Optimization of Ultrasound-Assisted Extraction, HPLC and UHPLC-ESI-Q-TOF-MS/MS Analysis of Main Macamides and Macaenes from Maca (Cultivars of Lepidium meyenii Walp)
Abstract
:1. Introduction
2. Results and Discussion
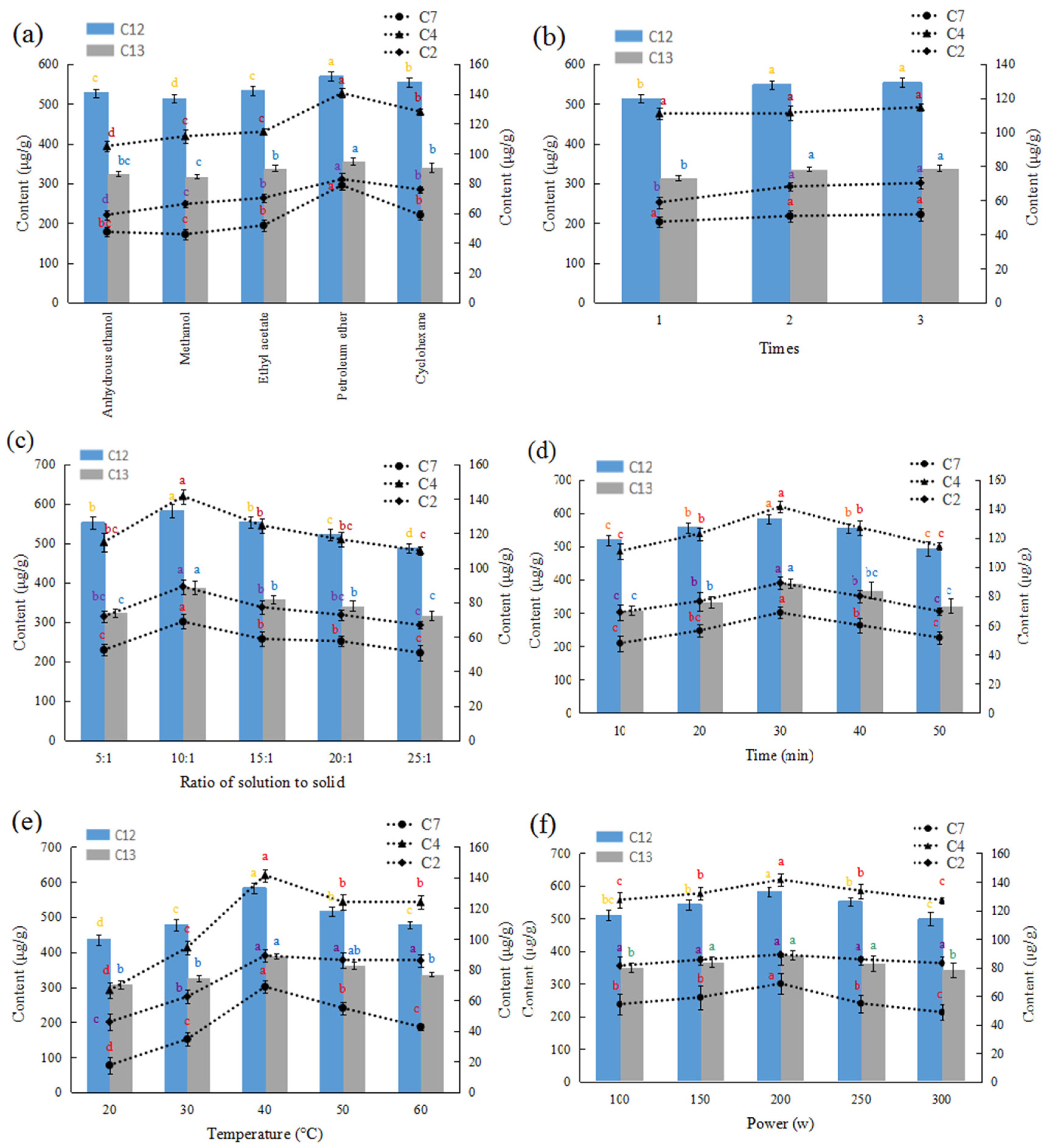
2.1. Single Factor Test
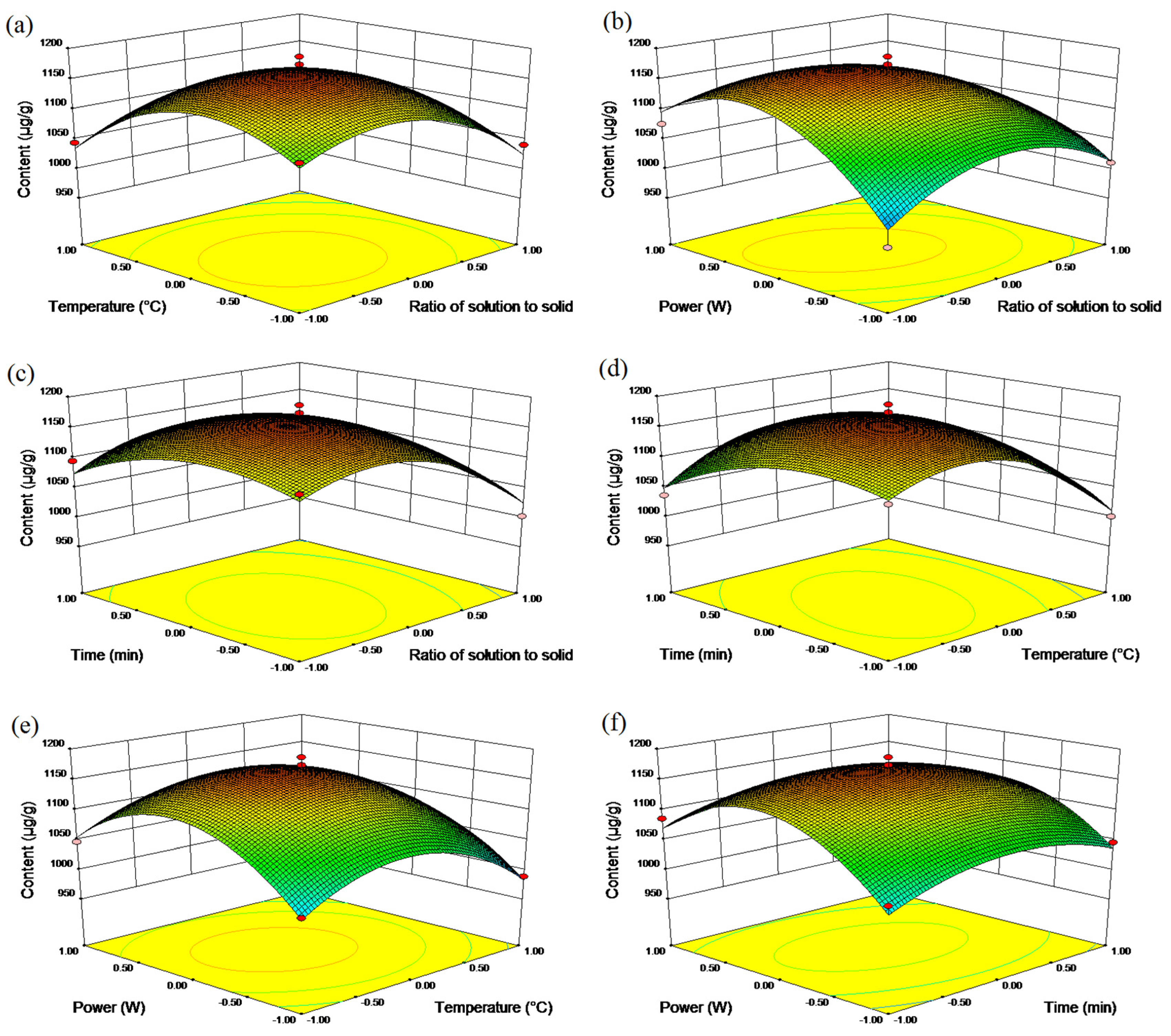
2.2. Statistical Analysis and Model Fitting using RSM
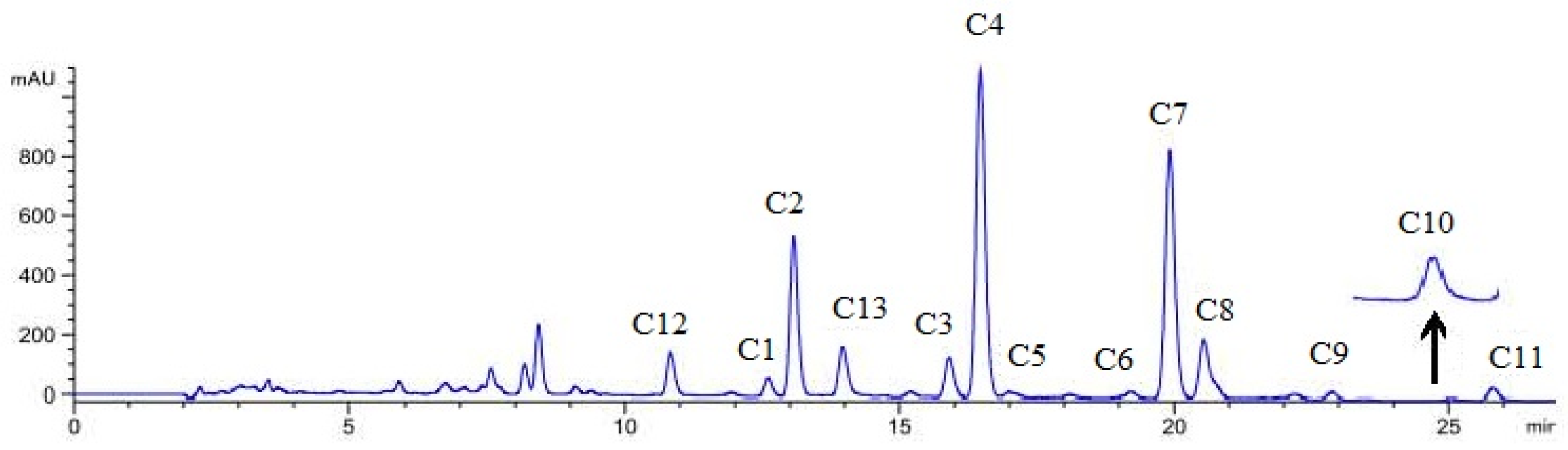
2.3. Qualitative Analysis
2.4. Quantitative Analysis
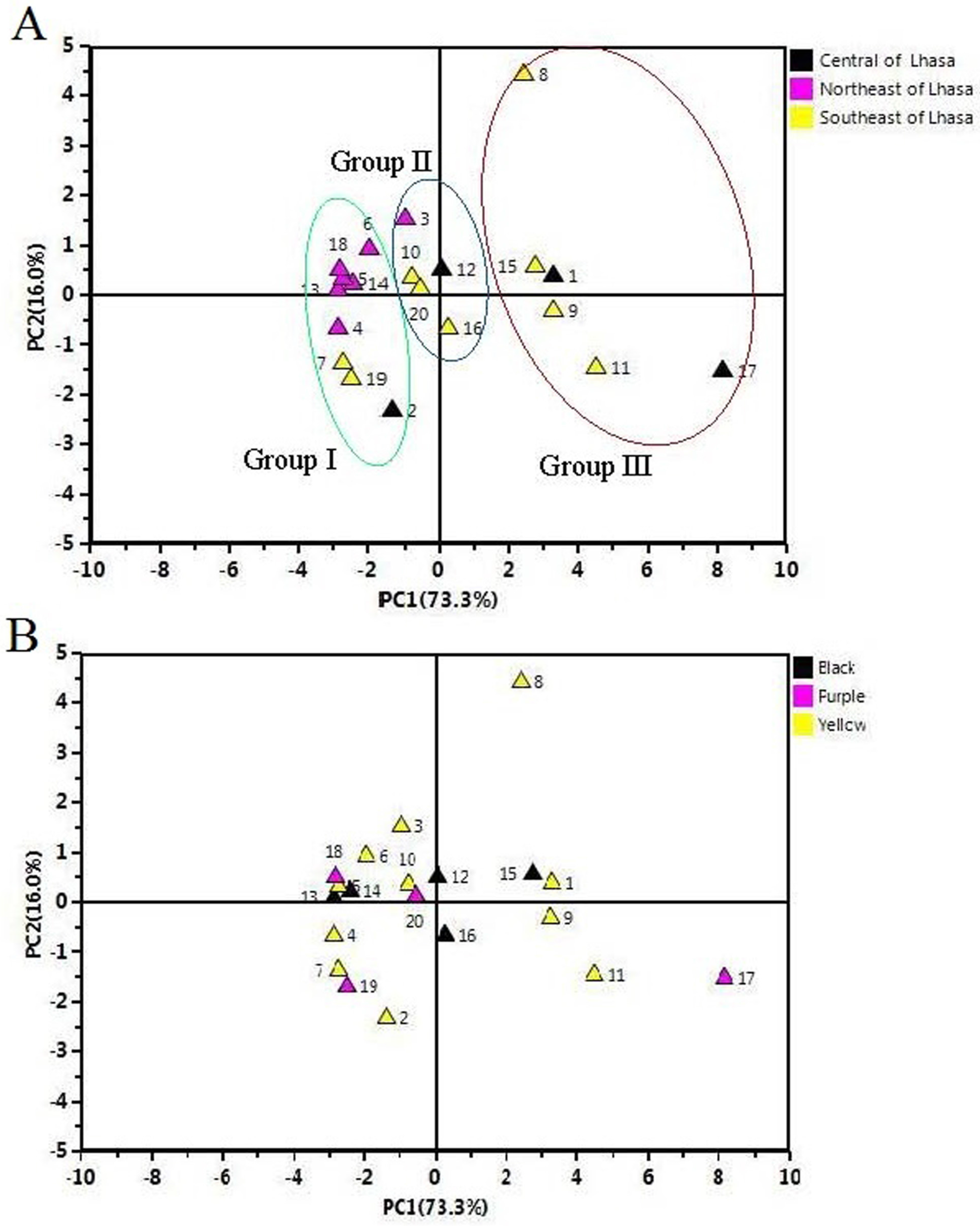
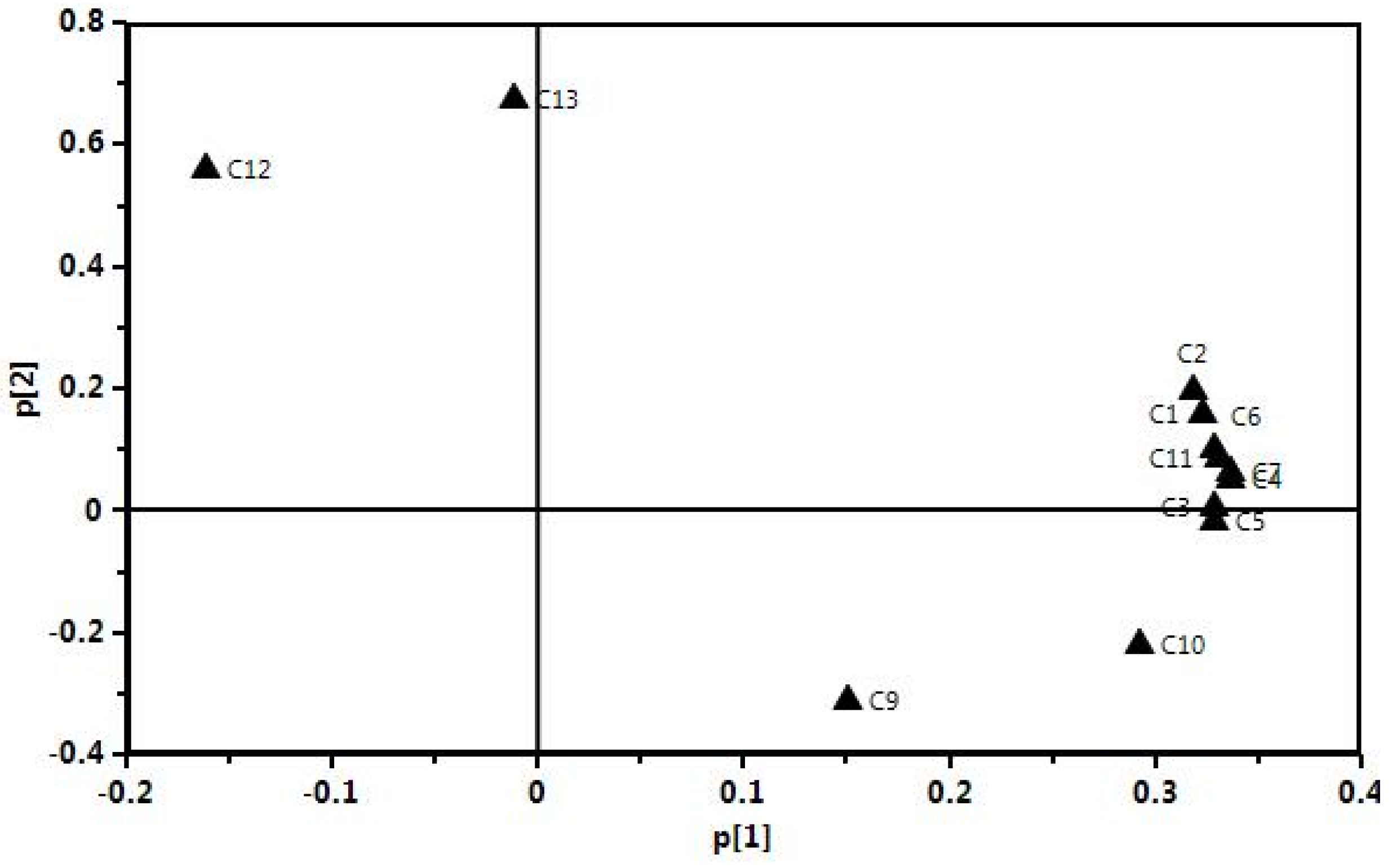
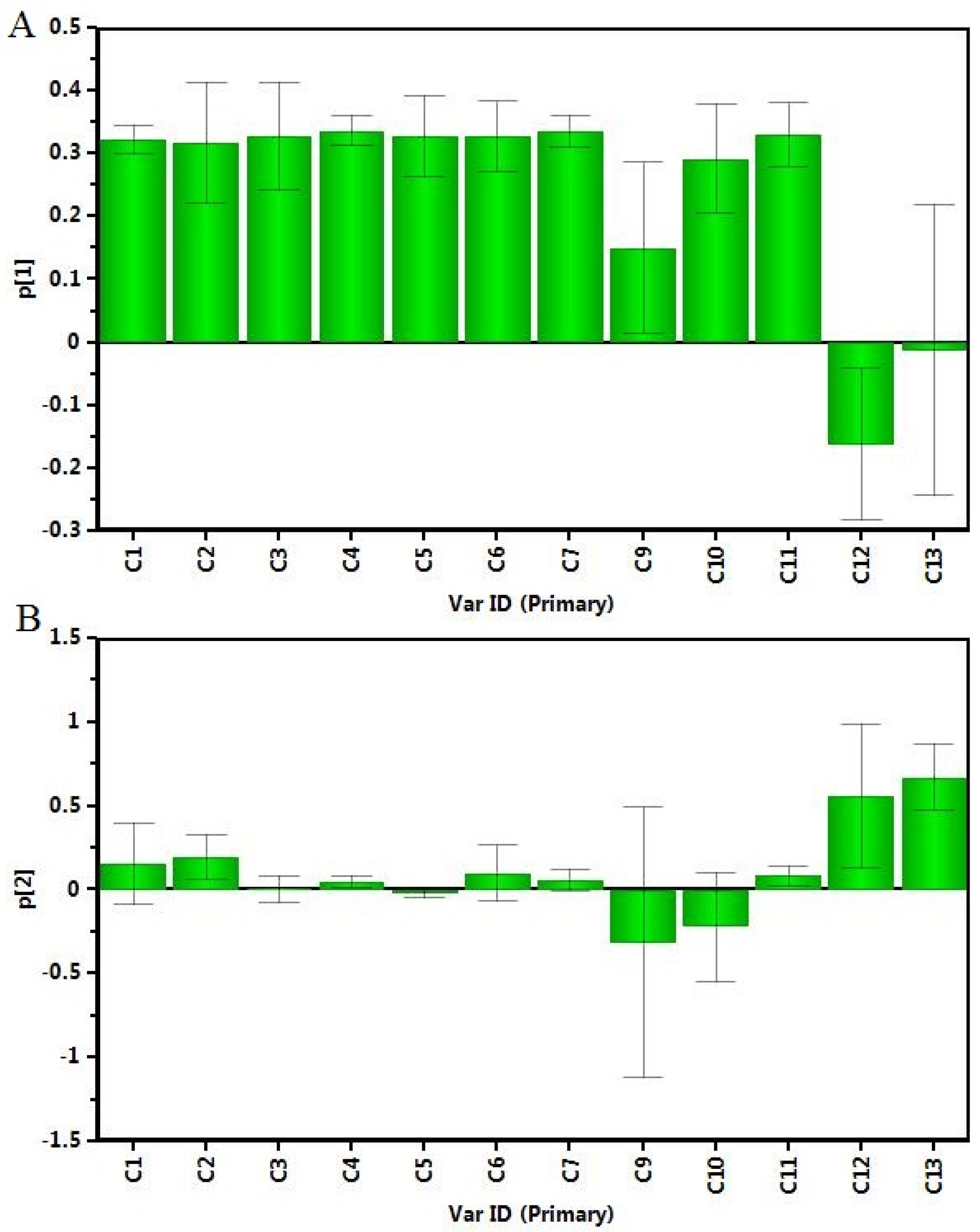
2.5. Principal Component Analysis (PCA) of the Samples
3. Materials and Methods
3.1. Plant Materials
3.2. Chemicals and Reagents
3.3. Equipment
3.4. Ultrasound-Assisted Extraction
3.5. Single Factor Experiment
3.6. Experimental Design and Data Dnalysis
3.7. Chemical Characterization of Extracts Obtained at Optimized Conditions
3.7.1. Qualitative Analysis
3.7.2. Quantitative Analysis
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McCollom, M.M.; Villinski, J.R.; McPhail, K.L.; Craker, L.E.; Gafner, S. Analysis of macamides in samples of Maca (Lepidium meyenii) by HPLC-UV-MS/MS. Phytochem. Anal. 2005, 16, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jin, W.; Lv, X.; Dai, P.; Ao, Y.; Wu, M.; Deng, W.; Yu, L. Effects of macamides on endurance capacity and anti-fatigue property in prolonged swimming mice. Pharm. Biol. 2016, 54, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Almukadi, H.; Wu, H.; Böhlke, M.; Kelley, C.J.; Maher, T.J.; Pino-Figueroa, A. The macamide N-3-methoxybenzyl-linoleamide is a time-dependent fatty acid amide hydrolase (FAAH) inhibitor. Mol. Neurobiol. 2013, 48, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Kelley, C.J.; Pino-Figueroa, A.; Vu, H.D.; Maher, T.J. Macamides and their synthetic analogs: Evaluation of in vitro FAAH inhibition. Bioorg. Med. Chem. 2013, 21, 5188–5197. [Google Scholar] [CrossRef] [PubMed]
