Immunomodulatory Activity of Octenyl Succinic Anhydride Modified Porang (Amorphophallus oncophyllus) Glucomannan on Mouse Macrophage-Like J774.1 Cells and Mouse Primary Peritoneal Macrophages
Abstract
:1. Introduction
2. Results and Discussion
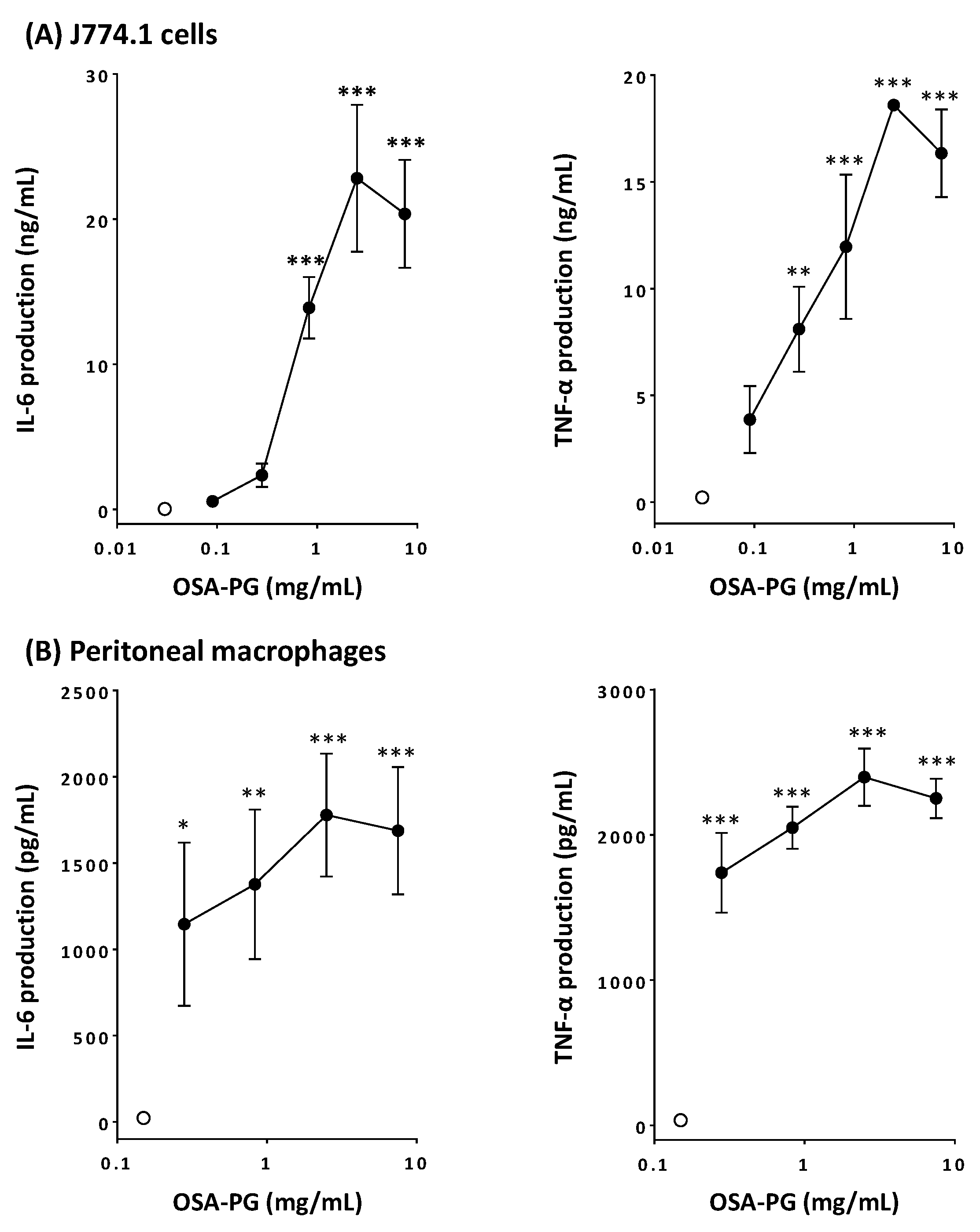
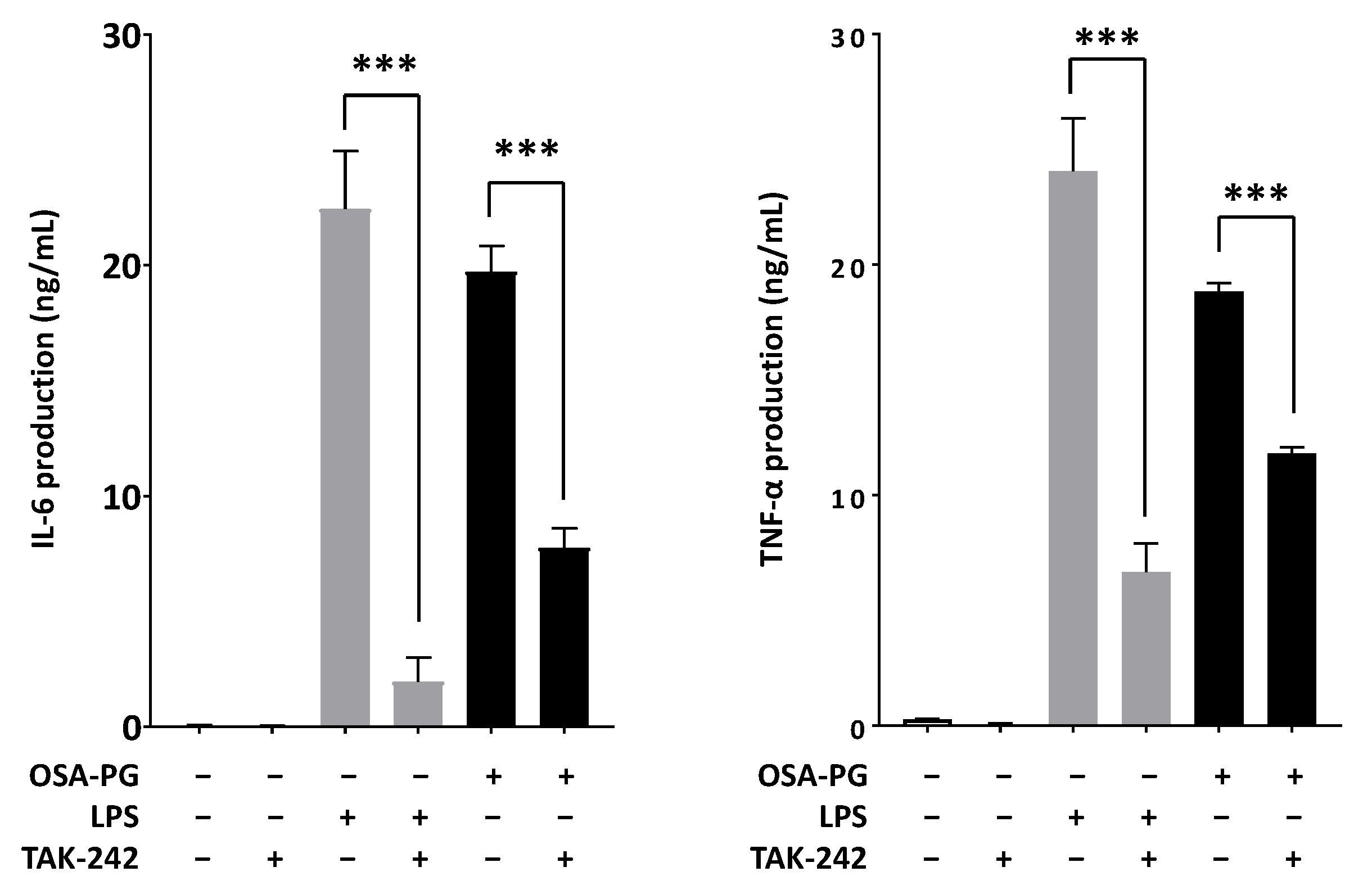
2.1. Effect of OSA-PG on Cytokine Production by J774.1 Cells and Peritoneal Macrophages
2.2. Effect of OSA-PG on Transcription of Cytokine Genes in J774.1 Cells and Peritoneal Macrophages
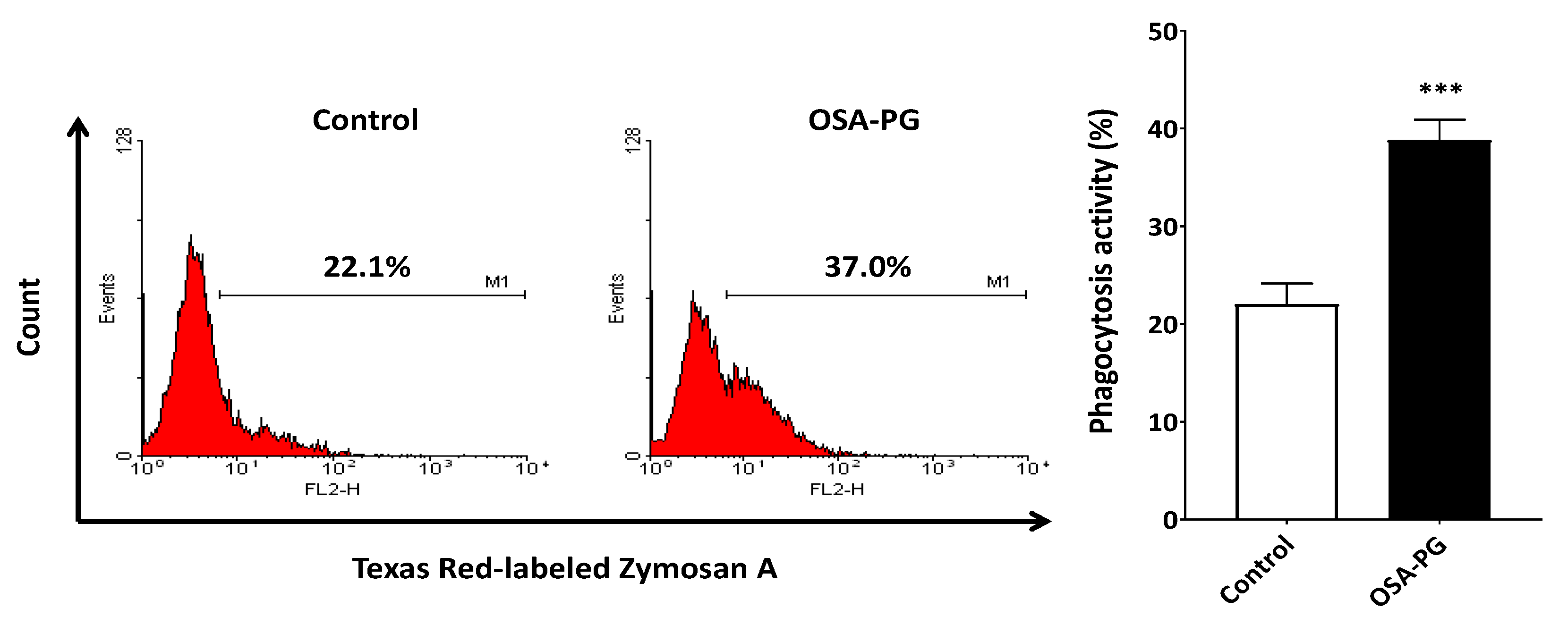
2.3. Effect of OSA-PG on Phagocytosis Activity of J774.1 Cells
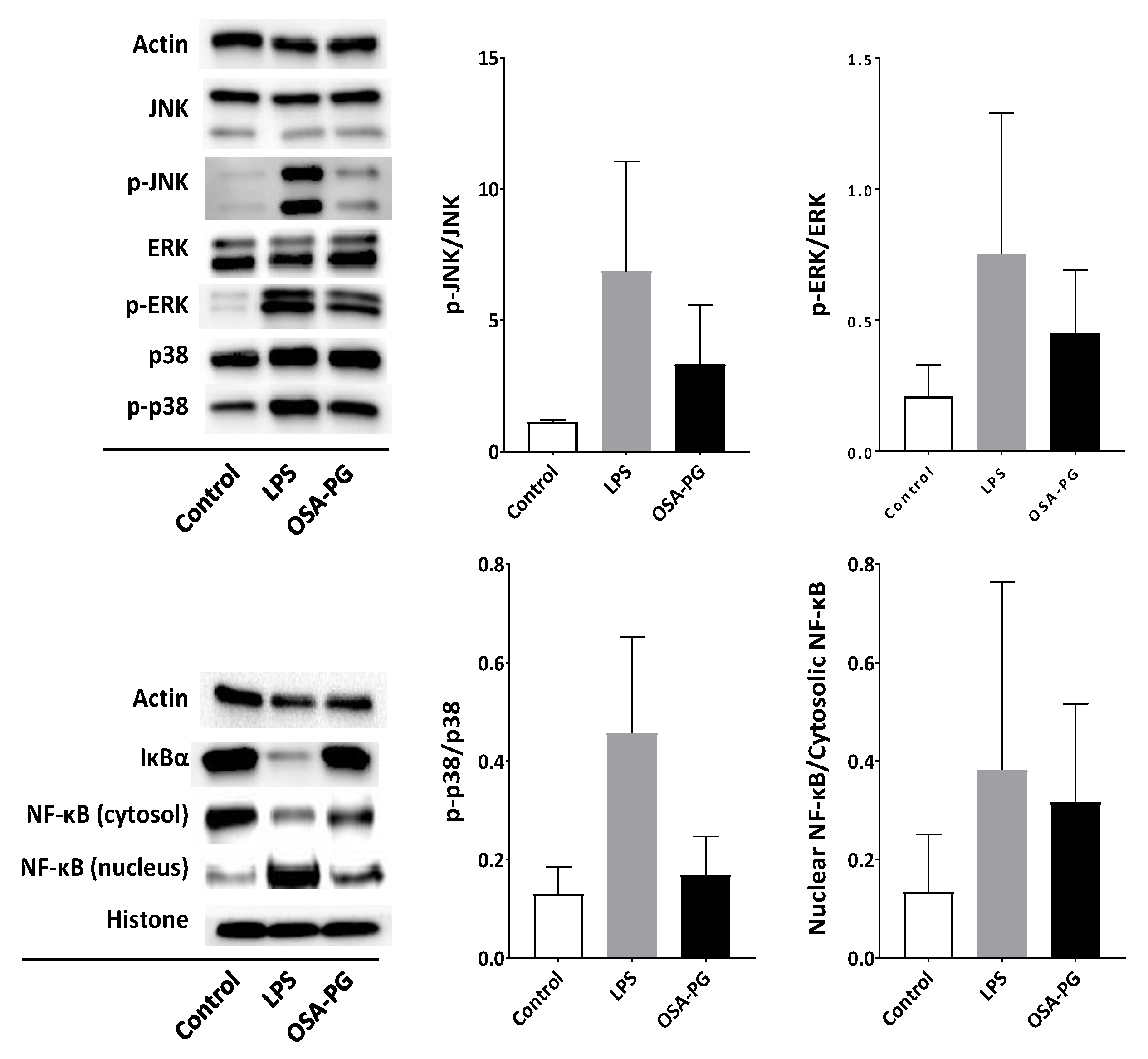
2.4. Effect of OSA-PG on Nuclear Factor-κB (NF-κB) and MAPK Signaling Cascades
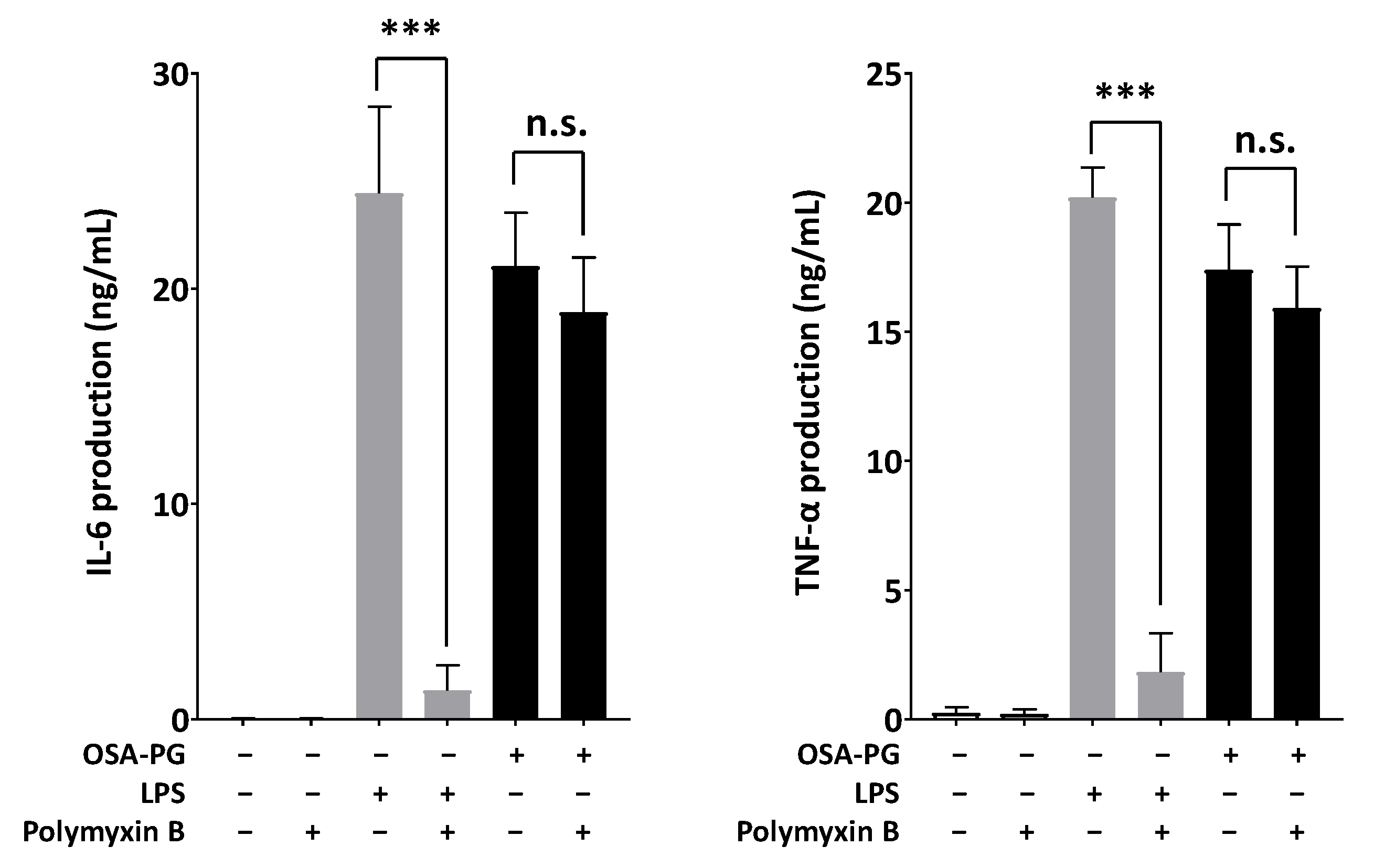
2.5. Effect of Polymyxin B-Treated OSA-PG on Cytokine Production
2.6. Effect of OSA-PG on TLR4
3. Materials and Methods
3.1. Reagents
3.2. Cells and Cell Culture
3.3. Preparation of OSA-PG
3.4. Cytokine Production Assay
3.5. Real-Time RT-PCR
3.6. Phagocytosis Activity
3.7. Immunoblot Analysis
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Harmayani, E.; Aprilia, V.; Marsono, Y. Characterization of glucomannan from Amorphophallus oncophyllus and its prebiotic activity in vivo. Carbohydr. Polym. 2014, 112, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Yanuriati, A.; Marseno, D.W.; Rochmadi; Harmayani, E. Characteristics of glucomannan isolated from fresh tuber of Porang (Amorphophallus muelleri Blume). Carbohydr. Polym. 2017, 156, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghazzewi, F.H.; Khanna, S.; Tester, R.F.; Piggott, J. The potential use of hydrolysed konjac glucomannan as a prebiotic. J. Sci. Food Agric. 2007, 87, 1758–1766. [Google Scholar] [CrossRef]
