Margination of Fluorescent Polylactic Acid–Polyaspartamide based Nanoparticles in Microcapillaries In Vitro: the Effect of Hematocrit and Pressure
Abstract
:1. Introduction
2. Results
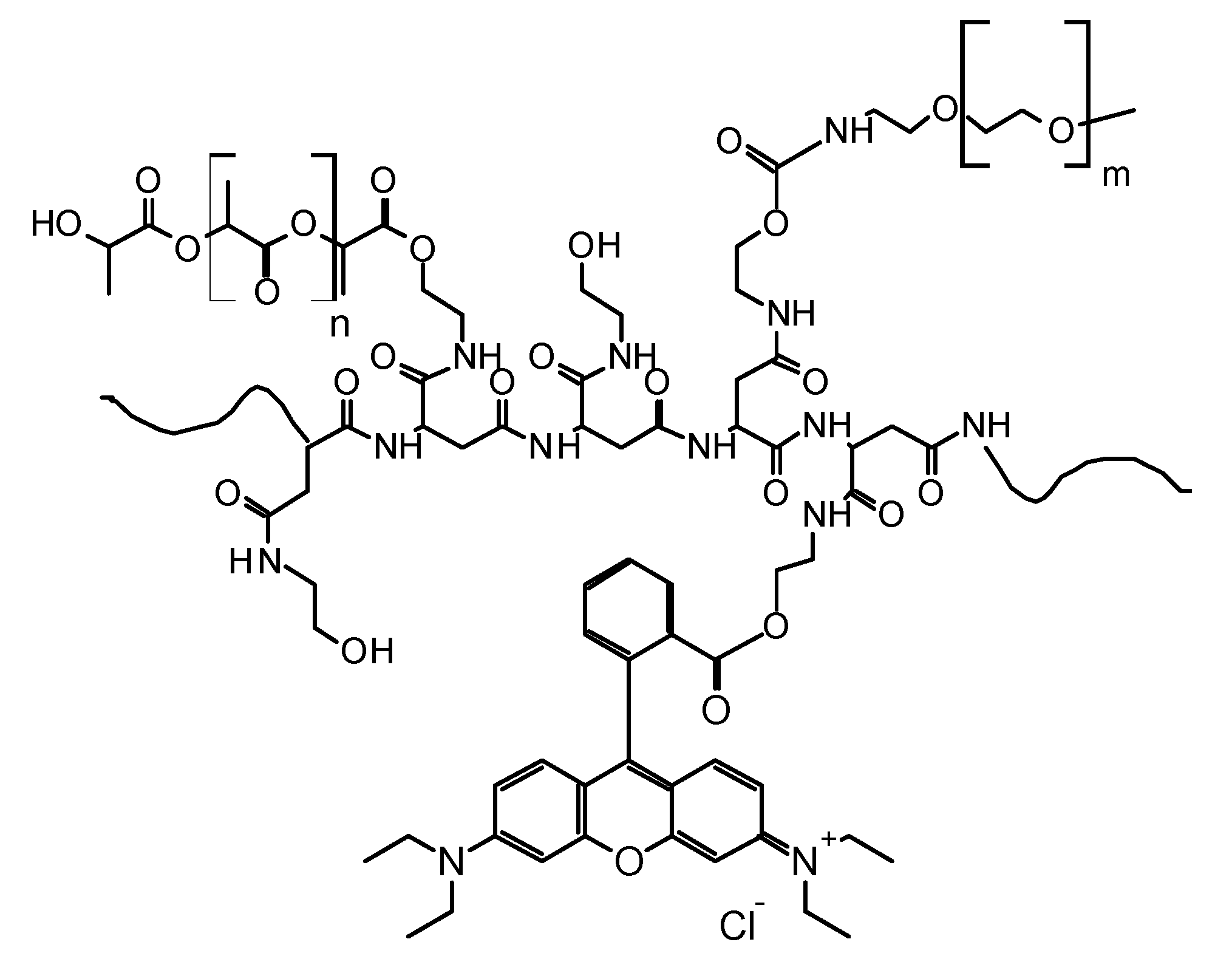
2.1. Design of Fluorescent Polymeric Nanoparticles (FNPs)
2.2. Evaluation of FNPs Margination
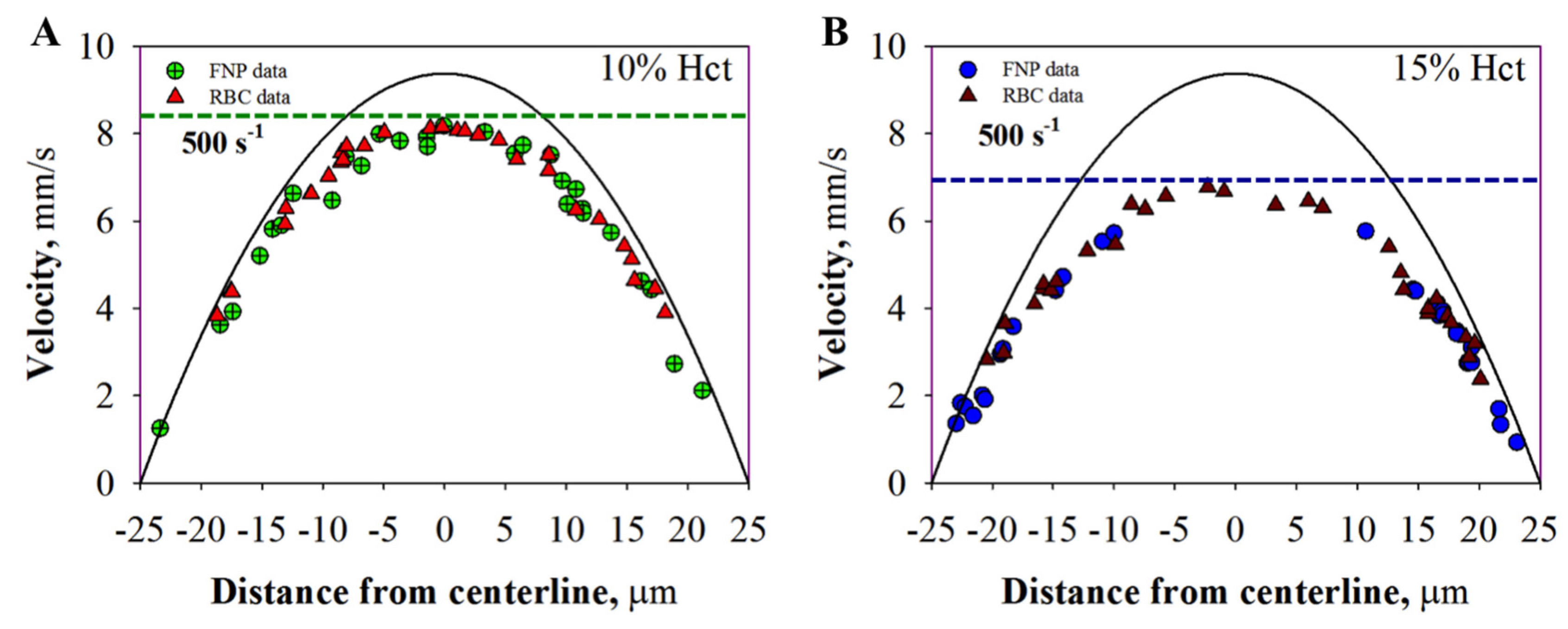
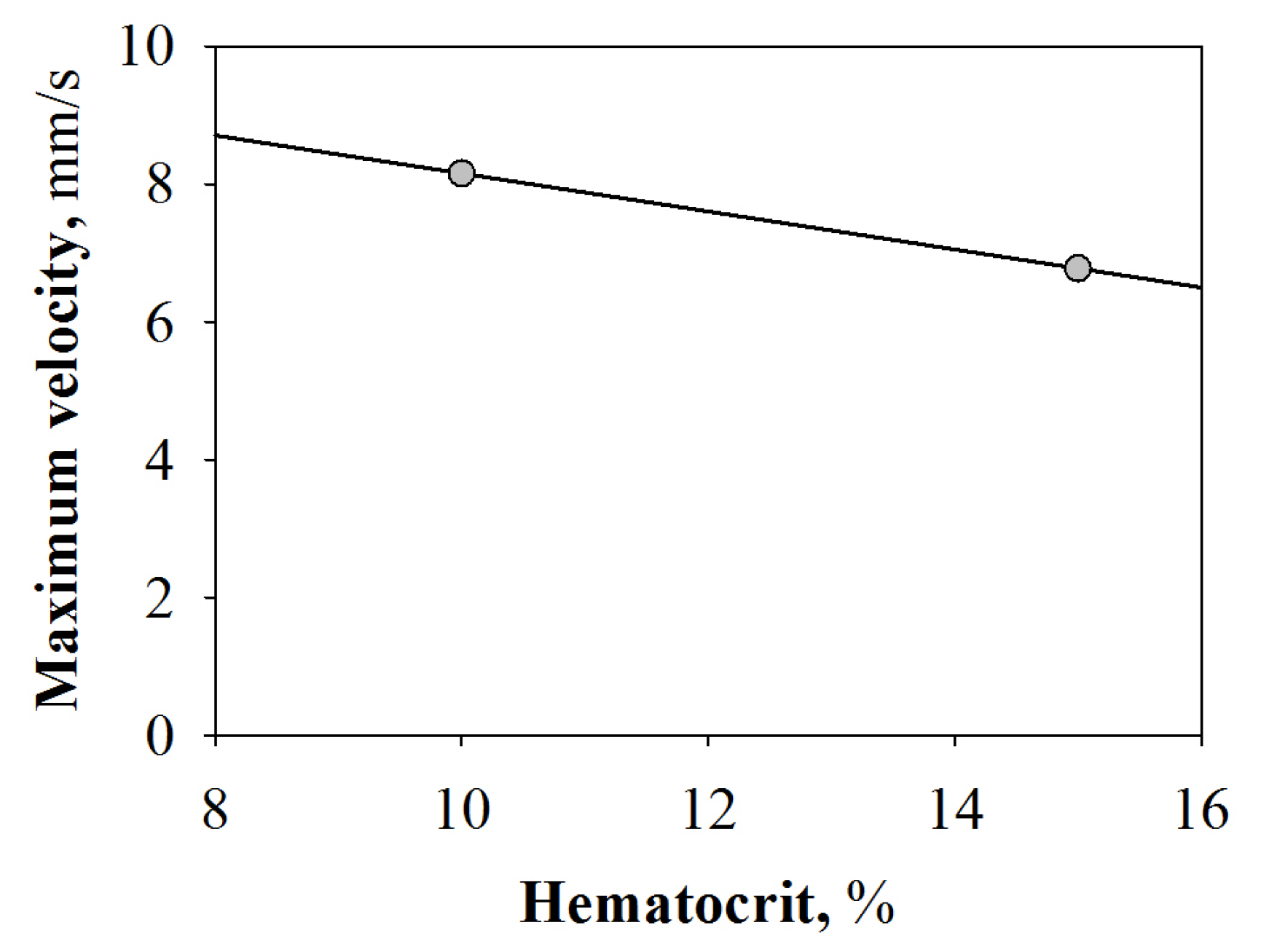
2.2.1. Velocity Profile
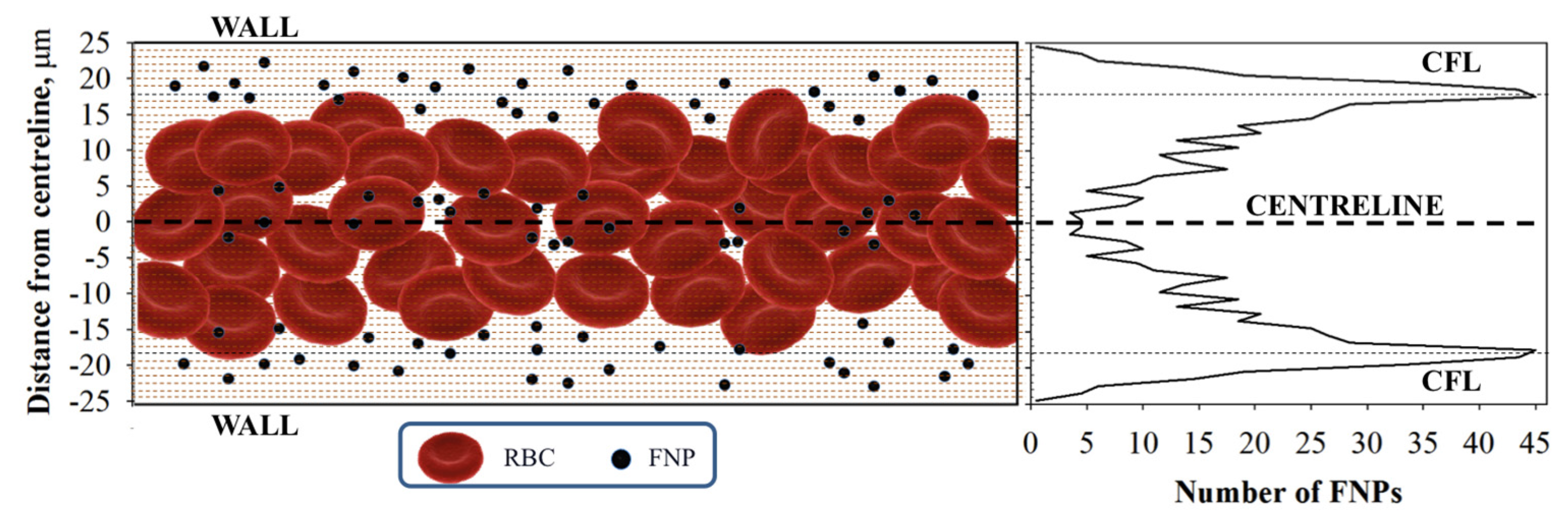
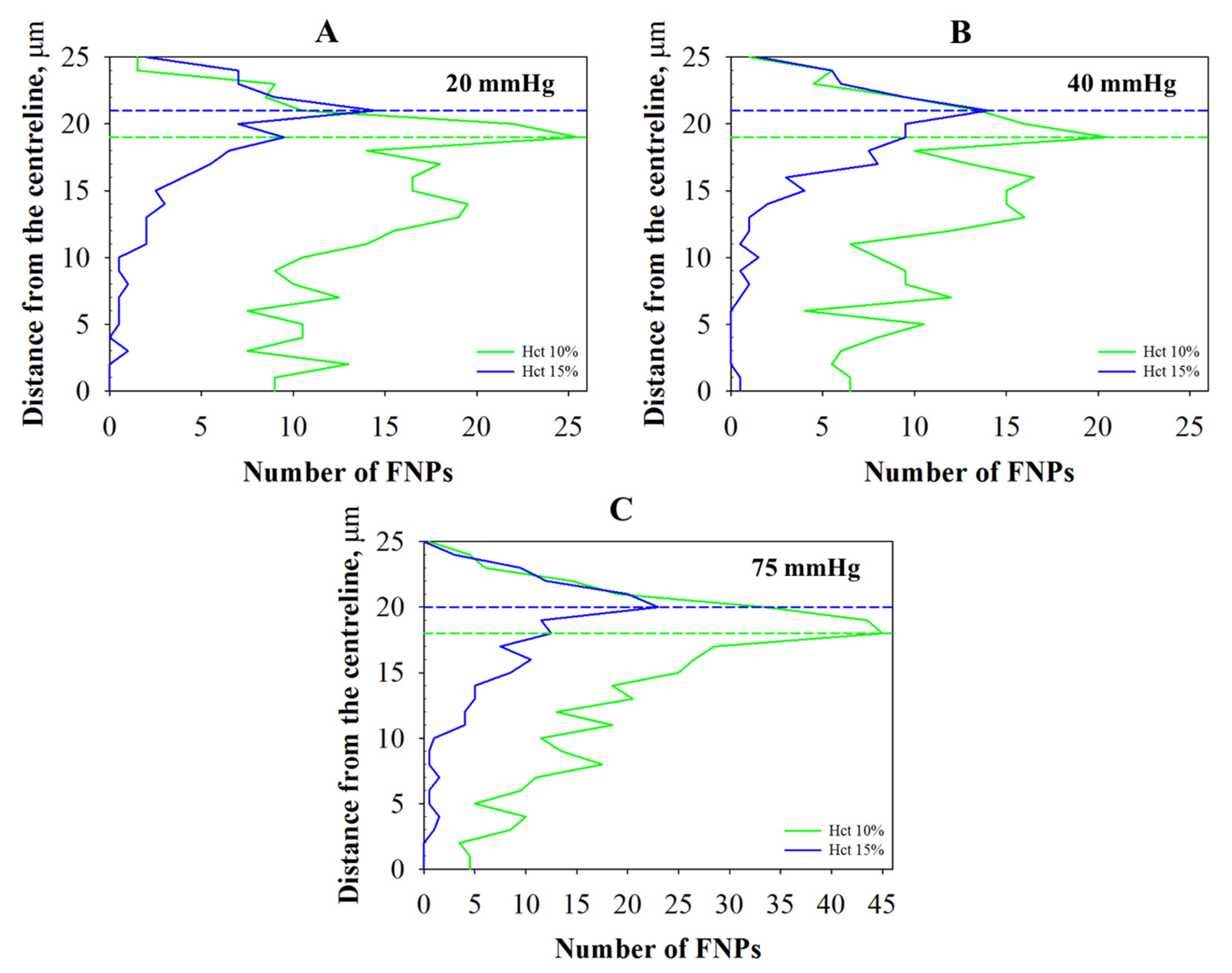
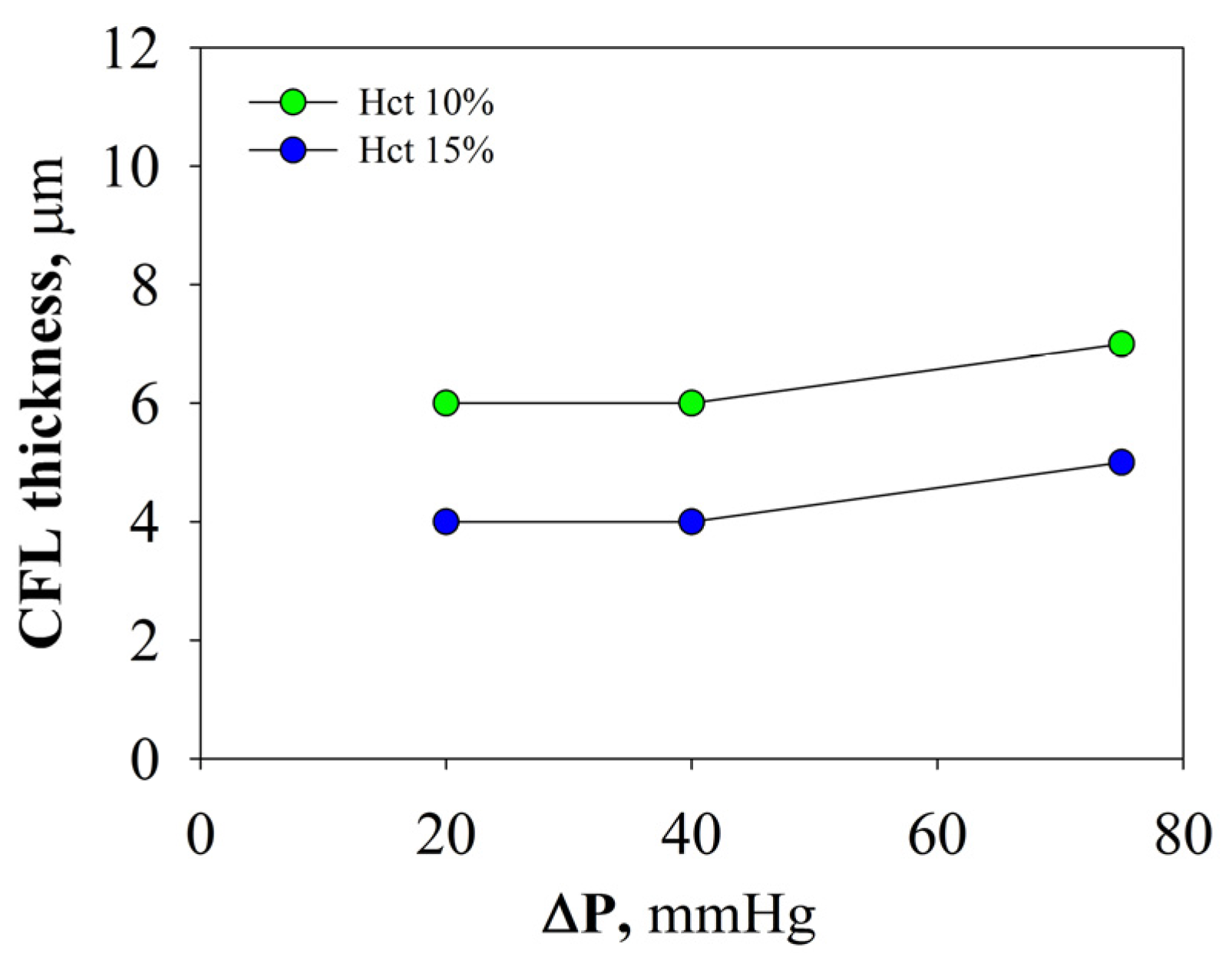
2.2.2. Radial Distribution
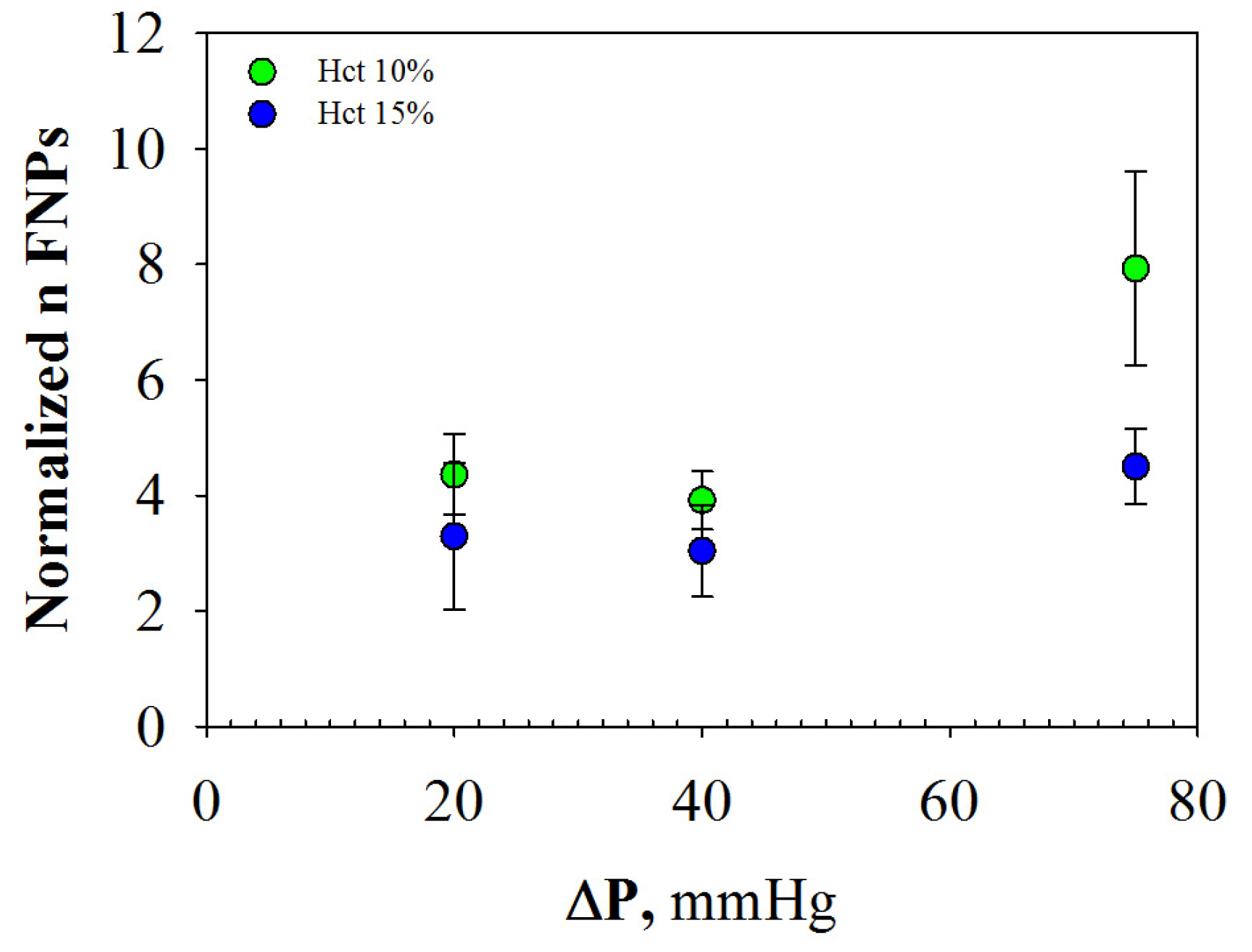
2.2.3. Margination
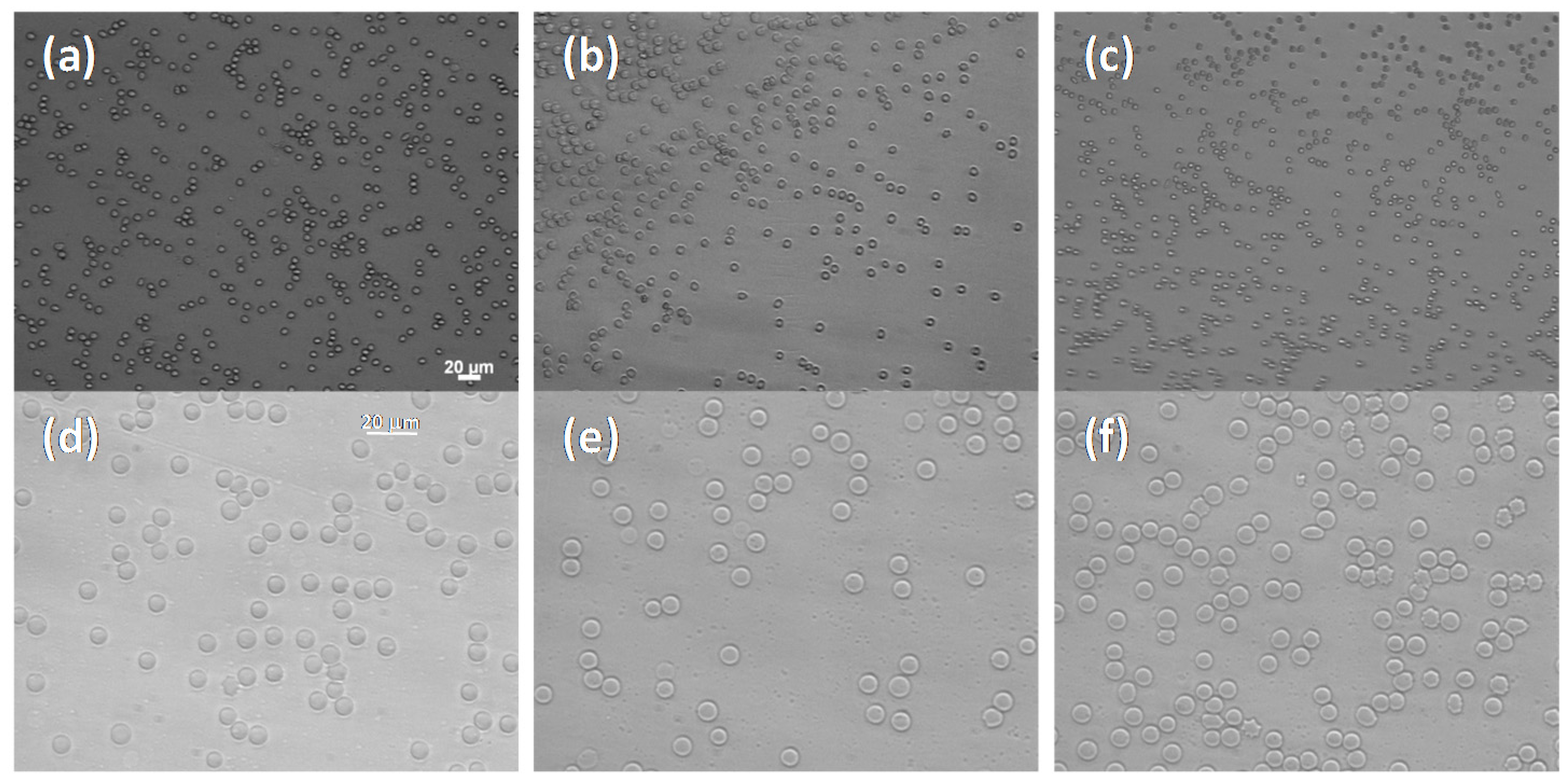
2.3. Hemocompatibility
3. Materials and Methods
3.1. Synthesis of PHEA-RhB-PLA-PEG Graft Copolymer and Characterization
3.2. High Pressure Homogenization (HPH) for Nanoparticle Preparation
3.3. FNPs Characterization: Mean Size and ζ Potential
3.4. Blood Samples
3.5. FNP and RBC/FNP Suspension
3.6. Experimental Set Up
3.7. Hemolytic Test and Erythro-Aggregation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Muthu, M.S.; Singh, S. Targeted nanomedicines: Effective treatment modalities for cancer, AIDS and brain