- Hajdu, Z.; Nicolussi, S.; Rau, M.; Lorántfy, L.; Forgo, P.; Hohmann, J.; Csupor, D.; Gertsch, J. Identification of endocannabinoid system-modulating N-alkylamides from Heliopsis helianthoides var. scabra and Lepidium meyenii. J. Nat. Prod. 2014, 77, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Pino-Figueroa, A.; Nguyen, D.; Maher, T.J. Neuroprotective effects of Lepidium meyenii (Maca). Ann. N. Y. Acad. Sci. 2010, 1199, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, P.; Brantner, A.; Wang, H.; Shu, X.; Yang, J.; Si, N.; Han, L.; Zhao, H.; Bian, B. Chemical profiling analysis of Maca using UHPLC-ESI-Orbitrap MS coupled with UHPLC-ESI-QqQ MS and the neuroprotective study on its active ingredients. Sci. Rep. 2017, 7, 44660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, L.; Ao, M.; Jin, W. Effect of ethanol extract of Lepidium meyenii Walp. on osteoporosis in ovariectomized rat. J. Ethnopharmacol. 2006, 105, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.L.; He, K.; Kim, C.H.; Rogers, L.; Shao, Y.; Huang, Z.Y.; Lu, Y.; Yan, S.J.; Qien, L.C.; Zheng, Q.Y. Effect of a lipidic extract from lepidium meyenii on sexual behavior in mice and rats. Urology 2000, 55, 598–602. [Google Scholar] [CrossRef]
- Gonzales, C.; Rubio, J.; Gasco, M.; Nieto, J.; Yucra, S.; Gonzales, G.F. Effect of short-term and long-term treatments with three ecotypes of Lepidium meyenii (MACA) on spermatogenesis in rats. J. Ethnopharmacol. 2006, 103, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, F.; Jikyo, T.; Takeda, R.; Ogata, M. Lepidium meyenii (Maca) enhances the serum levels of luteinising hormone in female rats. J. Ethnopharmacol. 2014, 151, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.; Chirinos, R.; Barreto, O.; Noratto, G.; Pedreschi, R. Optimized methodology for the simultaneous extraction of glucosinolates, phenolic compounds and antioxidant capacity from maca (Lepidium meyenii). Ind. Crops Prod. 2013, 49, 747–754. [Google Scholar] [CrossRef]
- Zha, S.; Zhao, Q.; Chen, J.; Wang, L.; Zhang, G.; Zhang, H.; Zhao, B. Extraction, purification and antioxidant activities of the polysaccharides from maca (Lepidium meyenii). Carbohydr. Polym. 2014, 111, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zhang, H.; Zheng, H.; Zhang, J.; Gan, J. Optimization of glucosinolates extracted from maca (Lepidium meyenii) by combined ultrasonic and microwave extraction with response surface methodology. Adv. Mater. Res. 2012, 550–553, 1893–1899. