Lutein, a Natural Carotenoid, Induces α-1,3-Glucan Accumulation on the Cell Wall Surface of Fungal Plant Pathogens
Abstract
:1. Introduction
2. Results
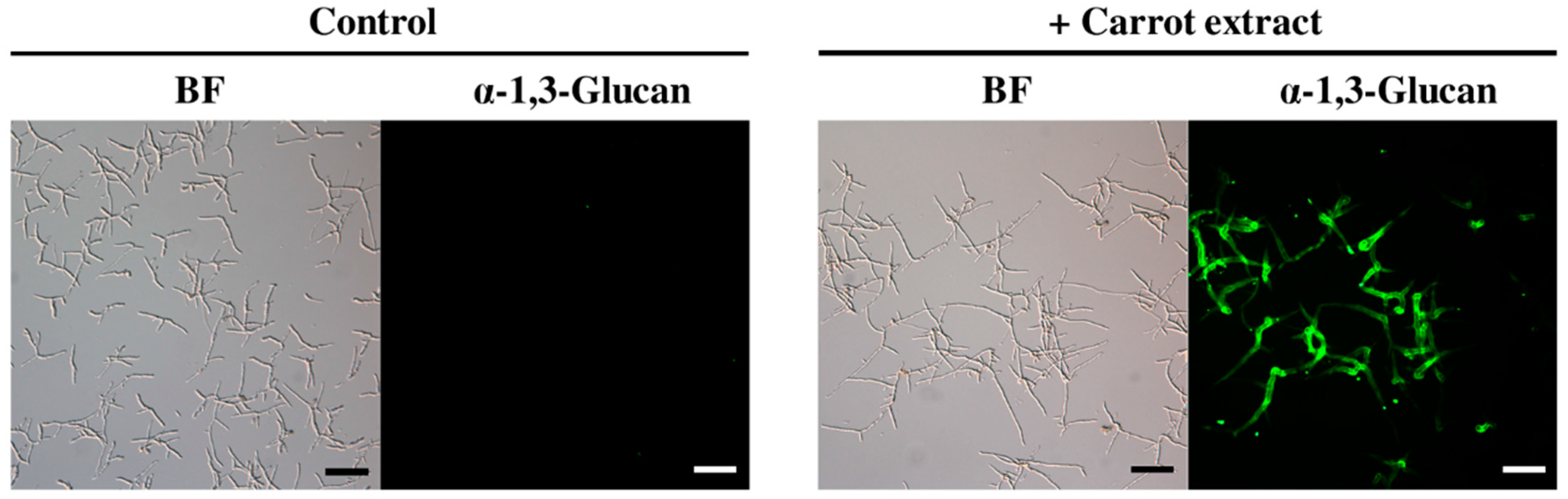
2.1. Isolation and Identification of Inducers of α-1,3-Glucan Accumulation in Colletotrichum Fioriniae from Carrot Leaves Subsection
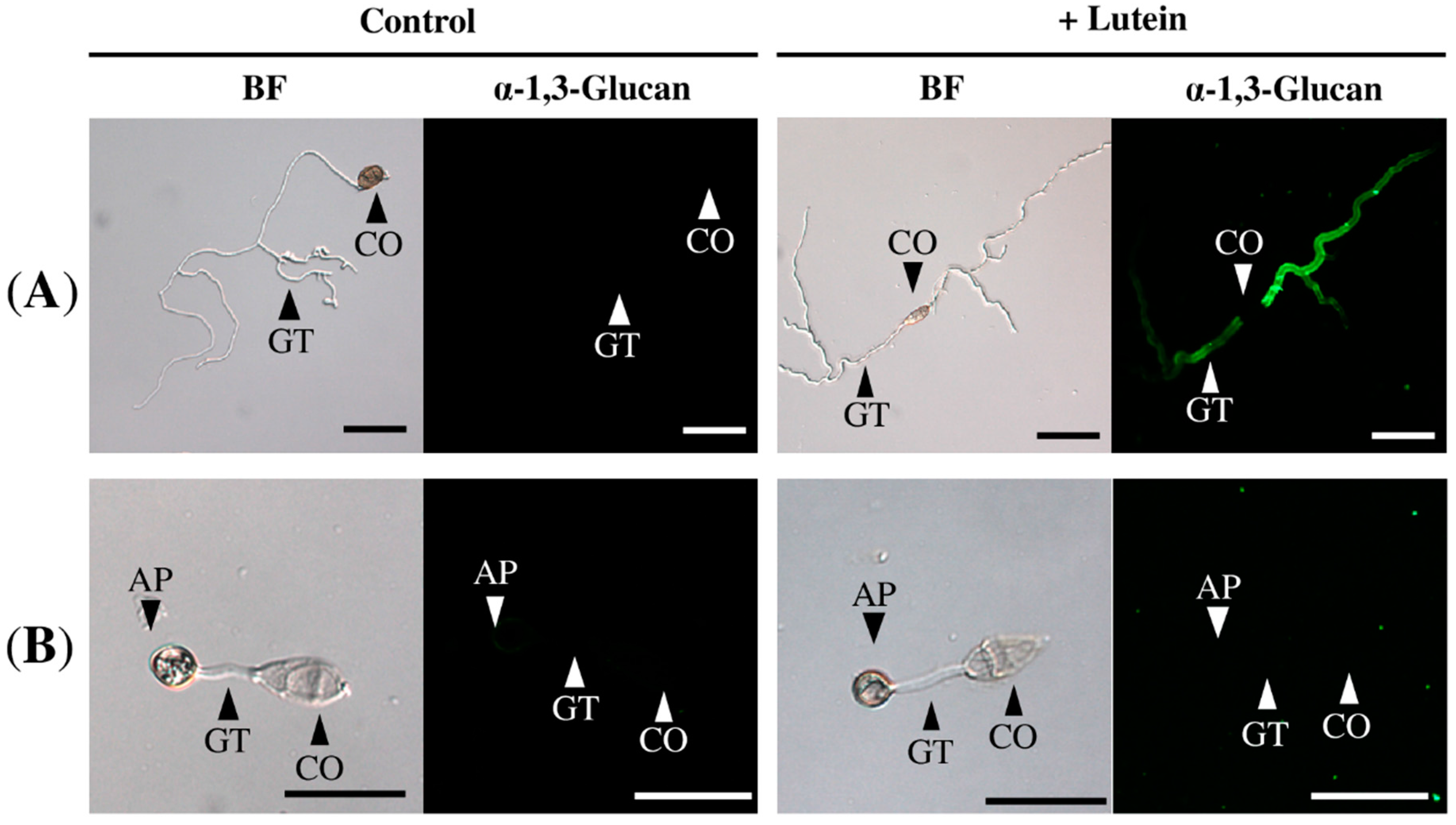
2.2. Lutein Induced the Accumulation of α-1,3-Glucan on the Surface in Various Colletotrichum Species
2.3. Lutein Induced α-1,3-Glucan Accumulation in Cochliobolus Miyabeanus but Not in Magnaporthe oryzae
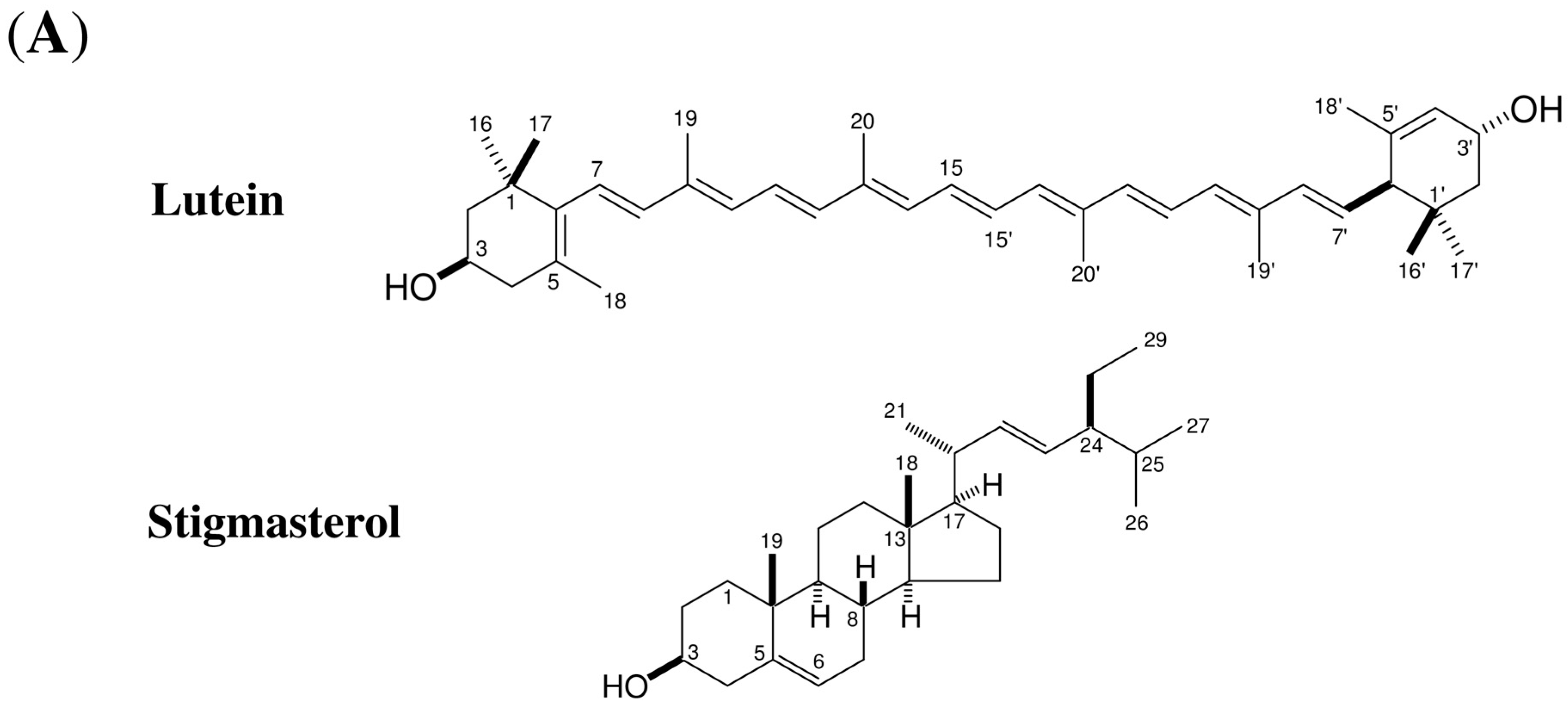
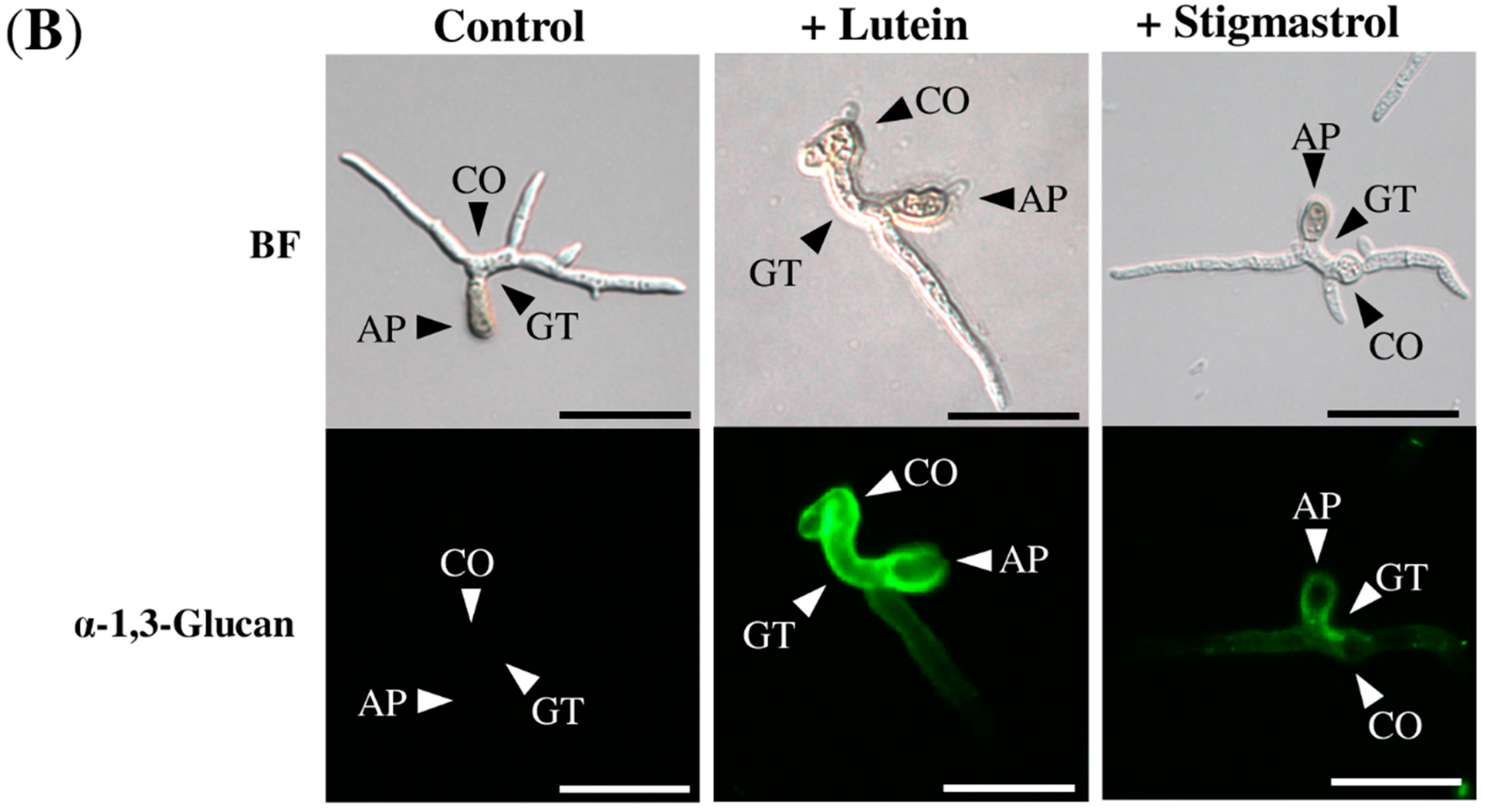
2.4. Stigmasterol Showed Induction Activity on Surface α-1,3-Glucan Accumulation in Various Fungi
2.5. Surface Accumulation of α-1,3-Glucan Protected the Cell Wall from Digestive Enzymes in Colletotrichum Fioriniae
3. Discussion
4. Experimental Section
4.1. Fungal Strains and Plant Materials
4.2. Chemicals
4.3. General