- Chua, M.; Baldwin, T.C.; Hocking, T.J.; Chan, K. Traditional uses and potential health benefits of Amorphophallus konjac K. Koch ex N.E.Br. J. Ethnopharmacol. 2010, 128, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Imeson, A. Food Stabilisers, Thickeners and Gelling Agents, 1st ed.; Wiley-Blackwell: Oxford, UK, 2009. [Google Scholar]
- Mikkonen, K.S.; Tenkanen, M.; Cooke, P.; Xu, C.; Rita, H.; Willför, S.; Holmbom, B.; Hicks, K.B.; Yadav, M.P. Mannans as stabilizers of oil-in-water beverage emulsions. LWT-Food Sci. Technol. 2009, 42, 849–855. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Xie, B.J.; Gan, X. Advance in the applications of konjac glucomannan and its derivatives. Carbohydr. Polym. 2005, 60, 27–31. [Google Scholar] [CrossRef]
- Meng, F.; Zheng, L.; Wang, Y.; Liang, Y.; Zhong, G. Preparation and properties of konjac glucomannan octenyl succinate modified by microwave method. Food Hydrocoll. 2014, 38, 205–210. [Google Scholar] [CrossRef]
- Sarkar, S.; Singhal, R.S. Esterification of guar gum hydrolysate and gum Arabic with n-octenyl succinic anhydride and oleic acid and its evaluation as wall material in microencapsulation. Carbohydr. Polym. 2014, 86, 1723–1731. [Google Scholar] [CrossRef]
- Sweedman, M.C.; Tizzotti, M.J.; Schäfer, C.; Gilbert, R.G. Structure and physicochemical properties of octenyl succinic anhydride modified starches: A review. Carbohydr. Polym. 2013, 92, 905–920. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Fu, X.; Luo, Z.G. Esterification of sugar beet pectin using octenyl succinic anhydride and its effect as an emulsion stabilizer. Food Hydrocoll. 2015, 49, 53–60. [Google Scholar] [CrossRef]
