Adenosine A1 and A2A Receptors in the Brain: Current Research and Their Role in Neurodegeneration
Abstract
:1. Introduction
2. Adenosine Signaling in the Brain
2.1. Regulation of Extracellular Adenosine Levels
2.2. Adenosine Receptors in Aging and Synaptic Plasticity
3. Role of A1Rs and A2ARs in Neurodegenerative Disease
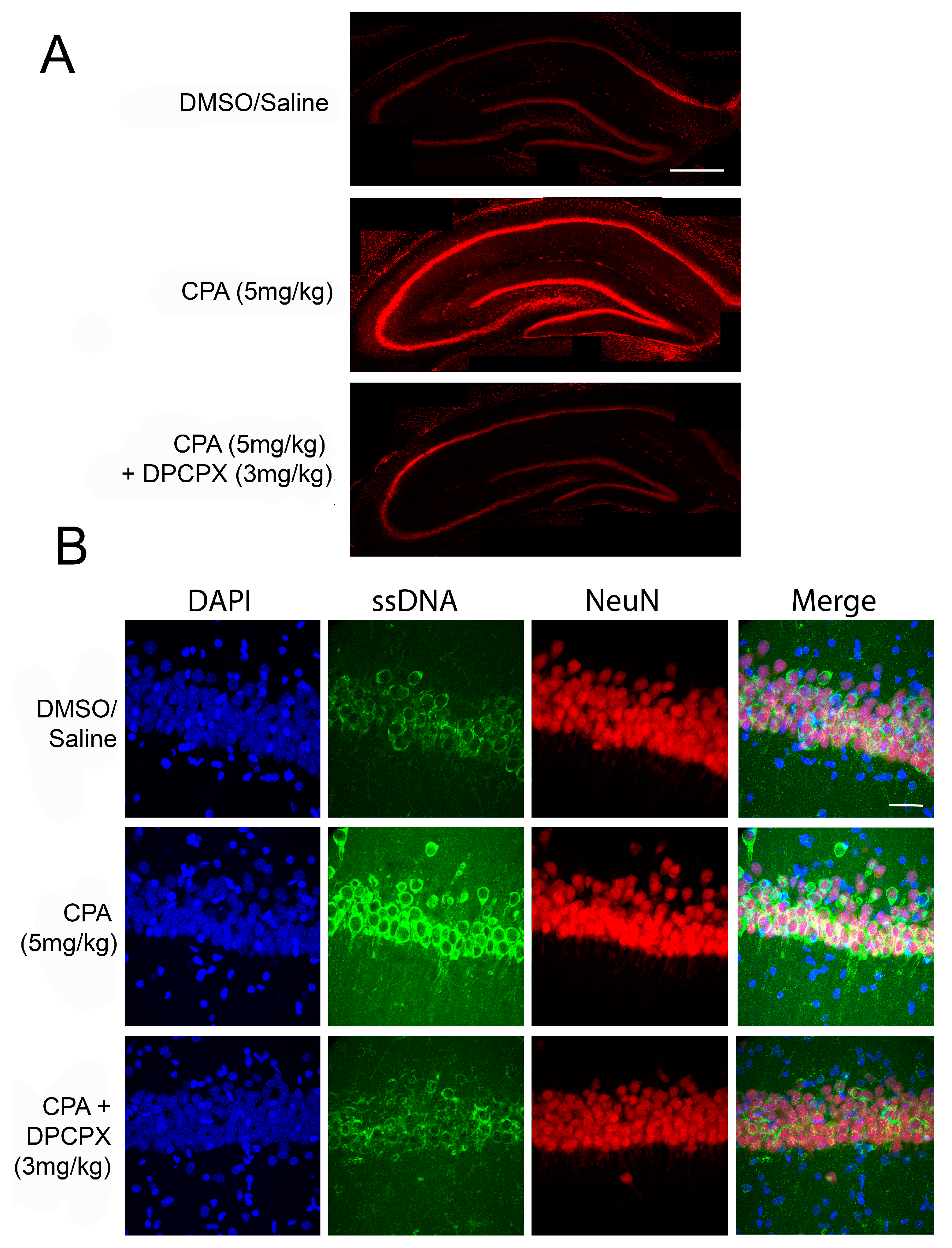
3.1. Clinical Testing of Adenosine Based Therapies
3.2. A1R Role in Neurodegeneration
3.3. A1R/A2AR Cross-Talk
4. Summary and Conclusions
5. Future Perspectives
Acknowledgments
Conflicts of Interest
References
- Fredholm, B.B.; Chen, J.-F.; Cunha, R.A.; Svenningsson, P.; Vaugeois, J.-M. Adenosine and brain function. Int. Rev. Neurobiol. 2005, 63, 191–270. [Google Scholar] [PubMed]
- De Mendonca, A.; Ribeiro, J. Adenosine and neuronal plasticity. Life Sci. 1996, 60, 245–251. [Google Scholar] [CrossRef]
- Dunwiddie, T.V.; Masino, S.A. The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci. 2001, 24, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Florian, C.; Vecsey, C.G.; Halassa, M.M.; Haydon, P.G.; Abel, T. Astrocyte-derived adenosine and A1 receptor activity contribute to sleep loss-induced deficits in hippocampal synaptic plasticity and memory in mice. J. Neurosci. 2011, 31, 6956–6962. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, F.E.; Xiong, W.; Zamzow, C.R. Astrocytes and neurons: Different roles in regulating adenosine levels. Neurol. Res. 2013, 27, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, L.I.; Sims, R.E.; Dale, N.; Haydon, P.G. Wakefulness affects synaptic and network activity by increasing extracellular astrocyte-derived adenosine. J. Neurosci. 2012, 32, 4417–4425. [Google Scholar] [CrossRef] [PubMed]
- Costenla, A.R.; Cunha, R.A.; De Mendonça, A. Caffeine, adenosine receptors, and synaptic plasticity. J. Alzheimer’s Dis. 2010, 20, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Costenla, A.R.; de Mendonca, A.; Ribeiro, J.A. Adenosine modulates synaptic plasticity in hippocampal slices from aged rats. Brain Res. 1999, 851, 228–234. [Google Scholar] [CrossRef]
- Costenla, A.R.; Diogenes, M.J.; Canas, P.M.; Rodrigues, R.J.; Nogueira, C.; Maroco, J.; Agostinho, P.M.; Ribeiro, J.A.; Cunha, R.A.; de Mendonca, A. Enhanced role of adenosine A(2A) receptors in the modulation of LTP in the rat hippocampus upon ageing. Eur. J. Neurosci. 2011, 34, 12–21. [Google Scholar] [CrossRef] [PubMed]
- De Mendonca, A.; Ribeiro, J.A. Adenosine and synaptic plasticity. Drug Dev. Res. 2001, 52, 283–290. [Google Scholar] [CrossRef]
- Yacoubi, M.E.; Ledent, C.; Ménard, J.F.; Parmentier, M.; Costentin, J.; Vaugeois, J.M. The stimulant effects of caffeine on locomotor behaviour in mice are mediated through its blockade of adenosine A2A receptors. Br. J. Pharmacol. 2000, 129, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Li, B.; Xi, X.; Suh, Y.; Martin, R.J. Role of Neuronal Energy Status in the Regulation of Adenosine 5′-Monophosphate-Activated Protein Kinase, Orexigenic Neuropeptides Expression, and Feeding Behavior. Endocrinology 2005, 146, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Reichert, C.F.; Maire, M.; Schmidt, C.; Cajochen, C. Sleep-wake regulation and its impact on working memory performance: The role of adenosine. Biology 2016, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Castillo, C.A.; Albasanz, J.L.; Leon, D.; Jordan, J.; Pallas, M.; Camins, A.; Martin, M. Age-related expression of adenosine receptors in brain from the senescence-accelerated mouse. Exp. Gerontol. 2009, 44, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Stockwell, J.; Cayabyab, F.S. Adenosine A1 receptor-mediated endocytosis of AMPA receptors contributes to impairments in long-term potentiation (LTP) in the middle-aged rat hippocampus. Neurochem. Res. 2016, 41, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Pedata, F.; Pugliese, A.M.; Corti, F.; Melani, A. Adenosine and Stroke. In Adenosine: A Key Link Between Metabolism and Brain Activity; Masino, S., Boison, D., Eds.; Springer: New York, NY, USA, 2013; pp. 273–306. [Google Scholar] [CrossRef]
- Boison, D. Adenosine and epilepsy: From therapeutic rationale to new therapeutic strategies. Neuroscientist 2005, 11, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Schwarzschild, M.A.; Agnati, L.; Fuxe, K.; Chen, J.-F.; Morelli, M. Targeting adenosine A2A receptors in Parkinson’s disease. Trends Neurosci. 2006, 29, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.A. Neuroprotection by adenosine in the brain: From A1 receptor activation to A2A receptor blockade. Purinergic Signal. 2005, 1, 111–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xiong, C.; Pancyr, C.; Stockwell, J.; Walz, W.; Cayabyab, F.S. Prolonged adenosine A1 receptor activation in hypoxia and pial vessel disruption focal cortical ischemia facilitates clathrin-mediated AMPA receptor endocytosis and long-lasting synaptic inhibition in rat hippocampal CA3-CA1 synapses: Differential regulation of GluA2 and GluA1 subunits by p38 MAPK and JNK. J. Neurosci. 2014, 34, 9621–9643. [Google Scholar] [PubMed]
- Stockwell, J.; Chen, Z.; Niazi, M.; Nosib, S.; Cayabyab, F.S. Protein phosphatase role in adenosine A1 receptor-induced AMPA receptor trafficking and rat hippocampal neuronal damage in hypoxia/reperfusion injury. Neuropharmacology 2016, 102, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Dunwiddie, T.V.; Haas, H.L. Adenosine increases synaptic facilitation in the in vitro rat hippocampus: Evidence for a presynaptic site of action. J. Physiol. 1985, 369, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Rebola, N.; Coelho, J.E.; Costenla, A.R.; Lopes, L.V.; Parada, A.; Oliveira, C.R.; Soares-da-Silva, P.; De Mendonca, A.; Cunha, R.A. Decrease of adenosine A1 receptor density and of adenosine neuromodulation in the hippocampus of kindled rats. Eur. J. Neurosci. 2003, 18, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Popoli, P.; Betto, P.; Reggio, R.; Ricciarello, G. Adenosine A2A receptor stimulation enhances striatal extracellular glutamate levels in rats. Eur. J. Pharmacol. 1995, 287, 215–217. [Google Scholar] [CrossRef]