disorders. Nanomedicine 2009, 4, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- D’Apolito, R.; Tomaiuolo, G.; Taraballi, F.; Minardi, S.; Kirui, D.; Liu, X.; Cevenini, A.; Palomba, R.; Ferrari, M.; Salvatore, F. Red blood cells affect the margination of microparticles in synthetic microcapillaries and intravital microcirculation as a function of their size and shape. J. Control. Release 2015, 217, 263–272. [Google Scholar]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Marth, W.; Aland, S.; Voigt, A. Margination of white blood cells: A computational approach by a hydrodynamic phase field model. J. Fluid Mech. 2016, 790, 389–406. [Google Scholar] [CrossRef]
- Fedosov, D.A.; Gompper, G. White blood cell margination in microcirculation. Soft Matter 2014, 10, 2961–2970. [Google Scholar] [CrossRef] [PubMed]
- Eichinger, C.D.; Fogelson, A.L.; Hlady, V. Functional assay of antiplatelet drugs based on margination of platelets in flowing blood. Biointerphases 2016, 11, 029805. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Fedosov, D.A.; Gompper, G. Margination of micro-and nano-particles in blood flow and its effect on drug delivery. Sci. Rep. 2014, 4, 4871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Apolito, R.; Bochicchio, S.; Dalmoro, A.; Barba, A.A.; Guido, S.; Tomaiuolo, G. Microfluidic investigation of the effect of liposome surface charge on drug delivery in microcirculation. Curr. Drug Deliv. 2017, 14, 231–238. [Google Scholar]
- Carboni, E.; Tschudi, K.; Nam, J.; Lu, X.; Ma, A.W. Particle margination and its implications on intravenous anticancer drug delivery. AAPS PharmSciTech 2014, 15, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Shaqfeh, E.S.G.; Narsimhan, V. Shear-induced particle migration and margination in a cellular suspension. Phys. Fluids 2012, 24, 011902. [Google Scholar] [CrossRef]
- D’Apolito, R.; Taraballi, F.; Minardi, S.; Liu, X.; Caserta, S.; Cevenini, A.; Tasciotti, E.; Tomaiuolo, G.; Guido, S. Microfluidic interactions between red blood cells and drug carriers by image analysis techniques. Med. Eng. Phys. 2016, 38, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Toy, R.; Hayden, E.; Shoup, C.; Baskaran, H.; Karathanasis, E. The effects of particle size, density and shape on margination of nanoparticles in microcirculation. Nanotechnology 2011, 22, 115101. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-R.; Choi, M.; Kopacz, A.M.; Yun, S.