4.4. Extraction and Isolation
4.5. Immunofluorescent Staining of Fungal Cell Wall α-1,3-Glucan
4.6. Sensitivity Assay to Cell Wall Digesting Enzymes
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Latgé, J.P. Tasting the Fungal Cell Wall. Cell. Mocrobiol. 2010, 12, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Free, S.J. Fungal Cell Wall Organization and Biosynthesis. Adv. Genet. 2013, 81, 33–82. [Google Scholar] [PubMed]
- Latgé, J.P.; Bauvais, A. Functional duality of the cell wall. Curr. Opin. Microbiol. 2014, 20, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, T.; Kuga, Y.; Yano, S.; Yoshimi, A.; Tachiki, T.; Abe, K.; Nishimura, M. Dynamics of Cell Wall Components of Magnaporthe grisea during Infectious Structure Development. Mol. Microbiol. 2009, 73, 553–570. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, T.; Sakaguchi, A.; Nishizawa, Y.; Kouzai, Y.; Minami, E.; Yano, S.; Koga, H.; Meshi, T.; Nishimura, M. Surface α-1,3-Glucan facilitates fungal stealth infection by interfering with innate immunity in plants. PLoS Pathog. 2012, 8e1002882. [Google Scholar] [CrossRef] [PubMed]
- Hyde, K.D.; Cai, L.; Cannon, P.F.; Crouch, J.A.; Crous, P.W.; Damm, U.; Goodwin, P.H.; Chen, H.; Johnston, P.R.; Jones, E.B.G.; et al. Colletotrichum—Names in current use. Fungal Divers. 2009, 39, 147–182. [Google Scholar]
- Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Crous, P.W. The Colletotrichum acutatum species complex. Stud. Mycol. 2012, 73, 37–113. [Google Scholar] [CrossRef] [PubMed]
- Cannon, P.F.; Damm, P.R.; Johnson, P.R.; Weir, B.S. Colletotrichum—Current status and future directions. Stud. Mycol. 2012, 73, 181–213. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; DellaPenna, D. Defining the Primary Route for Lutein Synthesis in Plants: The Role of Arabidopsis Carotenoid β-ring hydroxylase CYP97A3. Proc. Natl. Acad. Sci. USA 2006, 103, 3474–3479. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.-A. Plant sterols and the membrane environment. Trends Plant Sci. 1998, 3, 170–174. [Google Scholar] [CrossRef]