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, J.; Li, H.; Wang, Y.Z.; Li, W.Y. Simultaneous analysis of macamides in maca (Lepidium meyenii) with different drying process by liquid chromatography tandem mass spectrometry. Food Anal. Methods 2016, 9, 1686–1695. [Google Scholar] [CrossRef]
- Chain, F.E.; Grau, A.; Martins, J.C.; Catalan, C.A.N. Macamides from wild ‘Maca’, Lepidium meyenii Walpers (Brassicaceae). Phytochem. Lett. 2014, 8, 145–148. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Qi, Y.; Li, S.; Pan, Y.; Li, Y. Circulating ultrasound-assisted extraction, countercurrent chromatography, and liquid chromatography for the simultaneous extraction, isolation, and analysis of the constituents of Uncaria tomentosa. J. Chromatogr. A 2015, 1388, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Chavan, Y.; Singhal, R.S. Ultrasound-assisted extraction (UAE) of bioactives from arecanut (Areca catechu L.) and optimization study using response surface methodology. Innov. Food Sci. Emerg. Technol. 2013, 17, 106–113. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; Wang, J.; Liu, Z.; Yang, G.; Geng, G. Ultrasound-assisted solvent extraction of swainsonine from Oxytropis ochrocephala Bunge. J. Med. Plants Res. 2011, 5, 890–894. [Google Scholar]
- Li, Y.; Zeng, R.J.; Lu, Q.; Wu, S.S.; Chen, J.Z. Ultrasound/microwave-assisted extraction and comparative analysis of bioactive/toxic indole alkaloids in different medicinal parts of Gelsemium elegans Benth by ultra-high performance liquid chromatography with MS/MS. J. Sep. Sci. 2014, 37, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Z.; Zhang, Z.J.; Hou, T.Y.; Li, X.J.; Chen, T. Optimization of ultrasound-assisted hexane extraction of perilla oil using response surface methodology. Ind. Crops Prod. 2015, 76, 18–24. [Google Scholar] [CrossRef]