-H.; Liu, W.K.; Decuzzi, P. On the near-wall accumulation of injectable particles in the microcirculation: Smaller is not better. Sci. Rep. 2013, 3, 2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craparo, E.F.; Porsio, B.; Mauro, N.; Giammona, G.; Cavallaro, G. Polyaspartamide-polylactide graft copolymers with tunable properties for the realization of fluorescent nanoparticles for imaging. Macromol. Rapid Commun. 2015, 36, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Giammona, G.; Carlisi, B.; Palazzo, S. Reaction of α,β-poly(N-Hydroxyethyl)-dl-Aspartamide with Derivatives of Carboxylic Acids. J. Polym. Sci. Part A Polym. Chem. 1987, 25, 2813–2818. [Google Scholar] [CrossRef]
- Craparo, E.F.; Cavallaro, G.; Bondì, M.L.; Giammona, G. Preparation of polymeric nanoparticles by photo-crosslinking of an acryloylated polyaspartamide in w/o microemulsion. Macromol. Chem. Phys. 2004, 205, 1955–1964. [Google Scholar] [CrossRef]
- Craparo, E.F.; Licciardi, M.; Conigliaro, A.; Palumbo, F.S.; Giammona, G.; Alessandro, R.; De Leo, G.; Cavallaro, G. Hepatocyte-targeted nanoparticles based on a polyaspartamide for potential theranostic applications. Polymer 2015, 70, 257–270. [Google Scholar] [CrossRef]