- Thompson, S.M.; Haas, H.L.; Gähwiler, B.H. Comparison of the actions of adenosine at pre-and postsynaptic receptors in the rat hippocampus in vitro. J. Physiol. 1992, 451, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Brust, T.B.; Cayabyab, F.S.; Zhou, N.; MacVicar, B.A. p38 mitogen-activated protein kinase contributes to adenosine A1 receptor-mediated synaptic depression in area CA1 of the rat hippocampus. J. Neurosci. 2006, 26, 12427–12438. [Google Scholar] [CrossRef] [PubMed]
- Brust, T.B.; Cayabyab, F.S.; MacVicar, B.A. C-Jun N-terminal kinase regulates adenosine A1 receptor-mediated synaptic depression in the rat hippocampus. Neuropharmacology 2007, 53, 906–917. [Google Scholar] [CrossRef] [PubMed]
- de Mendonça, A.; Sebastião, A.M.; Ribeiro, J.A. Inhibition of NMDA receptor-mediated currents in isolated rat hippocampal neurones by adenosine A1 receptor activation. Neuroreport 1995, 6, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Heurteaux, C.; Lauritzen, I.; Widmann, C.; Lazdunski, M. Essential role of adenosine, adenosine A1 receptors, and ATP-sensitive K+ channels in cerebral ischemic preconditioning. Proc. Natl. Acad. Sci. USA 1995, 92, 4666–4670. [Google Scholar] [CrossRef] [PubMed]
- Ochiishi, T.; Chen, L.; Yukawa, A.; Saitoh, Y.; Sekino, Y.; Arai, T.; Nakata, H.; Miyamoto, H. Cellular localization of adenosine A1 receptors in rat forebrain: Immunohistochemical analysis using adenosine A1 receptor-specific monoclonal antibody. J. Comp. Neurol. 1999, 411, 301–316. [Google Scholar] [CrossRef]
- Rebola, N.; Rodrigues, R.; Lopes, L.; Richardson, P.; Oliveira, C.; Cunha, R. Adenosine A1 and A2A receptors are co-expressed in pyramidal neurons and co-localized in glutamatergic nerve terminals of the rat hippocampus. Neuroscience 2005, 133, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, S. Molecular diversity of glutamate receptors and implications for brain function. Science 1992, 258, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.M.; Yin, H.Z.; Chiang, J.; Weiss, J.H. Ca2+-permeable AMPA/kainate and NMDA channels: High rate of Ca2+ influx underlies potent induction of injury. J. Neurosci. 1996, 16, 5457–5465. [Google Scholar] [PubMed]
- Bogenpohl, J.W.; Ritter, S.L.; Hall, R.A.; Smith, Y. Adenosine A2A receptor in the monkey basal ganglia: Ultrastructural localization and colocalization with the metabotropic glutamate receptor 5 in the striatum. J. Comp. Neurol. 2012, 520, 570–589. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.A.; Constantino, M.C.; Sebastiao, A.M.; Ribeiro, J.A. Modification of A1 and A2a adenosine receptor binding in aged striatum, hippocampus and cortex of the rat. Neuroreport 1995, 6, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.B.; Ribeiro, J.A.; Sebastiao, A.M. Enhancement of AMPA currents and GluR1 membrane expression through PKA-coupled adenosine A2A receptors. Hippocampus 2012, 22, 276–291. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, S.A.; Beal, P.R.; Yao, S.Y.; King, A.E.; Cass, C.E.; Young, J.D. The equilibrative nucleoside transporter family, SLC29. Pflugers Arch. 2004, 447, 735–743. [Google Scholar] [PubMed]
- Kong, W.; Engel, K.; Wang, J. Mammalian nucleoside transporters. Curr. Drug Metab. 2004, 5, 63–84. [Google Scholar] [CrossRef] [PubMed]
- Dale, N.; Pearson, T.; Frenguelli, B.G. Direct measurement of adenosine release during hypoxia in the CA1 region of the rat hippocampal slice. J. Physiol. 2000, 526, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Adenosine and its receptors: Multiple modulatory functions and potential therapeutic targets for neurologic disease. Neurology 2008, 70, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.W.; Rothman, S.M. Adenosine inhibits excitatory but not inhibitory synaptic transmission in the hippocampus. J. Neurosci. 1991, 11, 1375–1380. [Google Scholar] [PubMed]
- Huang, C.C.; Liang, Y.C.; Hsu, K.S. A role for extracellular adenosine in time-dependent reversal of long-term potentiation by low-frequency stimulation at hippocampal CA1 synapses. J. Neurosci. 1999, 19, 9728–9738. [Google Scholar] [PubMed]
- Andersen, B.T.; Gillespie, D.G.; Mi, Z.; Dubey, R.K.; Jackson, E.K. Role of adenosine A1 receptors in modulating extracellular adenosine levels. J. Pharmacol. Exp. Ther. 1999, 291, 76–80. [Google Scholar]