- Cheng, J.H.; Hu, Y.N.; Luo, Z.G.; Chen, W.; Chen, H.M.; Peng, X.C. Preparation and properties of octenyl succinate β-cyclodextrin and its application as an emulsion stabilizer. Food Chem. 2017, 218, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Tester, R.F.; Al-Ghazzewi, F.H. Beneficial health characteristics of native and hydrolysed konjac (Amorphophallus konjac) glucomannan. J. Sci. Food Agric. 2016, 96, 3283–3291. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xia, J.; Wang, Y.; Xie, B. Structure characterization and its antiobesity of ball-milled konjac flour. Eur. Food Res. Technol. 2005, 221, 814–820. [Google Scholar] [CrossRef]
- Chen, H.L.; Sheu, W.H.H.; Tai, T.S.; Liaw, Y.P.; Chen, Y.C. Konjac supplement alleviated hypercholesterolemia and hyperglycemia in type 2 diabetic subjects—A randomized double-blind trial. J. Am. Coll. Nutr. 2003, 22, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghazzewi, F.H.; Tester, R.F. Efficacy of cellulase and mannanase hydrolysates of konjac glucomannan to promote the growth of lactic acid bacteria. J. Sci. Food Agric. 2012, 92, 2394–2396. [Google Scholar] [CrossRef] [PubMed]
- Onishi, N.; Kawamoto, S.; Suzuki, H.; Santo, H.; Aki, T.; Shigeta, S.; Hashimoto, K.; Hide, M.; Ono, K. Dietary pulverized konjac glucomannan suppresses scratching behavior and skin inflammatory immune responses in NC/Nga mice. Int. Arch. Allergy Immunol. 