- Craparo, E.F.; Porsio, B.; Sardo, C.; Giammona, G.; Cavallaro, G. Pegylated polyaspartamide–polylactide-based nanoparticles penetrating cystic fibrosis artificial mucus. Biomacromolecules 2016, 17, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Craparo, E.F.; Ferraro, M.; Pace, E.; Bondì, M.L.; Giammona, G.; Cavallaro, G. Polyaspartamide-based nanoparticles loaded with fluticasone propionate and the in vitro evaluation towards cigarette smokeeffects. Nanomaterials 2017, 7, 222. [Google Scholar] [CrossRef] [PubMed]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena; John Wiley & Sons: New York, NY, USA, 2007. [Google Scholar]
- Fedosov, D.A.; Caswell, B.; Popel, A.; Karniadakis, G.E. Blood flow and cell-free layer in microvessels. Microcirculation 2010, 17, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Aarts, P.; Vandenbroek, S.A.T.; Prins, G.W.; Kuiken, G.D.C.; Sixma, J.J.; Heethaar, R.M. Blood-platelets are concentrated near the wall and red blood-cells, in the center in flowing blood. Arteriosclerosis 1988, 8, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Tilles, A.W.; Eckstein, E.C. The near-wall excess of platelet-sized particles in blood flow: Its dependence on hematocrit and wall shear rate. Microvasc. Res. 1987, 33, 211–223. [Google Scholar] [CrossRef]