- Ribeiro, J.A. What can adenosine neuromodulation do for neuroprotection? Curr. Drug Targets-CNS Neurol. Disord. 2005, 4, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Xiong, W.; Zhang, D.; Soylu, H.; Sun, C.; Albensi, B.C.; Parkinson, F.E. Regulation of adenosine levels during cerebral ischemia. Acta Pharmacol. Sin. 2013, 34, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xiong, W.; Albensi, B.C.; Parkinson, F.E. Expression of human equilibrative nucleoside transporter 1 in mouse neurons regulates adenosine levels in physiological and hypoxic-ischemic conditions. J. Neurochem. 2011, 118, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Latini, S.; Pedata, F. Adenosine in the central nervous system: Release mechanisms and extracellular concentrations. J. Neurochem. 2001, 79, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, K.; Carriere, I.; de Mendonca, A.; Portet, F.; Dartigues, J.F.; Rouaud, O.; Barberger-Gateau, P.; Ancelin, M.L. The neuroprotective effects of caffeine: A prospective population study (the Three City Study). Neurology 2007, 69, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Meneses, A.; Manuel-Apolinar, L.; Rocha, L.; Castillo, E.; Castillo, C. Expression of the 5-HT receptors in rat brain during memory consolidation. Behav. Brain Res. 2004, 152, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Sebastiao, A.M.; Cunha, R.A.; de Mendonca, A.; Ribeiro, J.A. Modification of adenosine modulation of synaptic transmission in the hippocampus of aged rats. Br. J. Pharmacol. 2000, 131, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Teyler, T.J.; Robbins, N. Aging differentially alters forms of long-term potentiation in rat hippocampal area CA1. J. Neurophysiol. 1998, 79, 334–341. [Google Scholar] [PubMed]
- Rex, C.S.; Kramar, E.A.; Colgin, L.L.; Lin, B.; Gall, C.M.; Lynch, G. Long-term potentiation is impaired in middle-aged rats: Regional specificity and reversal by adenosine receptor antagonists. J. Neurosci. 2005, 25, 5956–5966. [Google Scholar] [CrossRef] [PubMed]
- Leon, D.; Albasanz, J.L.; Ruiz, M.A.; Martin, M. Chronic caffeine or theophylline intake during pregnancy inhibits A1 receptor function in the rat brain. Neuroscience 2005, 131, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Horita, T.K.; Kobayashi, M.; Mori, A.; Jenner, P.; Kanda, T. Effects of the adenosine A2A antagonist istradefylline on cognitive performance in rats with a 6-OHDA lesion in prefrontal cortex. Psychopharmacology 2013, 230, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Mizuno, Y.; Japanese Istradefylline Study Group. A long-term study of istradefylline safety and efficacy in patients with Parkinson disease. Clin. Neuropharmacol. 2015, 38, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Pinna, A. Adenosine A2A receptor antagonists in Parkinson’s disease: Progress in clinical trials from the newly approved istradefylline to drugs in early development and those already discontinued. CNS Drugs 2014, 28, 455–474. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.-I.; Tashiro, T.; Kawai-Uchida, M.; Mori, A.; Jenner, P.; Kanda, T. The Adenosine A 2A-Receptor Antagonist Istradefylline Enhances the Motor Response of L-DOPA Without Worsening Dyskinesia in MPTP-Treated Common Marmosets. J. Pharmacol. Sci. 2014, 124, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Atack, J.R.; Shook, B.C.; Rassnick, S.; Jackson, P.F.; Rhodes, K.; Drinkenburg, W.H.; Ahnaou, A.; te Riele, P.; Langlois, X.; Hrupka, B. JNJ-40255293, a Novel Adenosine A2A/A1 Antagonist with Efficacy in Preclinical Models of Parkinson’s Disease. ACS Chem. Neurosci. 2014, 5, 1005–1019. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, A.M.; Traini, C.; Cipriani, S.; Gianfriddo, M.; Mello, T.; Giovannini, M.G.; Galli, A.; Pedata, F. The adenosine A2A receptor antagonist ZM241385 enhances neuronal survival after oxygen-glucose deprivation in rat CA1 hippocampal slices. Br. J. Pharmacol. 2009, 157, 818–830. [Google Scholar] [CrossRef] [PubMed]
- Yuzlenko, O.; Kiec-Kononowicz, K. Potent adenosine A1 and A2A receptors antagonists: Recent developments. Curr. Med. Chem. 2006, 13, 3609–3625. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-F.; Xu, K.; Petzer, J.P.; Staal, R.; Xu, Y.-H.; Beilstein, M.; Sonsalla, P.K.; Castagnoli, K.; Castagnoli, N., Jr.; Schwarzschild, M.A. Neuroprotection by caffeine and A (2A) adenosine receptor inactivation in a model of Parkinson’s disease. J. Neurosci. 2001, 21, 143. [Google Scholar]
- Costa, M.S.; Botton, P.H.; Mioranzza, S.; Souza, D.O.; Porciuncula, L.O. Caffeine prevents age-associated recognition memory decline and changes brain-derived neurotrophic factor and tirosine kinase receptor (TrkB) content in mice. Neuroscience 2008, 153, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, J.; Rocha, A.; Nunes, F.; Costa, M.S.; Schein, V.; Kazlauckas, V.; Kalinine, E.; Souza, D.O.; Cunha, R.A.; Porciúncula, L.O. Caffeine Consumption Prevents Memory Impairment, Neuronal Damage, and Adenosine A 2A Receptors Upregulation in the Hippocampus of a Rat Model of Sporadic Dementia. J. Alzheimer’s Dis. 2013, 34, 509–518. [Google Scholar]
- Flaten, V.; Laurent, C.; Coelho, J.E.; Sandau, U.; Batalha, V.L.; Burnouf, S.; Hamdane, M.; Humez, S.; Boison, D.; Lopes, L.V. From epidemiology to pathophysiology: What about caffeine in Alzheimer’s disease? Biochem. Soc. Trans. 2014, 42, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Oliver, M.; Díaz-Ríos, M. Using caffeine and other adenosine receptor antagonists and agonists as therapeutic tools against neurodegenerative diseases: A review. Life Sci. 2014, 101, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Di Luca, D.; Orrú, M.; Xu, Y.; Chen, J.-F.; Schwarzschild, M. Neuroprotection by caffeine in the MPTP model of parkinson’s disease and its dependence on adenosine A 2A receptors. Neuroscience 2016, 322, 129–137. [Google Scholar] [CrossRef] [PubMed]
- De Sarro, G.; De Sarro, A.; Di Paola, E.D.; Bertorelli, R. Effects of adenosine receptor agonists and antagonists on audiogenic seizure-sensible DBA/2 mice. Eur. J. Pharmacol. 1999, 371, 137–145. [Google Scholar] [CrossRef]
- Huber, A.; Güttinger, M.; Möhler, H.; Boison, D. Seizure suppression by adenosine A2A receptor activation in a rat model of audiogenic brainstem epilepsy. Neurosci. Lett. 2002, 329, 289–292. [Google Scholar] [CrossRef]
- Zhang, G.; Franklin, P.H.; Murray, T.F. Activation of adenosine A1 receptors underlies anticonvulsant effect of CGS21680. Eur. J. Pharmacol. 1994, 255, 239–243. [Google Scholar] [CrossRef]
