Doping-Promoted Solar Water Oxidation on Hematite Photoanodes
Abstract
:1. Introduction
2. Metal Element Doping
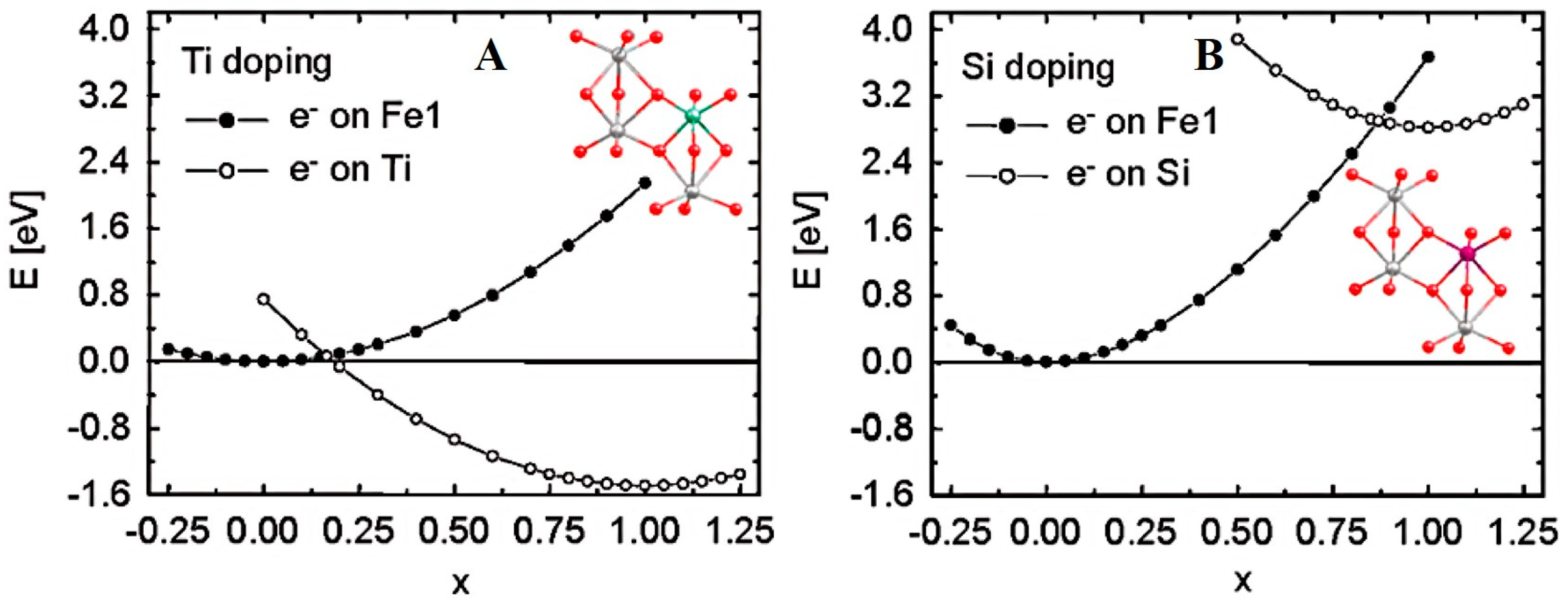
2.1. n-Type Doping
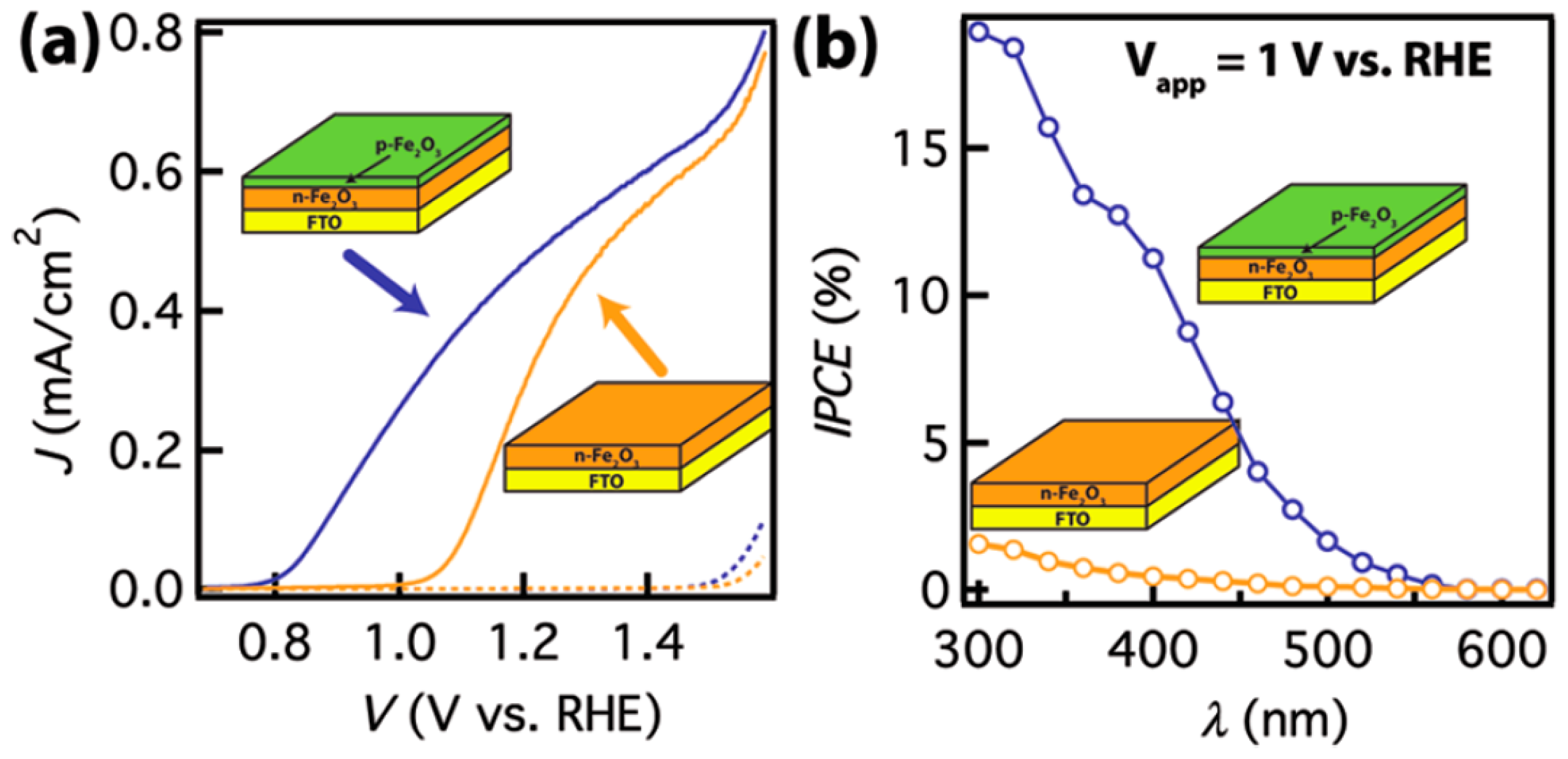