- Tao, Y.; Wu, D.; Zhang, Q.A.; Sun, D.W. Ultrasound-assisted extraction of phenolics from wine lees: Modeling, optimization and stability of extracts during storage. Ultrason. Sononchem. 2014, 21, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Ghitescu, R.E.; Volf, I.; Carausu, C.; Bühlmann, A.M.; Gilca, I.A.; Popa, V.I. Optimization of ultrasound-assisted extraction of polyphenols from spruce wood bark. Ultrason. Sonochem. 2015, 22, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, B.; Luo, C.; Luo, Z.; Huang, D. Analysis of macaenes and macamides in maca plant by high performance liquid chromatography-mass. Fenxi Yiqi 2014, 15, 44–49. [Google Scholar]
- Pan, Y.; Zhang, J.; Li, H.; Wang, Y.Z.; Li, W.Y. Characteristic fingerprinting based on macamides for discrimination of maca (Lepidium meyenii) by LC/MS/MS and multivariate statistical analysis. J. Sci. Food Agric. 2016, 96, 4475–4483. [Google Scholar] [CrossRef] [PubMed]
- Ganzera, M.; Zhao, J.; Muhammad, I.; Khan, I.A. Chemical profiling and standardization of Lepidium meyenii (Maca) by reversed phase high performance liquid chromatography. Chem. Pharm. Bull. 2002, 50, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Desgrouas, C.; Baghdikian, B.; Mabrouki, F.; Bory, S.; Taudon, N.; Parzy, D.; Ollivier, E. Rapid and green extraction, assisted by microwave and ultrasound of cepharanthine from Stephania rotunda Lour. Sep. Purif. Technol. 2014, 123, 9–14. [Google Scholar] [CrossRef]
- Dong, B.; Yuan, X.; Zhao, Q.; Feng, Q.; Liu, B.; Guo, Y.; Zhao, B. Ultrasound-assisted aqueous two-phase extraction of phenylethanoid glycosides from Cistanche deserticola Y.C. Ma stems. J. Sep. Sci. 2015, 38, 1194–1203. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Shao, D.; Yuan, Y.; Wang, Z.; Qiang, C. Ultrasound-assisted extraction, HPLC analysis, and antioxidant activity of polyphenols from unripe apple. J. Sep. Sci. 2012, 35, 2138–2145. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the used plant materials are available from the authors. |
| Run No. | X1: Ratio of Solution to Solid (mL/g) | X2 : Extraction Temperature (°C) | X3: Extraction Time (min) | X4: Extraction Power (W) | Content (μg/g) |
|---|---|---|---|---|---|
| 1 | 0 (10:1) | 0 (40) | 0 (30) | 0 (200) | 1173.80 |
| 2 | 0 (10:1) | −1 (30) | 0 (30) | –1 (150) | 1017.79 |
| 3 | 0 (10:1) | 0 (40) | 0 (30) | 0 (200) | 1186.45 |
| 4 | –1 (5:1) | 0 (40) | 0 (30) | 1 (250) | 1076.28 |