- Malhotra, J.; Gupta, Y.K. Effect of adenosine receptor modulation on pentylenetetrazole-induced seizures in rats. Br. J. Pharmacol. 1997, 120, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Safran, N.; Shneyvays, V.; Balas, N.; Jacobson, K.A.; Nawrath, H.; Shainberg, A. Cardioprotective effects of adenosine A1 and A 3 receptor activation during hypoxia in isolated rat cardiac myocytes. Mol. Cell. Biochem. 2001, 217, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Etherington, L.-A.V.; Frenguelli, B.G. Endogenous adenosine modulates epileptiform activity in rat hippocampus in a receptor subtype-dependent manner. Eur. J. Neurosci. 2004, 19, 2539–2550. [Google Scholar] [CrossRef] [PubMed]
- Laghi Pasini, F.; Guideri, F.; Picano, E.; Parenti, G.; Petersen, C.; Varga, A.; Di Perri, T. Increase in plasma adenosine during brain ischemia in man: A study during transient ischemic attacks, and stroke. Brain Res. Bull. 2000, 51, 327–330. [Google Scholar] [CrossRef]
- Liu, B.; Liao, M.; Mielke, J.G.; Ning, K.; Chen, Y.; Li, L.; El-Hayek, Y.H.; Gomez, E.; Zukin, R.S.; Fehlings, M.G. Ischemic insults direct glutamate receptor subunit 2-lacking AMPA receptors to synaptic sites. J. Neurosci. 2006, 26, 5309–5319. [Google Scholar] [CrossRef] [PubMed]
- Obrietan, K.; Belousov, A.B.; Heller, H.C.; van den Pol, A.N. Adenosine pre-and postsynaptic modulation of glutamate-dependent calcium activity in hypothalamic neurons. J. Neurophysiol. 1995, 74, 2150–2162. [Google Scholar] [PubMed]
- Moschovos, C.; Kostopoulos, G.; Papatheodoropoulos, C. Endogenous adenosine induces NMDA receptor-independent persistent epileptiform discharges in dorsal and ventral hippocampus via activation of A2 receptors. Epilepsy Res. 2012, 100, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Semah, F.; Picot, M.C.; Adam, C.; Broglin, D.; Arzimanoglou, A.; Bazin, B.; Cavalcanti, D.; Baulac, M. Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology 1998, 51, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Harbord, M.G.; Manson, J.I. Temporal lobe epilepsy in childhood: Reappraisal of etiology and outcome. Pediatr. Neurol. 1987, 3, 263–268. [Google Scholar] [CrossRef]
- Jokeit, H.; Ebner, A. Long term effects of refractory temporal lobe epilepsy on cognitive abilities: A cross sectional study. J. Neurol. Neurosurg. Psychiatry 1999, 67, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Rosim, F.E.; Persike, D.S.; Nehlig, A.; Amorim, R.P.; de Oliveira, D.M.; Fernandes, M.J. Differential neuroprotection by A(1) receptor activation and A(2A) receptor inhibition following pilocarpine-induced status epilepticus. Epilepsy Behav. 2011, 22, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.E. Perampanel: A Review in Drug-Resistant Epilepsy. Drugs 2015, 75, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Armentero, M.T.; Pinna, A.; Ferré, S.; Lanciego, J.L.; Müller, C.E.; Franco, R. Past, present and future of A2A adenosine receptor antagonists in the therapy of Parkinson’s disease. Pharmacol. Ther. 2011, 132, 280–299. [Google Scholar] [CrossRef] [PubMed]
- LeWitt, P.A.; Guttman, M.; Tetrud, J.W.; Tuite, P.J.; Mori, A.; Chaikin, P.; Sussman, N.M. Adenosine A2A receptor antagonist istradefylline (KW-6002) reduces “off” time in Parkinson’s disease: A double-blind, randomized, multicenter clinical trial (6002-US-005). Ann. Neurol. 2008, 63, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.; Olanow, C.W.; Greenamyre, J.T.; Bezard, E. Slowing of neurodegeneration in Parkinson’s disease and Huntington’s disease: Future therapeutic perspectives. Lancet 2014, 384, 545–555. [Google Scholar] [CrossRef]
- Olanow, C.W.; Schapira, A.H. Therapeutic prospects for Parkinson disease. Ann. Neurol. 2013, 74, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Dungo, R.; Deeks, E.D. Istradefylline: First global approval. Drugs 2013, 73, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Hasegawa, K.; Kondo, T.; Kuno, S.; Yamamoto, M. Clinical efficacy of istradefylline (KW-6002) in Parkinson’s disease: A randomized, controlled study. Mov. Disord. 2010, 25, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Lang, A.E.; Munhoz, R.P.; Charland, K.; Pelletier, A.; Moscovich, M.; Filla, L.; Zanatta, D.; Romenets, S.R.; Altman, R. Caffeine for treatment of Parkinson disease A randomized controlled trial. Neurology 2012, 79, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Wills, A.M.A.; Eberly, S.; Tennis, M.; Lang, A.E.; Messing, S.; Togasaki, D.; Tanner, C.M.; Kamp, C.; Chen, J.F.; Oakes, D. Caffeine consumption and risk of dyskinesia in CALM-PD. Mov. Disord. 2013, 28, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Ferré, S. Adenosine-dopamine interactions in the ventral striatum Implications for the treatment of schizophrenia. Psychopharmacology 1997, 133, 107–120. [Google Scholar] [PubMed]
- Fuxe, K.; Guidolin, D.; Agnati, L.F.; Borroto-Escuela, D.O. Dopamine heteroreceptor complexes as therapeutic targets in Parkinson’s disease. Expert Opin. Ther. Targets 2015, 19, 377–398. [Google Scholar] [CrossRef] [PubMed]
- Floran, B.; Barajas, C.; Floran, L.; Erlij, D.; Aceves, J. Adenosine A1 receptors control dopamine D1-dependent [3 H] GABA release in slices of substantia nigra pars reticulata and motor behavior in the rat. Neuroscience 2002, 115, 743–751. [Google Scholar] [CrossRef]
- Augusto, E.; Matos, M.; Sévigny, J.; El-Tayeb, A.; Bynoe, M.S.; Müller, C.E.; Cunha, R.A.; Chen, J.-F. Ecto-5′-nucleotidase (CD73)-mediated formation of adenosine is critical for the striatal adenosine A2A receptor functions. J. Neurosci. 2013, 33, 11390–11399. [Google Scholar] [CrossRef] [PubMed]