2007, 144, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Oomizu, S.; Yanase, Y.; Onishi, N.; Uchida, K.; Mihara, S.; Ono, K.; Kameyoshi, Y.; Hide, M. Hydrolyzed konjac glucomannan suppresses IgE production in mice B cells. Int. Arch. Allergy Immunol. 2010, 152, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Hume, D.A. The mononuclear phagocyte system. Curr. Opin. Immunol. 2006, 18, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Katsiari, C.G.; Liossis, S.N.C.; Sfikakis, P.P. The pathophysiologic role of monocytes and macrophages in systemic lupus erythematosus: A reappraisal. Semin. Arthritis Rheum. 2010, 39, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Aderem, A.; Ulevitch, R.J. Toll-like receptors in the induction of the innate immune response. Nature 2000, 406, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Luo, Z.; Liu, D.; Ning, Z.; Yang, J.; Ren, J. Structure characterization of a novel polysaccharide from Dictyophora indusiata and its macrophage immunomodulatory activities. J. Agric. Food Chem. 2015, 63, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Wijanarti, S.; Putra, A.B.N.; Nishi, K.; Harmayani, E.; Sugahara, T. Immunostimulatory activity of snake fruit peel extract on murine macrophage-like J774.1 cells. Cytotechnology 2016, 68, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Che, T.M.; Johnson, R.W.; Kelley, K.W.; Dawson, K.A.; Moran, C.A.; Pettigrew, J.E. Effects of mannan oligosaccharide on cytokine secretions by porcine alveolar macrophages and serum cytokine concentrations in nursery pigs. J. Anim. Sci. 2012, 90, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, F.J.; Liénard, D.; Matter, M.; Rüegg, C. Efficiency of recombinant human TNF in human cancer therapy. Cancer Immun. 2006, 6, 6. [Google Scholar] [PubMed]