| 5 | 0 (10:1) | –1 (30) | 0 (30) | 1 (250) | 1047.24 |
| 6 | 0 (10:1) | 0 (40) | –1 (20) | 1 (250) | 1086.08 |
| 7 | 0 (10:1) | 0 (40) | 0 (30) | 0 (200) | 1158.12 |
| 8 | 0 (10:1) | 0 (40) | 0 (30) | 0 (200) | 1164.44 |
| 9 | 0 (10:1) | 1 (50) | 1 (40) | 0 (200) | 1038.88 |
| 10 | 0 (10:1) | 1 (50) | 0 (30) | 1 (250) | 985.32 |
| 11 | 1 (15:1) | 0 (40) | 1 (40) | 0 (200) | 1032.04 |
| 12 | 0 (10:1) | 0 (40) | 1 (40) | –1 (150) | 1046.16 |
| 13 | –1 (5:1) | 0 (40) | 0 (30) | –1 (150) | 970.92 |
| 14 | –1 (5:1) | 1 (50) | 0 (30) | 0 (200) | 1044.56 |
| 15 | 0 (10:1) | 1 (50) | 0 (30) | –1 (150) | 988.48 |
| 16 | 1 (15:1) | 1 (50) | 0 (30) | 0 (200) | 1001.09 |
| 17 | –1 (5:1) | –1 (30) | 0 (30) | 0 (200) | 1099.08 |
| 18 | 0 (10:1) | 0 (40) | 1 (40) | 1 (250) | 1047.16 |
| 19 | 1 (15:1) | 0 (40) | –1 (20) | 0 (200) | 1002.16 |
| 20 | 0 (10:1) | 0 (40) | –1 (20) | –1 (150) | 1036.44 |
| 21 | 0 (10:1) | 1 (50) | –1 (20) | 0 (200) | 1000.48 |
| 22 | 1 (15:1) | 0 (40) | 0 (30) | –1 (150) | 1010.80 |
| 23 | 0 (10:1) | –1 (30) | 1 (40) | 0 (200) | 1036.68 |
| 24 | 1 (15:1) | –1 (30) | 0 (30) | 0 (200) | 1041.04 |
| 25 | 0 (10:1) | –1 (30) | –1 (20) | 0 (200) | 1108.80 |
| 26 | 1 (15:1) | 0 (40) | 0 (30) | 1 (250) | 966.24 |
| 27 | 0 (10:1) | 0 (40) | 0 (30) | 0 (200) | 1163.08 |
| 28 | –1 (5:1) | 0 (40) | –1 (20) | 0 (200) | 1124.24 |
| 29 | –1 (5:1) | 0 (40) | 1 (40) | 0 (200) | 1095.32 |
| Source | Sum of Squares | DF | Mean Square | F-Value | P-Value |
|---|---|---|---|---|---|
| Model | 1.02 × 105 | 14 | 7.91 × 103 | 22.28 | <0.0001 *** |
| X1 | 1.06 × 104 | 1 | 1.06 × 104 | 29.92 | <0.0001 *** |
| X2 | 7.10 × 103 | 1 | 7.10 × 103 | 19.99 | 0.0005 ** |
| X3 | 3.20 × 102 | 1 | 3.20 × 102 | 0.90 | 0.3586 |
| X4 | 1.58 × 103 | 1 | 1.58 × 103 | 4.45 | 0.0533 |
| X1X2 | 53.12 | 1 | 5.3.12 | 0.15 | 0.7049 |
| X1X3 | 8.64 × 102 | 1 | 8.64 × 102 | 2.43 | 0.1410 |
| X1X4 | 5.62 × 103 | 1 | 5.62 × 103 | 15.82 | 0.0014 ** |
| X2X3 | 3.05 × 103 | 1 | 3.05 × 103 | 8.60 | 0.0109 * |
| X2X4 | 2.66 × 102 | 1 | 2.66 × 102 | 0.75 | 0.4015 |
| X3X4 | 5.91 × 102 | 1 | 5.91 × 102 | 1.67 | 0.2177 |
| X12 | 2.68 × 104 | 1 | 2.68 × 104 | 75.42 | <0.0001 *** |