- Fuxe, K.; Ferré, S.; Canals, M.; Torvinen, M.; Terasmaa, A.; Marcellino, D.; Goldberg, S.R.; Staines, W.; Jacobsen, K.X.; Lluis, C. Adenosine A2A and dopamine D2 heteromeric receptor complexes and their function. J. Mol. Neurosci. 2005, 26, 209. [Google Scholar] [CrossRef]
- Torvinen, M.; Marcellino, D.; Canals, M.; Agnati, L.F.; Lluis, C.; Franco, R.; Fuxe, K. Adenosine A2A receptor and dopamine D3 receptor interactions: Evidence of functional A2A/D3 heteromeric complexes. Mol. Pharmacol. 2005, 67, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Soriano, A.; Ventura, R.; Molero, A.; Hoen, R.; Casadó, V.; Cortés, A.; Fanelli, F.; Albericio, F.; Lluís, C.; Franco, R. Adenosine A2A receptor-antagonist/dopamine D2 receptor-agonist bivalent ligands as pharmacological tools to detect A2A-D2 receptor heteromers. J. Med. Chem 2009, 52, 5590–5602. [Google Scholar] [CrossRef] [PubMed]
- Fuxe, K.; Marcellino, D.; Genedani, S.; Agnati, L. Adenosine A2A receptors, dopamine D2 receptors and their interactions in Parkinson’s disease. Mov. Disord. 2007, 22, 1990–2017. [Google Scholar] [CrossRef] [PubMed]
- Ferré, S.; Karcz-Kubicha, M.; Hope, B.T.; Popoli, P.; Burgueño, J.; Gutiérrez, M.A.; Casadó, V.; Fuxe, K.; Goldberg, S.R.; Lluis, C. Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: Implications for striatal neuronal function. Proc. Natl. Acad. Sci. USA 2002, 99, 11940–11945. [Google Scholar] [CrossRef] [PubMed]
- Nishi, A.; Liu, F.; Matsuyama, S.; Hamada, M.; Higashi, H.; Nairn, A.C.; Greengard, P. Metabotropic mGlu5 receptors regulate adenosine A2A receptor signaling. Proc. Natl. Acad. Sci. USA 2003, 100, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Dı́az-Cabiale, Z.; Vivó, M.; Del Arco, A.; O’Connor, W.T.; Harte, M.K.; Müller, C.E.; Martı́nez, E.; Popoli, P.; Fuxe, K.; Ferré, S. Metabotropic glutamate mGlu5 receptor-mediated modulation of the ventral striopallidal GABA pathway in rats. Interactions with adenosine A2A and dopamine D2 receptors. Neurosci. Lett. 2002, 324, 154–158. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Ijzerman, A.P.; Jacobson, K.A.; Linden, J.; Müller, C.E. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and Classification of Adenosine Receptors—An Update. Pharmacol. Rev. 2011, 63, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Van den Boogaard, M.; Ramakers, B.P.; van Alfen, N.; van der Werf, S.P.; Fick, W.F.; Hoedemaekers, C.W.; Verbeek, M.M.; Schoonhoven, L.; van der Hoeven, J.G.; Pickkers, P. Endotoxemia-induced inflammation and the effect on the human brain. Crit. Care 2010, 14, R81. [Google Scholar] [CrossRef] [PubMed]
- Ramakers, B.P.; Riksen, N.P.; van den Broek, P.; Franke, B.; Peters, W.H.M.; van der Hoeven, J.G.; Smits, P.; Pickkers, P. Circulating adenosine increases during human experimental endotoxemia but blockade of its receptor does not influence the immune response and subsequent organ injury. Crit. Care 2011, 15, R3. [Google Scholar] [CrossRef] [PubMed]
- Desmet, W.; Bogaert, J.; Dubois, C.; Sinnaeve, P.; Adriaenssens, T.; Pappas, C.; Ganame, J.; Dymarkowski, S.; Janssens, S.; Belmans, A. High-dose intracoronary adenosine for myocardial salvage in patients with acute ST-segment elevation myocardial infarction. Eur. Heart J. 2011, 32, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Dorado, D.; García-del-Blanco, B.; Otaegui, I.; Rodríguez-Palomares, J.; Pineda, V.; Gimeno, F.; Ruiz-Salmerón, R.; Elizaga, J.; Evangelista, A.; Fernandez-Avilés, F.; et al. Intracoronary injection of adenosine before reperfusion in patients with ST-segment elevation myocardial infarction: A randomized controlled clinical trial. Int. J. Cardiol. 2014, 177, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Rauck, R.L.; North, J.; Eisenach, J.C. Intrathecal clonidine and adenosine: Effects on pain and sensory processing in patients with chronic regional pain syndrome. Pain 2015, 156, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.S.; Minkowitz, H.; Osborn, T.; Ogunnaike, B.; Candiotti, K.; Viscusi, E.; Gu, J.; Creed, M.R.; Gan, T.J. Phase 2, double-blind, placebo-controlled, dose-response trial of intravenous adenosine for perioperative analgesia. J. Am. Soc. Anesthesiol. 2008, 109, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Pandey, C.K.; Nath, S.S.; Pandey, V.K.; Karna, S.T.; Tandon, M. Perioperative ischaemia-induced liver injury and protection strategies: An expanding horizon for anaesthesiologists. Indian J. Anaesth. 2013, 57, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Field, J.J.; Lin, G.; Okam, M.M.; Majerus, E.; Keefer, J.; Onyekwere, O.; Ross, A.; Campigotto, F.; Neuberg, D.; Linden, J. Sickle cell vaso-occlusion causes activation of iNKT cells that is decreased by the adenosine A2A receptor agonist regadenoson. Blood 2013, 121, 3329–3334. [Google Scholar] [CrossRef] [PubMed]
- David, M.; Akerman, L.; Ziv, M.; Kadurina, M.; Gospodinov, D.; Pavlotsky, F.; Yankova, R.; Kouzeva, V.; Ramon, M.; Silverman, M. Treatment of plaque-type psoriasis with oral CF101: Data from an exploratory randomized phase 2 clinical trial. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Fishman, P.; Cohen, S. The A3 adenosine receptor (A3AR): Therapeutic target and predictive biological marker in rheumatoid arthritis. Clin. Rheumatol. 2016, 35, 2359–2362. [Google Scholar] [CrossRef] [PubMed]
- Stemmer, S.M.; Benjaminov, O.; Medalia, G.; Ciuraru, N.B.; Silverman, M.H.; Bar-Yehuda, S.; Fishman, S.; Harpaz, Z.; Farbstein, M.; Cohen, S. CF102 for the treatment of hepatocellular carcinoma: A phase I/II, open-label, dose-escalation study. Oncologist 2013, 18, 25–26. [Google Scholar] [CrossRef] [PubMed]