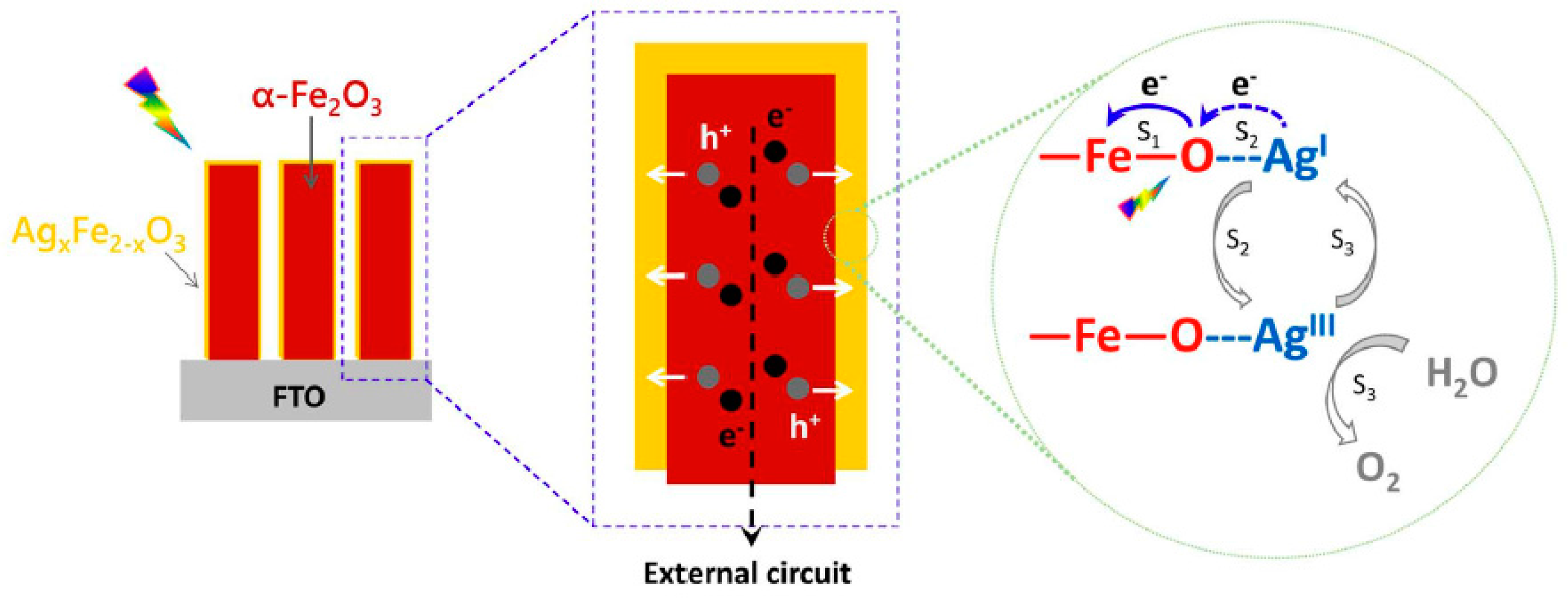
2.2. p-Type Doping
3. Nonmetal Element Doping