| X22 | 3.27 × 104 | 1 | 3.27 × 104 | 92.21 | <0.0001 *** |
| X32 | 1.06 × 104 | 1 | 1.06 × 104 | 29.85 | <0.0001 *** |
| X42 | 4.95 × 104 | 1 | 4.95 × 104 | 139.42 | <0.0001 *** |
| Residual | 4.97 × 103 | 14 | 3.55 × 102 | ||
| Lack of Fit | 4.47 × 103 | 10 | 4.47 × 102 | 3.56 | 0.1160 |
| Pure Error | 5.02 × 102 | 4 | 1.25 × 102 | ||
| Cor Total | 1.16 × 105 | 28 |
| Compounds | Retention Time (min) | Chemical Structure | Molecular Formula | Measured Value (m/z) | Main Fragment Ions |
|---|---|---|---|---|---|
| C1 | 6.388 |  | C26H39NO2 | 398.3073 [M + H]+ | 138.0920,121.0653 |
| C2 | 6.643 |  | C25H37NO | 368.2968 [M + H]+ | 108.0812, 91.0549 |
| C3 | 9.006 |  | C26H41NO2 | 400.3227 [M + H]+ | 121.0645 |
| C4 | 9.316 |  | C25H39NO | 370.3127 [M + H]+ | 91.0547 |
| C5 | 9.912 |  | C22H37NO | 332.2953 [M + H]+ | 91.0551 |
| C6 | 11.061 |  | C24H41NO2 | 376.3225 [M + H]+ | 138.0918, 121.0652 |
| C7 | 11.447 |  | C23H39NO | 346.3125 [M + H]+ | 91.0548 |
| C8 | 13.005 |  | C25H41NO | 372.3276 [M + H]+ | 108.0808, 91.0547 |
| C9 | 14.507 |  | C24H41NO | 360.3282 [M + H]+ | 91.0548 |
| C10 | 17.335 |  | C26H45NO2 | 404.3546 [M + H]+ | 138.0915, 121.0653 |
| C11 | 17.876 |  | C25H43NO | 374.3437 [M + H]+ | 91.0549 |
| C12 | 2.673 |  | C18H30O2 | 277.2173 [M − H]− | |
| C13 | 3.91 |  | C18H32O2 | 279.2334 [M − H]− |
| Analytes | Content (μg/g) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yellow Maca | |||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| C1 | 48.37 ± 0.66 | 12.03 ± 0.26 | 20.13 ± 0.83 | 2.09 ± 0.17 | 5.25 ± 0.44 | 11.77 ± 0.47 | 3.36 ± 0.25 | 65.93 ± 0.70 | 50.48 ± 0.60 | 13.48 ± 0.73 | 49.78 ± 0.45 |
| C2 | 319.54 ± 5.65 | 71.55 ± 2.33 | 179.46 ± 5.20 | 4.07 ± 0.14 | 27.13 ± 0.55 | 80.34 ± 2.80 | 11.74 ± 0.25 | 520.73 ± 0.45 | 306.60 ± 4.76 | 158.87 ± 4.67 | 484.85 ± 4.51 |
| C3 | 77.37 ± 1.41 | 9.79 ± 0.16 | 15.39 ± 0.66 | 1.19 ± 0.03 | 3.08 ± 0.12 | 11.80 ± 0.45 | 2.24 ± 0.08 | 55.75 ± 1.43 | 68.52 ± 1.43 | 15.27 ± 0.31 | 63.85 ± 1.21 |
| C4 | 792.77 ± 10.44 | 95.80 ± 4.17 | 200.59 ± 6.53 | 15.49 ± 0.14 | 40.97 ± 0.27 | 137.33 ± 3.61 | 27.20 ± 0.52 | 693.99 ± 3.36 | 628.00 ± 9.96 | 285.06 ± 13.85 | 951.90 ± 9.73 |