- Domeeka, A.D.; Lori, A.F.; Denise, M.K.; Robert, B.R. Indirect Modulation of Dopamine D2 Receptors as Potential Pharmacotherapy for Schizophrenia: I. Adenosine Agonists. Ann. Pharmacother. 1999, 33, 480–488. [Google Scholar]
- Akhondzadeh, S.; Shasavand, E.; Jamilian, H.R.; Shabestari, O.; Kamalipour, A. Dipyridamole in the treatment of schizophrenia: Adenosine-dopamine receptor interactions. J. Clin. Pharm. Ther. 2000, 25, 131–137. [Google Scholar] [CrossRef] [PubMed]
- El Messaoudi, S.; Wouters, C.; Van Swieten, H.; Pickkers, P.; Noyez, L.; Kievit, P.; Abbink, E.; Rasing-Hoogveld, A.; Bouw, T.; Peters, J. Effect of dipyridamole on myocardial reperfusion injury: A double-blind randomized controlled trial in patients undergoing elective coronary artery bypass surgery. Clin. Pharmacol. Ther. 2016, 99, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.A.; Shulman, L.M.; Trugman, J.M.; Roberts, J.W.; Mori, A.; Ballerini, R.; Sussman, N.M. Study of istradefylline in patients with Parkinson’s disease on levodopa with motor fluctuations. Mov. Disord. 2008, 23, 2177–2185. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.A.; Stocchi, F.; Rascol, O.; Huyck, S.B.; Capece, R.; Ho, T.W.; Sklar, P.; Lines, C.; Michelson, D.; Hewitt, D. Preladenant as an adjunctive therapy with levodopa in parkinson disease: Two randomized clinical trials and lessons learned. JAMA Neurol. 2015, 72, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Hattori, N.; Kikuchi, M.; Adachi, N.; Hewitt, D.; Huyck, S.; Saito, T. Adjunctive preladenant: A placebo-controlled, dose-finding study in Japanese patients with Parkinson’s disease. Parkinsonism Relat. Disord. 2016, 32, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Iskandrian, A.E.; Bateman, T.M.; Belardinelli, L.; Blackburn, B.; Cerqueira, M.D.; Hendel, R.C.; Lieu, H.; Mahmarian, J.J.; Olmsted, A.; Underwood, S.R. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: Results of the ADVANCE phase 3 multicenter international trial. J. Nuclear Cardiol. 2007, 14, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.D.; Nguyen, P.; Staehr, P.; Underwood, S.R.; Iskandrian, A.E.; Investigators, A.-M.T. Effects of age, gender, obesity, and diabetes on the efficacy and safety of the selective A 2A agonist regadenoson versus adenosine in myocardial perfusion imaging: Integrated ADVANCE-MPI trial results. JACC Cardiovasc. Imaging 2008, 1, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Aljaroudi, W.; Hermann, D.; Hage, F.; Heo, J.; Iskandrian, A.E. Safety of regadenoson in patients with end-stage renal disease. Am. J. Cardiol. 2010, 105, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Manwani, D.; Frenette, P.S. Vaso-occlusion in sickle cell disease: Pathophysiology and novel targeted therapies. Blood 2013, 122, 3892–3898. [Google Scholar] [CrossRef] [PubMed]
- Charache, S.; Terrin, M.L.; Moore, R.D.; Dover, G.J.; Barton, F.B.; Eckert, S.V.; McMahon, R.P.; Bonds, D.R. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N. Engl. J. Med. 1995, 332, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Ashley-Koch, A.; Yang, Q.; Olney, R.S. Sickle hemoglobin (Hb S) allele and sickle cell disease: A HuGE review. Am. J. Epidemiol. 2000, 151, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Hage, F.G.; Heo, J.; Franks, B.; Belardinelli, L.; Blackburn, B.; Wang, W.; Iskandrian, A.E. Differences in heart rate response to adenosine and regadenoson in patients with and without diabetes mellitus. Am. Heart J. 2009, 157, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Givertz, M.M.; Postmus, D.; Hillege, H.L.; Mansoor, G.A.; Massie, B.M.; Davison, B.A.; Ponikowski, P.; Metra, M.; Teerlink, J.R.; Cleland, J.G.F. Renal function trajectories and clinical outcomes in acute heart failure. Circ. Heart Fail. 2014, 7, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Tromp, J.; Meyer, S.; Mentz, R.J.; O’Connor, C.M.; Metra, M.; Dittrich, H.C.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; Davison, B. Acute heart failure in the young: Clinical characteristics and biomarker profiles. Int. J. Cardiol. 2016, 221, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Ter Maaten, J.M.; Damman, K.; Hanberg, J.S.; Givertz, M.M.; Metra, M.; O’Connor, C.M.; Teerlink, J.R.; Ponikowski, P.; Cotter, G.; Davison, B. Hypochloremia, Diuretic Resistance, and Outcome in Patients With Acute Heart FailureClinical Perspective. Circ. Heart Fail. 2016, 9, e003109. [Google Scholar] [CrossRef] [PubMed]
- Streng, K.W.; ter Maaten, J.M.; Cleland, J.G.; O’Connor, C.M.; Davison, B.A.; Metra, M.; Givertz, M.M.; Teerlink, J.R.; Ponikowski, P.; Bloomfield, D.M. Associations of Body Mass Index With Laboratory and Biomarkers in Patients With Acute Heart Failure. Circ. Heart Fail. 2017, 10, e003350. [Google Scholar] [CrossRef] [PubMed]
- Lane, S.D.; Green, C.E.; Steinberg, J.L.; Ma, L.; Schmitz, J.M.; Rathnayaka, N.; Bandak, S.D.; Ferre, S.; Moeller, F.G. Cardiovascular and subjective effects of the novel adenosine A2A receptor antagonist SYN115 in cocaine dependent individuals. J. Addict. Res. Ther. 2012. [Google Scholar] [CrossRef]
- Moeller, F.G.; Steinberg, J.L.; Lane, S.D.; Kjome, K.L.; Ma, L.; Ferre, S.; Schmitz, J.; Green, C.E.; Bandak, S.I.; Renshaw, P.F. Increased orbitofrontal brain activation after administration of a selective adenosine A2a antagonist in cocaine dependent subjects. Front. Psychiatry 2012, 3, 44. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.A.; Olanow, C.W.; Kieburtz, K.D.; Pourcher, E.; Docu-Axelerad, A.; Lew, M.; Kozyolkin, O.; Neale, A.; Resburg, C.; Meya, U. Tozadenant (SYN115) in patients with Parkinson’s disease who have motor fluctuations on levodopa: A phase 2b, double-blind, randomised trial. Lancet Neurol. 2014, 13, 767–776. [Google Scholar] [CrossRef]