- Padmore, T.; Stark, C.; Turkevich, L.A.; Champion, J.A. Quantitative analysis of the role of fiber length on phagocytosis and inflammatory response by alveolar macrophages. Biochim. Biophys. Acta 2017, 1861, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Sigola, L.B.; Fuentes, A.L.; Millis, L.M.; Vapenik, J.; Murira, A. Effects of Toll-like receptor ligands on RAW 264.7 macrophage morphology and zymosan phagocytosis. Tissue Cell 2016, 48, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Kumalasari, I.D.; Nishi, K.; Putra, A.B.N.; Sugahara, T. Activation of macrophages stimulated by the bengkoang fiber extract through toll-like receptor 4. Food Funct. 2014, 5, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Putra, A.B.N.; Nishi, K.; Shiraishi, R.; Doi, M.; Sugahara, T. Jellyfish collagen stimulates production of TNF-α and IL-6 by J774.1 cells through activation of NF-κB and JNK via TLR4 signaling pathway. Mol. Immunol. 2014, 58, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Jung, J.Y.; Shin, J.S.; Shin, K.S.; Cho, C.W.; Rhee, Y.K.; Hong, H.D.; Lee, K.T. Immunostimulatory polysaccharide isolated from the leaves of Diospyros kaki Thumb modulate macrophage via TLR2. Int. J. Biol. Macromol. 2015, 79, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wu, X.; Wang, Q.; Cai, N.; Zhang, H.; Jiang, Z.; Wan, M.; Oda, T. Immunomodulatory effects of alginate oligosaccharides on murine macrophage RAW264.7 cells and their structure-activity relationships. J. Agric. Food Chem. 2014, 62, 3168–3176. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.J. Signal transduction by the JNK group of MAP kinases. Cell 2000, 103, 239–252. [Google Scholar] [CrossRef]