| C5 | 23.01 ± 0.57 | 6.97 ± 0.19 | 3.12 ± 0.11 | - | - | - | - | 13.79 ± 0.42 | 21.54 ± 1.00 | 5.33 ± 0.17 | 17.82 ± 0.14 |
| C6 | 58.64 ± 1.27 | - | 28.48 ± 0.56 | - | - | 12.81 ± 0.21 | - | 50.15 ± 1.09 | 66.17 ± 1.40 | 39.39 ± 1.05 | 66.34 ± 0.56 |
| C7 | 935.14 ± 9.48 | 91.79 ± 0.44 | 266.04 ± 5.52 | 10.33 ± 0.20 | 26.13 ± 0.58 | 80.65 ± 2.30 | 20.81 ± 0.30 | 776.32 ± 12.46 | 880.22 ± 21.17 | 346.25 ± 6.39 | 1018.49 ± 8.52 |
| C9 | 20.52 ± 0.62 | 62.31 ± 2.36 | 6.45 ± 0.28 | 0.89 ± 0.02 | 3.20 ± 0.11 | 2.85 ± 0.11 | 1.80 ± 0.05 | 9.84 ± 0.22 | 19.50 ± 0.45 | 4.72 ± 0.14 | 14.12 ± 0.20 |
| C10 | 4.19 ± 0.21 | - | - | - | - | - | - | - | 10.80 ± 0.30 | - | 21.82 ± 0.99 |
| C11 | 70.02 ± 0.86 | 15.54 ± 0.59 | 34.48 ± 1.71 | - | - | 18.66 ± 0.89 | - | 58.19 ± 0.52 | 70.96 ± 2.53 | 21.79 ± 0.17 | 78.99 ± 2.80 |
| C12 | 308.31 ± 5.96 | 380.98 ± 11.13 | 653.97 ± 16.75 | 473.95 ± 13.96 | 571.64 ± 2.87 | 529.31 ± 16.42 | 341.36 ± 1.85 | 875.03 ± 24.73 | 325.54 ± 6.18 | 420.43 ± 12.48 | 220.65 ± 2.87 |
| C13 | 330.89 ± 13.42 | 197.32 ± 6.71 | 340.79 ± 5.30 | 216.99 ± 3.37 | 286.53 ± 10.33 | 346.17 ± 10.77 | 193.73 ± 2.82 | 476.05 ± 11.29 | 272.68 ± 4.82 | 304.74 ± 9.04 | 185.17 ± 2.84 |
| Total | 2988.77 ± 50.55 | 944.08 ± 28.34 | 1748.90 ± 43.45 | 725.00 ± 18.02 | 963.93 ± 15.27 | 1231.69 ± 35.73 | 602.24 ± 6.12 | 3595.77 ± 56.67 | 2721.01 ± 60.60 | 1615.33 ± 49.00 | 3173.78 ± 34.82 |
| Analytes | Content (μg/g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Black Maca | Purple Maca | ||||||||
| 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
| C1 | 21.84 ± 0.99 | 2.63 ± 0.18 | 10.24 ± 0.33 | 32.11 ± 0.54 | 29.71 ± 0.24 | 81.74 ± 0.89 | 3.92 ± 0.13 | 5.36 ± 0.39 | 13.33 ± 0.55 |
| C2 | 174.65 ± 4.42 | 3.44 ± 0.08 | 53.37 ± 1.83 | 367.70 ± 7.06 | 219.94 ± 3.61 | 539.51 ± 6.30 | 13.97 ± 0.53 | 19.80 ± 0.29 | 145.42 ± 1.37 |
| C3 | 36.09 ± 0.84 | 0.87 ± 0.03 | 6.69 ± 0.21 | 36.03 ± 0.57 | 42.44 ± 1.73 | 143.13 ± 1.34 | 2.84 ± 0.11 | 4.13 ± 0.03 | 14.58 ± 0.61 |
| C4 | 444.00 ± 8.57 | 13.66 ± 0.57 | 69.51 ± 2.32 | 624.32 ± 10.70 | 343.07 ± 6.77 | 1383.67 ± 8.02 | 31.22 ± 1.42 | 38.18 ± 0.64 | 252.42 ± 0.47 |
| C5 | 10.84 ± 0.17 | - | - | 26.22 ± 0.52 | 7.50 ± 0.47 | 42.18 ± 0.46 | - | - | 10.44 ± 0.41 |