- Tamargo, J.; López-Sendón, J. Novel therapeutic targets for the treatment of heart failure. Nat. Rev. Drug Discov. 2011, 10, 536–555. [Google Scholar] [CrossRef] [PubMed]
- Cayabyab, F.S.; Gowribai, K.; Walz, W. Involvement of matrix metalloproteinases-2 and -9 in the formation of a lacuna-like cerebral cavity. J. Neurosci. Res. 2013, 91, 920–933. [Google Scholar] [CrossRef] [PubMed]
- Walz, W.; Cayabyab, F.S. Neutrophil infiltration and matrix metalloproteinase-9 in lacunar infarction. Neurochem. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Selbie, L.A.; Hill, S.J. G protein-coupled-receptor cross-talk: The fine-tuning of multiple receptor-signalling pathways. Trends Pharmacol. Sci. 1998, 19, 87–93. [Google Scholar] [CrossRef]
- Werry, T.D.; Wilkinson, G.F.; Willars, G.B. Mechanisms of cross-talk between G-protein-coupled receptors resulting in enhanced release of intracellular Ca2+. Biochem. J. 2003, 374, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.V.; Cunha, R.A.; Ribeiro, J.A. Cross talk between A1 and A2A adenosine receptors in the hippocampus and cortex of young adult and old rats. J. Neurophysiol. 1999, 82, 3196–3203. [Google Scholar] [PubMed]
- Blanquet, P.R. Casein kinase 2 as a potentially important enzyme in the nervous system. Prog. Neurobiol. 2000, 60, 211–246. [Google Scholar] [CrossRef]
- Rebholz, H.; Nishi, A.; Liebscher, S.; Nairn, A.C.; Flajolet, M.; Greengard, P. CK2 negatively regulates Gαs signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 14096–14101. [Google Scholar] [CrossRef] [PubMed]

| Drug Name | MoA | Preclinical Trial | Results |
|---|---|---|---|
| 2-HE-NECA | A2AR agonist | Epilepsy prone transgenic mouse strain (DBA/2 strain) [67] | Suppresses seizure activity of both tonic and clonic extension seizures |
| CCPA | A1R agonist | Epilepsy prone rats (GEPR-9 strain) [68] | 2. Suppresses seizure activity |
| CGS 21680 | A2AR agonist | 1. Bicuculline methiodide-induced motor seizures in male Sprague-Dawley rats [69] | 1. Inefficient antagonist of bicuculline-induced seizures |
| 2.Epilepsy prone transgenic mice (DBA/2 strain) [67] | 2. Suppresses seizure activity, of both tonic and clonic extension seizures | ||
| Cl-IB-MECA | A3R agonist | Epilepsy prone mice (DBA/2 strain) [67] | Ineffective anti-epileptic |
| CPA | A1R agonist | Pentylenetetrazole-induced seizures in Wistar rats [70] | Significant protection against pentylenetetrazole-induced seizures |
| CPCA | A2AR agonist | 1. Pentylenetetrazole-induced seizures in Wistar rats [70] | 1. Ineffective anti-epileptic |
| 2. Epilepsy prone rats (GEPR-9 strain) [70] | 2. Suppresses seizure activity | ||
| DMPX | A2AR antagonist | Pentylenetetrazole-induced seizures in Wistar rats [71] | Kept protection afforded by CPA against Pentylenetetrazole-induced seizures |
| DPCPX | A1R antagonist | Pentylenetetrazole-induced seizures in Wistar rats [70] | Reverse protection afforded by CPA against Pentylenetetrazole-induced seizures |
| MRS1523 | A3R antagonist | Ex vivo seizure activity in hippocampal slices from Sprague-Dawley rats [72] | Reduced both seizure duration and intensity |
| Theophylline | Nonspecific adenosine receptor antagonist | Pentylenetetrazole-induced seizures in Wistar rats [70] | Reverse protection afforded by CPA against Pentylenetetrazole-induced seizures |
| ZM 241385 | A2AR antagonist | Ex vivo seizure activity in hippocampal slices from Sprague-Dawley rats [72] | Shorten the duration of epileptiform activity |
| Drug Name | MOA | Clinical Trial | Results |
|---|---|---|---|
| Adenosine | Non selective agonist | 1. The role of adenosine in the release of VEGF and Cytokines, Phase 1 [101] | 1. (NCT00580905) * Terminated |
| 2. A possible therapeutic role for adenosine during inflammation, Phase 1 [102,103] | 2. (NCT00513110) Completed | ||
| 3. Prophylactic intra-coronary adenosine to prevent post coronary artery stenting myonecrosis, Phase 3 [101] | 3. (NCT00612521) Terminated | ||
| 4. Postconditioning with adenosine for ST-elevated myocardial infarction, Phase 2 [104] | 4. (NCT00284323) Ongoing | ||
| 5. Myocardial protection with adenosine during primary percutaneous coronary intervention in patients with ST-elevated myocardial infarction, Phase 3 [105] | 5. (NCT00781404) Completed | ||
| 6. Clonidine versus adenosine to treat neuropathic pain, Phase 2 [106] | 6. (NCT00349921) Completed | ||
| 7. Dose response of adenosine for perioperative pain, Phase 2 [107] | 7. (NCT00298636) Completed | ||
| 8. Perioperative ischemia-induced liver injury and protection strategies [108] | 8. (NCT00760708) Ongoing | ||
| Apadenoson | A2AR agonist | Adenosine 2A agonist lexiscan in children and adults with sickle cell disease, Phase 1 [109] | (NCT01085201) Completed |
| Caffeine | Non selective antagonist | 1. Caffeine for motor manifestations of Parkinson’s disease, Phase 2 | 1. (NCT01190735) Completed |
| 2. Study investigating caffeine for excessive daytime somnolence if Parkinson’s disease, Phase 2 & 3 | 2. (NCT00459420) Completed | ||
| 3. Caffeine as a therapy for Parkinson’s disease, Phase 3 [88] | 3. (NCT01738178) Ongoing | ||
| CF-101 | A3R agonist | 1. Safety and efficacy study of CF101 to treat Psoriasis, Phase 2 [110] | 1. (NCT00428974) Completed |
| 2. Oral CF101 tablet and methotrexate treatment in Rheumatoid arthritis patients, Phase 2 [111] | 2. (NCT00556894) Completed | ||
| CF-102 | A3R agonist | A phase 1-2 Study of CF102 in patients with advanced hepatocellular carcinoma, Phase 1 & 2 [112] | (NCT00790218) Completed |
