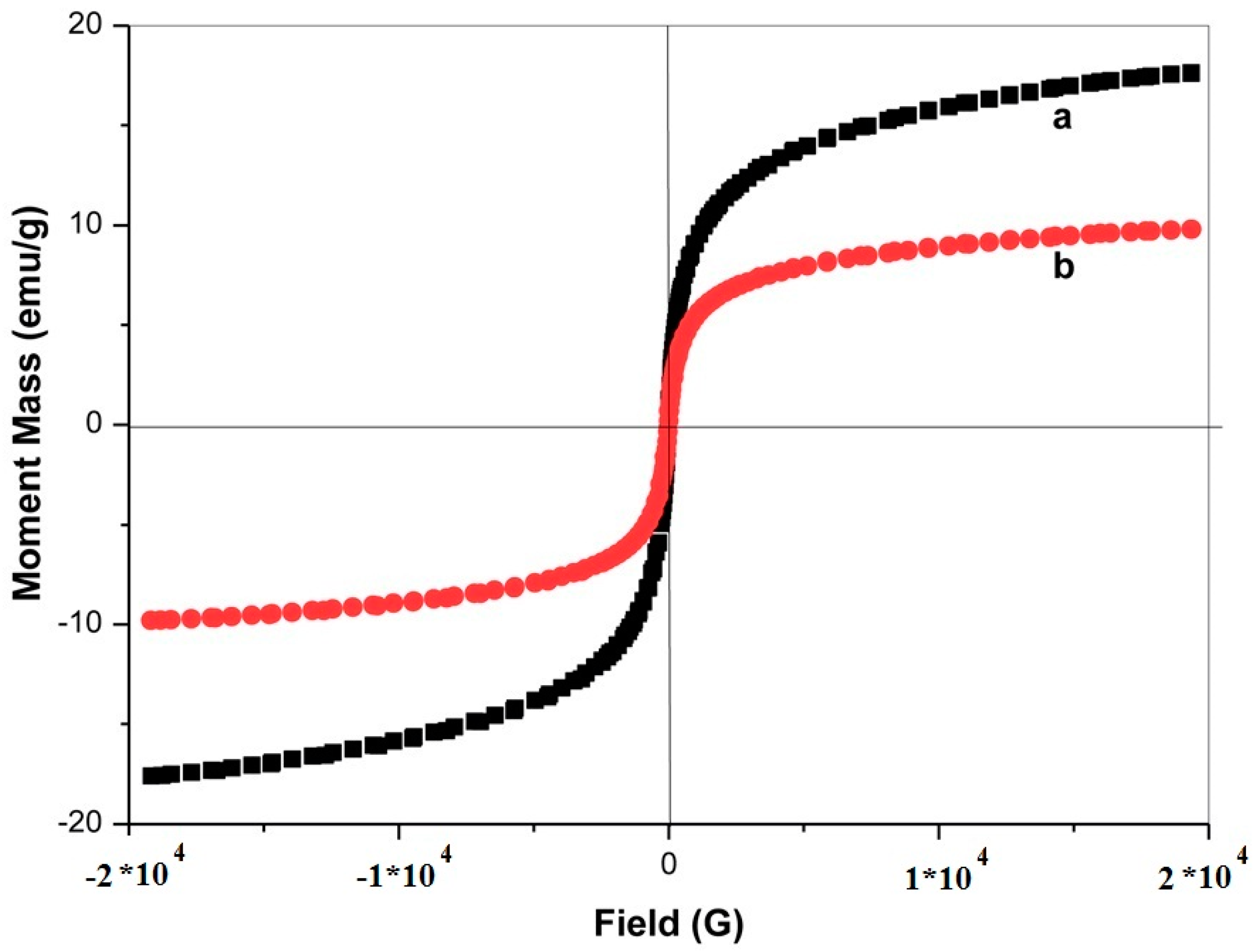
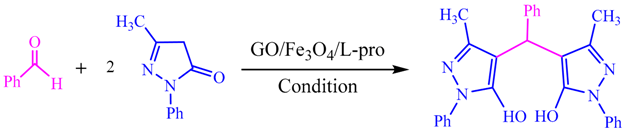
| Entry | Conditions | Temperature (°C) | GO/Fe3O4/l-pro (g) | Time (min) | Yield (%) a |
|---|---|---|---|---|---|
| 1 | neat | 100 | 0.02 | 40 | 60 |
| 2 | CH2Cl2 | reflux | 0.02 | 45 | 65 |
| 3 | CH3CN | reflux | 0.02 | 40 | 55 |
| 4 | THF | 65 | 0.02 | 45 | 60 |
| 5 | DMF | 100 | 0.02 | 45 | 65 |
| 6 | H2O/DMF | 100 | 0.02 | 20 | 75 |
| 7 | H2O | reflux | 0.02 | 25 | 80 |
| 8 | CH3CH2OH | reflux | - | 120 | 40 |
| 9 | CH3CH2OH | reflux | 0.02 | 20 | 92 |
| 10 | CH3CH2OH | reflux | 0.05 | 10 | 98 |
| 11 | CH3CH2OH | reflux | 0.1 | 10 | 95 |
| Entry | Ar | Product | Time (min) | Yield a (%) | m.p. (°C) | |
|---|---|---|---|---|---|---|
| Reported | Found | |||||
| 1 | C6H5- | 3a | 10 | 98 | 171–172 | 170–172 |
| 2 | 4-Me-C6H4- | 3b | 13 | 94 | 202–204 | 203–205 |
| 3 | 4-Cl-C6H4- | 3c | 8 | 93 | 213–215 | 213–215 |
| 4 | 2-Cl-C6H4- | 3d | 10 | 91 | 236–237 | 233–235 |
| 5 | 2,4-(Cl)2-C6H3- | 3e | 10 | 94 | 227–229 | 227–229 |
| 6 | 4-NO2-C6H4- | 3f | 5 | 96 | 224–226 | 225–227 |
| 7 | 3-NO2-C6H4- | 3g | 8 | 90 | 149–150 | 152–154 |
| 8 | 4-OH-C6H4- | 3h | 15 | 92 | 152–153 | 153–155 |
| 9 | 3-OH-C6H4- | 3i | 14 | 87 | 165–168 | 169–170 |
| 10 | 3,4-(MeO)2-C6H3- | 3j | 7 | 90 | 195–197 | 194–196 |
| 11 | 4-MeS-C6H4- | 3k | 15 | 89 | 201–203 | 205–207 |
| 12 | 4-CN-C6H4- | 3l | 15 | 95 | 210–212 | 210–212 |
| 13 | 2-OH-C6H4- | 3m | 15 | 89 | 230–231 | 232–234 |
| 14 | 4-F-C6H4- | 3n | 13 | 93 | 180–182 | 180–182 |
| 15 | 4-iPr-C6H4- | 3o | 12 | 87 | 132–134 | 132–134 |
| 16 | 4-MeO-C6H4- | 3p | 10 | 91 | 172–174 | 173–175 |
| 17 | 2-Br-C6H4- | 3q | 8 | 94 | 198–200 | 198–200 |
| 18 | 3-Br-C6H4- | 3r | 5 | 89 | 173–175 | 170–173 |
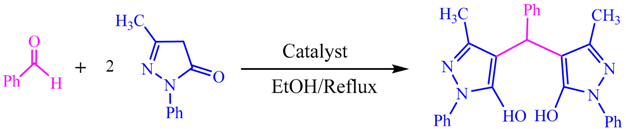
| Entry | Catalyst | Time (min) | Yield (%) |
|---|---|---|---|
| 1 | GO/Fe3O4/l-pro (0.05 g) | 10 | 98 |
| 2 | GO (0.05 g) | 120 | 70 |
| 3 | Fe3O4 nanoparticle (0.05 g) | 90 | 80 |
| 4 | l-Proline (0.01 g) | 45 | 90 |