- Xia, Y.; Makris, C.; Su, B.; Li, E.; Yang, J.; Nemerow, G.R.; Karin, M. MEK kinase 1 is critically required for c-Jun N-terminal kinase activation by proinflammatory stimuli and growth factor-induced cell migration. Proc. Natl. Acad. Sci. USA 2000, 97, 5243–5248. [Google Scholar] [CrossRef] [PubMed]
- Esteban, E.; Ferrer, R.; Alsina, L.; Artigas, A. Immunomodulation in sepsis: The role of endotoxin removal by polymyxin B-immobilized cartridge. Mediators Inflamm. 2013, 2013, 507539. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Browning, J.D., Jr.; Eichen, P.A.; Lu, C.H.; Mossine, V.V.; Rottinghaus, G.E.; Folk, W.R.; Sun, G.Y.; Lubahn, D.B.; Fritsche, K.L. Immuno-stimulatory activity of a polysaccharide-enriched fraction of Sutherlandia frutescens occurs by the toll-like receptor-4 signaling pathway. J. Ethnopharmacol. 2015, 172, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. Pattern recognition receptors: Doubling up for the innate immune response. Cell 2002, 111, 927–930. [Google Scholar] [CrossRef]
- Xu, X.; Yan, H.; Zhang, X. Structure and immuno-stimulating activities of a new heteropolysaccharide from Lentinula edodes. J. Agric. Food Chem. 2012, 60, 11560–11566. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Liu, H.T.; Wei, P.; Xu, Q.S.; Bai, X.F.; Du, Y.G.; Yu, C. Chitosan oligosaccharides inhibit LPS-induced over-expression of IL-6 and TNF-α in RAW264.7 macrophage cells through blockade of mitogen-activated protein kinase (MAPK) and PI3K/Akt signaling pathways. Carbohydr. Polym. 2011, 84, 1391–1398. [Google Scholar] [CrossRef]