- Tan, J.; Thomas, A.; Liu, Y. Influence of red blood cells on nanoparticle targeted delivery in microcirculation. Soft Matter 2012, 8, 1934–1946. [Google Scholar] [CrossRef] [PubMed]
- Saadatmand, M.; Ishikawa, T.; Matsuki, N.; Abdekhodaie, M.J.; Imai, Y.; Ueno, H.; Yamaguchi, T. Fluid particle diffusion through high-hematocrit blood flow within a capillary tube. J. Biomech. 2011, 44, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Rusconi, R.; Stone, H.A. Shear-induced diffusion of platelike particles in microchannels. Phys. Rev. Lett. 2008, 101, 254502. [Google Scholar] [CrossRef] [PubMed]
- Fedosov, D.A.; Fornleitner, J.; Gompper, G. Margination of white blood cells in microcapillary flow. Phys. Rev. Lett. 2012, 108, 028104. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Munn, L.L. Determinants of leukocyte margination in rectangular microchannels. PLoS ONE 2009, 4, e7104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldsmith, H.L.; Spain, S. Margination of leukocytes in blood flow through small tubes. Microvasc. Res. 1984, 27, 204–222. [Google Scholar] [CrossRef]
- Namdee, K.; Thompson, A.J.; Charoenphol, P.; Eniola-Adefeso, O. Margination Propensity of Vascular-Targeted Spheres from Blood Flow in a Microfluidic Model of Human Microvessels. Langmuir 2013, 29, 2530–2535. [Google Scholar] [CrossRef] [PubMed]
- Craparo, E.F.; Cavallaro, G.; Bondi, M.L.; Mandracchia, D.; Giammona, G. PEGylated Nanoparticles Based on a Polyaspartamide. Preparation, Physico-Chemical Characterization, and Intracellular Uptake. Biomacromolecules 2006, 7, 3083–3092. [Google Scholar] [CrossRef] [PubMed]
- Craparo, E.F.; Teresi, G.; Ognibene, M.C.; Casaletto, M.P.; Bondì, M.L.; Cavallaro, G. Nanoparticles Based on Novel Amphiphilic Polyaspartamide Copolymers. J. Nanopart. Res. 2010, 12, 2629–2644. [Google Scholar] [CrossRef]
- Baskurt, O.; Boynard, M.; Cokelet, G.; Connes, P.; Cooke, B.M.; Forconi, S.; Liao, F.; Hardeman, M.; Jung, F.; Meiselman, H. New guidelines for hemorheological laboratory techniques. Clin. Hemorheol. Microcirc. 2009, 42, 75–97. [Google Scholar] [PubMed]
- Fung, Y. Biomechanics—Circulation; Springer: New York, NY, USA, 1997; p. 243. [Google Scholar]