| 5 | GO/Fe3O4 (0.05 g) | 40 | 89 |
| Entry | Catalyst | Condition | Time (min) | Yield (%) | Ref. |
|---|---|---|---|---|---|
| 1 | GO/Fe3O4/l-pro (0.05 g) | EtOH/reflux | 10 | 98 | This work |
| 2 | [Amb]l-prolinate (10 mol %) | EtOH/reflux | 11 | 97 | [30] |
| 3 | NiFe2O4@SiO2–H3PW12O40 | EtOH/reflux | 15 | 91 | [37] |
| 4 | Nano–SiO2/HClO4 | H2O/reflux | 20 | 94 | [38] |
| 5 | SBNPTT | EtOH/reflux | 30 | 90 | [39] |
| 6 | [Pyridine–SO3H]Cl (1 mol %) | Solvent-free /50 °C | 11 | 89 | [40] |
| 7 | Phosphomolybdic acid | EtOH/r.t. | 240 | 96 | [41] |
| 8 | AP-SiO2 (30 mol %) | CH3CN/r.t. | 10 | 98 | [42] |
| 9 | PEG-SO3H (1.5 mol %) | H2O/reflux | 30 | 92 | [43] |
| 10 | LiOH·H2O (10 mol %) | H2O/90 °C | 75 | 80 | [44] |
| 11 | [Dsim]AlCl4 (1 mol %) | Solvent-free/50 °C | 60 | 86 | [45] |
| 12 | SASPSPE ( 0.1 g) | EtOH/reflux | 18 | 90 | [46] |
| 13 | SBSSA (18 mol %) | EtOH/reflux | 120 | 80 | [47] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keshavarz, M.; Zarei Ahmady, A.; Vaccaro, L.; Kardani, M. Non-Covalent Supported of l-Proline on Graphene Oxide/Fe3O4 Nanocomposite: A Novel, Highly Efficient and Superparamagnetically Separable Catalyst for the Synthesis of Bis-Pyrazole Derivatives. Molecules 2018, 23, 330. https://doi.org/10.3390/molecules23020330
Keshavarz M, Zarei Ahmady A, Vaccaro L, Kardani M. Non-Covalent Supported of l-Proline on Graphene Oxide/Fe3O4 Nanocomposite: A Novel, Highly Efficient and Superparamagnetically Separable Catalyst for the Synthesis of Bis-Pyrazole Derivatives. Molecules. 2018; 23(2):330. https://doi.org/10.3390/molecules23020330
Chicago/Turabian StyleKeshavarz, Mosadegh, Amanollah Zarei Ahmady, Luigi Vaccaro, and Maryam Kardani. 2018. "Non-Covalent Supported of l-Proline on Graphene Oxide/Fe3O4 Nanocomposite: A Novel, Highly Efficient and Superparamagnetically Separable Catalyst for the Synthesis of Bis-Pyrazole Derivatives" Molecules 23, no. 2: 330. https://doi.org/10.3390/molecules23020330