- Cai, H.L.; Huang, X.J.; Nie, S.P.; Xie, M.Y.; Phillips, G.O.; Cui, S.W. Study on Dendrobium officinale O-acetyl-glucomannan (Dendronan®): Part III-immunomodulatory activity in vitro. Bioact. Carbohydr. Diet. Fibre 2015, 5, 99–105. [Google Scholar] [CrossRef]
- Putra, A.B.N.; Morishige, H.; Nishimoto, S.; Nishi, K.; Shiraishi, R.; Doi, M.; Sugahara, T. Effect of collagens from jellyfish and bovine Achilles tendon on the activity of J774.1 and mouse peritoneal macrophage cells. J. Funct. Foods 2012, 4, 504–512. [Google Scholar] [CrossRef]
- Ding, A.H.; Nathan, C.F.; Stuehr, D.J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J. Immunol. 1988, 141, 2407–2412. [Google Scholar] [PubMed]
- Vodovotz, Y.; Bogdan, C.; Paik, J.; Xie, Q.W.; Nathan, C. Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor β. J. Exp. Med. 1993, 178, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Schindler, H.; Lutz, M.B.; Röllinghoff, M.; Bogdan, C. The production of IFN-γ by IL-12/IL-18-activated macrophages requires STAT4 signaling and is inhibited by IL-4. J. Immunol. 2001, 166, 3075–3082. [Google Scholar] [CrossRef] [PubMed]
- Nishi, K.; Kondo, A.; Okamoto, T.; Nakano, H.; Daifuku, M.; Nishimoto, S.; Ochi, K.; Takaoka, T.; Sugahara, T. Immunostimulatory in vitro and in vivo effects of a water-soluble extract from kale. Biosci. Biotechnol. Biochem. 2011, 75, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Kanda, K.; Nishi, K.; Kadota, A.; Nishimoto, S.; Liu, M.C.; Sugahara, T. Nobiletin suppresses adipocyte differentiation of 3T3-L1 cells by an insulin and IBMX mixture induction. Biochim. Biophys. Acta 2012, 1820, 461–468. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compound OSA-PG are available from the authors. |