- Albrecht, K.; Gaehtgens, P.; Pries, A.; Heuser, M. The Fahraeus effect in narrow capillaries (i.d. 3.3 to 11.0 micron). Microvasc. Res. 1979, 18, 33–47. [Google Scholar] [CrossRef]
- Whitmore, R.L. The flow behaviour of blood in the circulation. Nature 1967, 215, 123–126. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Craparo, E.F.; D’Apolito, R.; Giammona, G.; Cavallaro, G.; Tomaiuolo, G. Margination of Fluorescent Polylactic Acid–Polyaspartamide based Nanoparticles in Microcapillaries In Vitro: the Effect of Hematocrit and Pressure. Molecules 2017, 22, 1845. https://doi.org/10.3390/molecules22111845
Craparo EF, D’Apolito R, Giammona G, Cavallaro G, Tomaiuolo G. Margination of Fluorescent Polylactic Acid–Polyaspartamide based Nanoparticles in Microcapillaries In Vitro: the Effect of Hematocrit and Pressure. Molecules. 2017; 22(11):1845. https://doi.org/10.3390/molecules22111845
Chicago/Turabian StyleCraparo, Emanuela Fabiola, Rosa D’Apolito, Gaetano Giammona, Gennara Cavallaro, and Giovanna Tomaiuolo. 2017. "Margination of Fluorescent Polylactic Acid–Polyaspartamide based Nanoparticles in Microcapillaries In Vitro: the Effect of Hematocrit and Pressure" Molecules 22, no. 11: 1845. https://doi.org/10.3390/molecules22111845