- Levin, D.E. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: The cell wall integrity signaling pathway. Genetics 2011, 189, 1145–1175. [Google Scholar] [CrossRef] [PubMed]
- Yoshimi, A.; Miyazawa, K.; Abe, K. Cell wall structure and biogenesis in Aspergillus species. Biosci. Biotechnol. Biochem. 2016, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Condon, B.J.; Leng, Y.; Wu, D.; Bushley, K.E.; Ohm, R.A.; Otillar, R.; Martin, J.; Schackwitz, W.; Grimwood, J.; MohdZainudin, N.; et al. Comparative Genome Structure, Secondary Metabolite, and Effector Coding Capacity across Cochliobolus Pathogens. PLoS Genet. 2013, 9e1003233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Doorn, W.G.; Beers, E.P.; Dangl, J.L.; Franklin-Tong, V.E.; Gallois, P.; Hara-Nishimura, I.; Jones, A.M.; Kawai-Yamada, M.; Lam, E.; Mundy, J.; et al. Morphological classification of plant cell deaths. Cell Death Differ. 2011, 18, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: The samples of compounds used in this study are available from the authors upon request.



© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otaka, J.; Seo, S.; Nishimura, M. Lutein, a Natural Carotenoid, Induces α-1,3-Glucan Accumulation on the Cell Wall Surface of Fungal Plant Pathogens. Molecules 2016, 21, 980. https://doi.org/10.3390/molecules21080980
Otaka J, Seo S, Nishimura M. Lutein, a Natural Carotenoid, Induces α-1,3-Glucan Accumulation on the Cell Wall Surface of Fungal Plant Pathogens. Molecules. 2016; 21(8):980. https://doi.org/10.3390/molecules21080980
Chicago/Turabian StyleOtaka, Junnosuke, Shigemi Seo, and Marie Nishimura. 2016. "Lutein, a Natural Carotenoid, Induces α-1,3-Glucan Accumulation on the Cell Wall Surface of Fungal Plant Pathogens" Molecules 21, no. 8: 980. https://doi.org/10.3390/molecules21080980