| C6 | 30.97 ± 1.35 | - | - | 61.79 ± 1.62 | 35.09 ± 0.92 | 96.22 ± 4.01 | - | - | 34.60 ± 1.52 |
| C7 | 417.42 ± 8.67 | 9.81 ± 0.41 | 57.20 ± 0.80 | 1023.70 ± 20.95 | 383.68 ± 6.77 | 1571.22 ± 6.57 | 16.71 ± 0.44 | 29.05 ± 0.07 | 401.57 ± 8.72 |
| C9 | 12.53 ± 0.17 | 1.94 ± 0.07 | 3.62 ± 0.17 | 11.46 ± 0.31 | 6.95 ± 0.17 | 38.03 ± 0.64 | 3.61 ± 0.06 | 18.54 ± 0.13 | 6.63 ± 0.20 |
| C10 | 2.72 ± 0.13 | - | - | 6.99 ± 0.16 | 6.71 ± 0.31 | 19.71 ± 0.64 | - | - | - |
| C11 | 19.33 ± 0.20 | - | 10.15 ± 0.21 | 69.58 ± 1.07 | 21.46 ± 0.44 | 104.13 ± 2.10 | - | - | 34.23 ± 1.54 |
| C12 | 366.72 ± 17.91 | 531.46 ± 20.04 | 563.16 ± 4.04 | 417.84 ± 12.20 | 329.02 ± 5.07 | 173.85 ± 1.74 | 600.06 ± 22.97 | 338.18 ± 2.90 | 412.03 ± 16.42 |
| C13 | 365.56 ± 13.56 | 279.45 ± 7.35 | 269.40 ± 2.14 | 302.32 ± 7.46 | 244.40 ± 2.59 | 190.08 ± 8.40 | 298.12 ± 11.46 | 195.91 ± 2.05 | 290.77 ± 9.90 |
| Total | 1902.67 ± 56.98 | 843.26 ± 28.73 | 1043.34 ± 12.05 | 2980.06 ± 63.44 | 1669.97 ± 29.09 | 4383.40 ± 41.11 | 970.45 ± 37.12 | 649.15 ± 6.50 | 1616.02 ± 41.71 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-X.; Li, K.-K.; Pubu, D.; Jiang, S.-P.; Chen, B.; Chen, L.-R.; Yang, Z.; Ma, C.; Gong, X.-J. Optimization of Ultrasound-Assisted Extraction, HPLC and UHPLC-ESI-Q-TOF-MS/MS Analysis of Main Macamides and Macaenes from Maca (Cultivars of Lepidium meyenii Walp). Molecules 2017, 22, 2196. https://doi.org/10.3390/molecules22122196
Chen S-X, Li K-K, Pubu D, Jiang S-P, Chen B, Chen L-R, Yang Z, Ma C, Gong X-J. Optimization of Ultrasound-Assisted Extraction, HPLC and UHPLC-ESI-Q-TOF-MS/MS Analysis of Main Macamides and Macaenes from Maca (Cultivars of Lepidium meyenii Walp). Molecules. 2017; 22(12):2196. https://doi.org/10.3390/molecules22122196
Chicago/Turabian StyleChen, Shu-Xiao, Ke-Ke Li, Duoji Pubu, Si-Ping Jiang, Bin Chen, Li-Rong Chen, Zhen Yang, Chao Ma, and Xiao-Jie Gong. 2017. "Optimization of Ultrasound-Assisted Extraction, HPLC and UHPLC-ESI-Q-TOF-MS/MS Analysis of Main Macamides and Macaenes from Maca (Cultivars of Lepidium meyenii Walp)" Molecules 22, no. 12: 2196. https://doi.org/10.3390/molecules22122196