| Primer | Sequence (5′-3′) |
|---|---|
| TNF-α | 5′-CTACTCCCAGGTTCTCTTCAA-3′ (sense) |
| 5′-GCAGAGAGGAGGTTGACTTTC-3′ (antisense) | |
| IL-6 | 5′-AAGCCAGAGTCCTTCAGAGAGAT-3′ (sense) |
| 5′-TTGGATGGTCTTGGTCCTTAGC-3′ (antisense) | |
| β-Actin | 5′-CATCCGTAAAGACCTCTATGCCAAC-3′ (sense) |
| 5′-ATGGAGCCACCGATCCACA-3′ (antisense) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurusmatika, S.; Nishi, K.; Harmayani, E.; Pranoto, Y.; Sugahara, T. Immunomodulatory Activity of Octenyl Succinic Anhydride Modified Porang (Amorphophallus oncophyllus) Glucomannan on Mouse Macrophage-Like J774.1 Cells and Mouse Primary Peritoneal Macrophages. Molecules 2017, 22, 1187. https://doi.org/10.3390/molecules22071187
Gurusmatika S, Nishi K, Harmayani E, Pranoto Y, Sugahara T. Immunomodulatory Activity of Octenyl Succinic Anhydride Modified Porang (Amorphophallus oncophyllus) Glucomannan on Mouse Macrophage-Like J774.1 Cells and Mouse Primary Peritoneal Macrophages. Molecules. 2017; 22(7):1187. https://doi.org/10.3390/molecules22071187
Chicago/Turabian StyleGurusmatika, Sellen, Kosuke Nishi, Eni Harmayani, Yudi Pranoto, and Takuya Sugahara. 2017. "Immunomodulatory Activity of Octenyl Succinic Anhydride Modified Porang (Amorphophallus oncophyllus) Glucomannan on Mouse Macrophage-Like J774.1 Cells and Mouse Primary Peritoneal Macrophages" Molecules 22, no. 7: 1187. https://doi.org/10.3390/molecules22071187