| Dipyridamole | Adenosine uptake inhibitor | 1. “Normal coronary artery” with slow flow improved by adenosine injection, dipyridamole treatment, and clinical follow-up, Phase 1 | 1. (NCT00960817) Recruitment status unknown |
| 2. Clinical trial of dipyridamole in Schizophrenia [90,113,114] | 2. (NCT00349973) Completed | ||
| 3. Can dipyridamole induce protection against ischemia and reperfusion injury in patients undergoing elective coronary artery bypass grafting, Phase 4 [115] | 3. (NCT01295567) Competed | ||
| 4. Circulating adenosine levels before and after Intravenous (IV) persantine [101] | 4. (NCT00760708) Terminated | ||
| 5. A phase II trial comparing Z-102 with placebo in patients with moderate to severe rheumatoid arthritis, Phase 2 [101] | 5. (NCT01369745) Completed | ||
| GW493838 | A1R agonist | The study of GW493838, an adenosine A1 receptor agonist, in peripheral neuropathic pain, Phase 2 [101] | (NCT00376454) Completed |
| INO 8875 | A1R agonist | 1. A dose-escalation study designed to evaluate the tolerability, safety, pharmacokinetics, and efficacy of chronic topical ocular application of INO-8875 in adults with ocular hypertension or primary open-angle glaucoma, Phase 1 [101] | 1. (NCT01123785) Completed |
| 2. Study of trabodenoson in adults with ocular hypertension or primary open-angle glaucoma, Phase 3 | 2. (NCT02565173) Completed | ||
| Istradefylline | A2AR antagonist | 1. Study of Istradefylline for the treatments of Parkinson’s disease in patients taking levodopa, Phase 3 [83,116] | 1. (NCT00955526) Completed |
| 2. Long-term study of Istradefylline in Parkinson’s disease patients, Phase 3 [87] | 2. (NCT00957203) Completed | ||
| 3. A 12-week randomized study to evaluate oral Istradefylline in subjects with moderate to severe Parkinson’s disease, Phase 3 | 3. (NCT01968031) Completed | ||
| 4. The effects of mild Hepatic impairment on the pharmacokinetics of Istradefylline, Phase 1 [86] | 4. (NCT02256033) Completed | ||
| 5. An extension of Istradefylline in North American Parkinson’s disease patients who have completed study 6002-INT-001, Phase 3 | 5. (NCT00199381) Terminated | ||
| 6. The effects of rifampin on the metabolism of Istradefylline in healthy volunteers, Phase 1 | 6. (NCT02174250) Completed | ||
| Preladenant | A2AR antagonist | 1. A placebo- and active-controlled study of preladenant in early Parkinson’s disease, Phase 3 | 1. (NCT01155479) Terminated |
| 2a. A placebo- and active-controlled study of preladenant in subjects with moderate or severe Parkinson’s disease, Phase 3 [117] | 2.a (NCT01155466) Completed | ||
| 2b. An active-controlled extension study to NCT01155466 [P04938] and NCT01227265 [P07037], Phase 3 | 2.b (NCT01215227) Terminated | ||
| 3. A placebo controlled study of preladenant in participants with moderate to severe Parkinson’s disease, Phase 3 [117] | 3. (NCT01227265) Completed | ||
| 4. A dose finding study of preladenant for the treatment of Parkinson’s disease, Phase 2 [118] | 4. (NCT01294800) Completed | ||
| Regadenoson | A2AR agonist | 1. Advance MPI2: Study of regadenoson versus adenoscan in patients undergoing myocardial perfusion imaging, Phase 3 [119] | 1. (NCT00208312) Completed |
| 2. Myocardial perfusion magnetic resonance imaging using regadenoson, Phase 1 [119,120,121] | 2. (NTC00881218) Completed | ||
| 3a. Adenosine 2A agonist lexiscan in children and adults with sickle cell disease, Phase 1 [109] | 3.a (NCT01085201) Completed | ||
| 3b. A phase II trial of regadenoson in sickle cell anemia, Phase 2 [122] | 3.b (NCT01085201) Currently recruiting | ||
| 4. Microvascular blood flow in sickle cell anemia [123,124] | 4. (NCT01566890) Currently recruiting | ||
| 5. Regadenoson blood flow in type 1 diabetes, Phase 4 [125] | 5. (NCT01019486) Completed | ||
| Rolofylline | A1R antagonist | 1. Protect-1, A study of the selective A1 adenosine receptor antagonist KW-3902 for patients hospitalized with acute HF and volume overload to assess treatment effect on congestion and renal function, Phase 3 [126,127] | 1. (NCT00328692) Completed |
| 2. Protect-2, A study of the selective A1 adenosine receptor antagonist KW-3902 for patients hospitalized with acute HF and volume overload to assess treatment effect on congestion and renal function, Phase 3 [127,128,129] | 2. (NCT00354458) Completed | ||
| SYN-115 | A2AR antagonist | 1. An fMRI study of SYN-115 in cocaine dependent subjects [130,131] | 1. (NCT00783276) Completed |
| 2. Safety and efficacy study of SYN-115 in Parkinson’s disease patients using levodopa to treat end of dose wearing off, Phase 2 & 3 [132] | 2. (NCT01283594) Completed | ||
| Tonapofylline | A1R antagonist | Study to assess the safety and tolerability of IV tonapofylline in subjects with acute decompensated heart failure and renal insufficiency, Phase 2 [133] | (NCT00709865) Completed |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stockwell, J.; Jakova, E.; Cayabyab, F.S. Adenosine A1 and A2A Receptors in the Brain: Current Research and Their Role in Neurodegeneration. Molecules 2017, 22, 676. https://doi.org/10.3390/molecules22040676
Stockwell J, Jakova E, Cayabyab FS. Adenosine A1 and A2A Receptors in the Brain: Current Research and Their Role in Neurodegeneration. Molecules. 2017; 22(4):676. https://doi.org/10.3390/molecules22040676
Chicago/Turabian StyleStockwell, Jocelyn, Elisabet Jakova, and Francisco S. Cayabyab. 2017. "Adenosine A1 and A2A Receptors in the Brain: Current Research and Their Role in Neurodegeneration" Molecules 22, no. 4: 676. https://doi.org/10.3390/molecules22040676