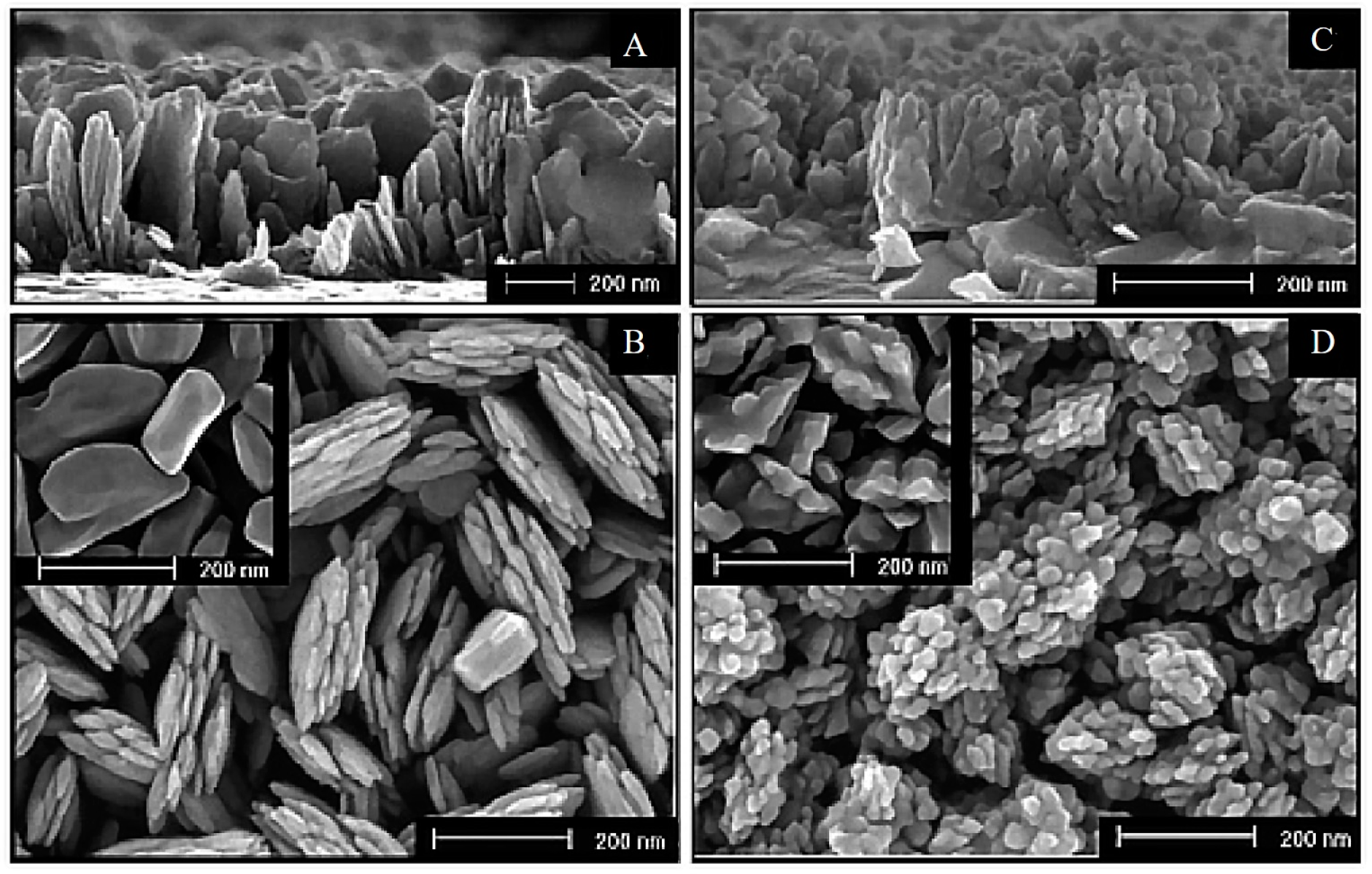
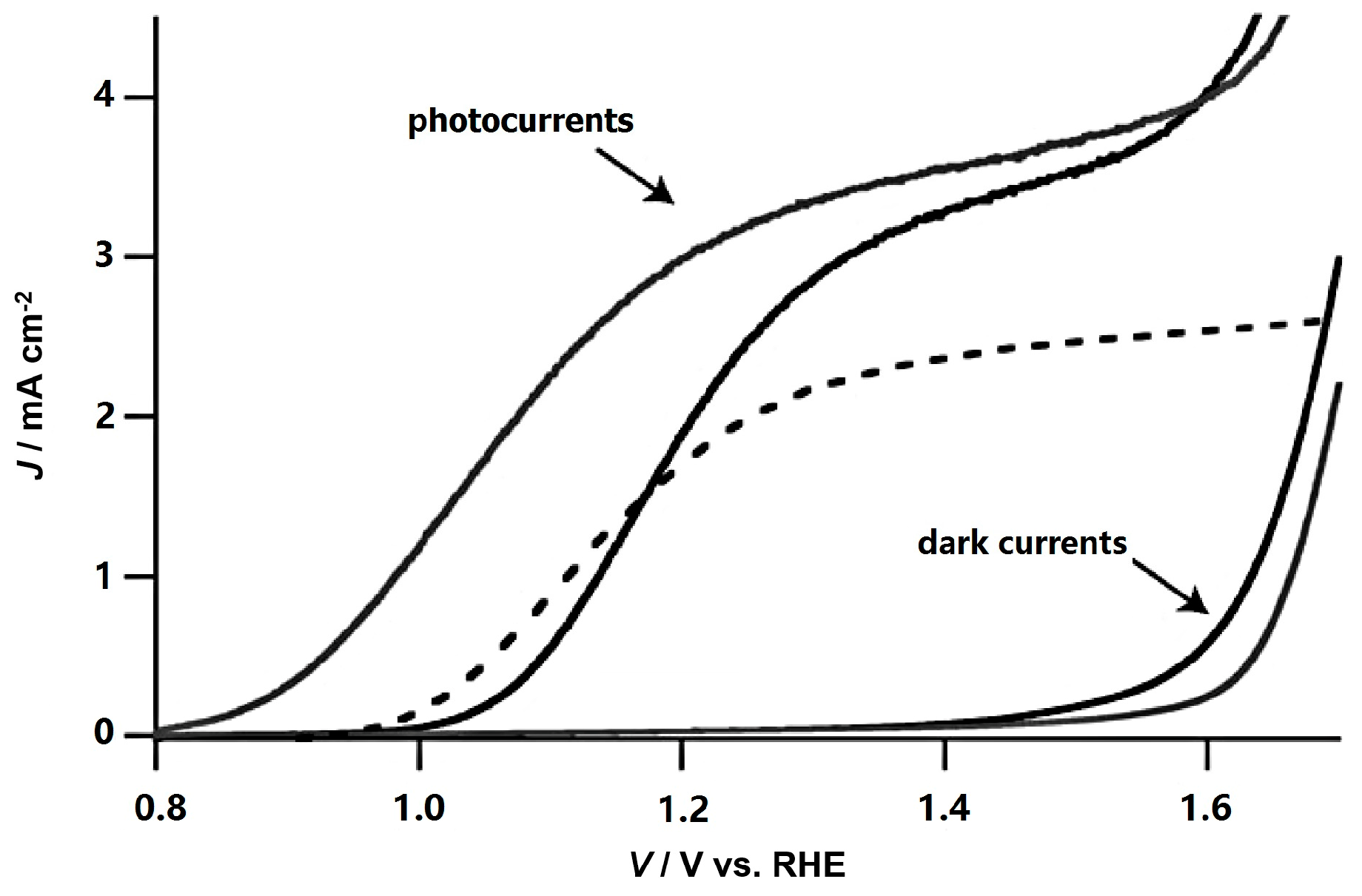
3.1. Si-Doping
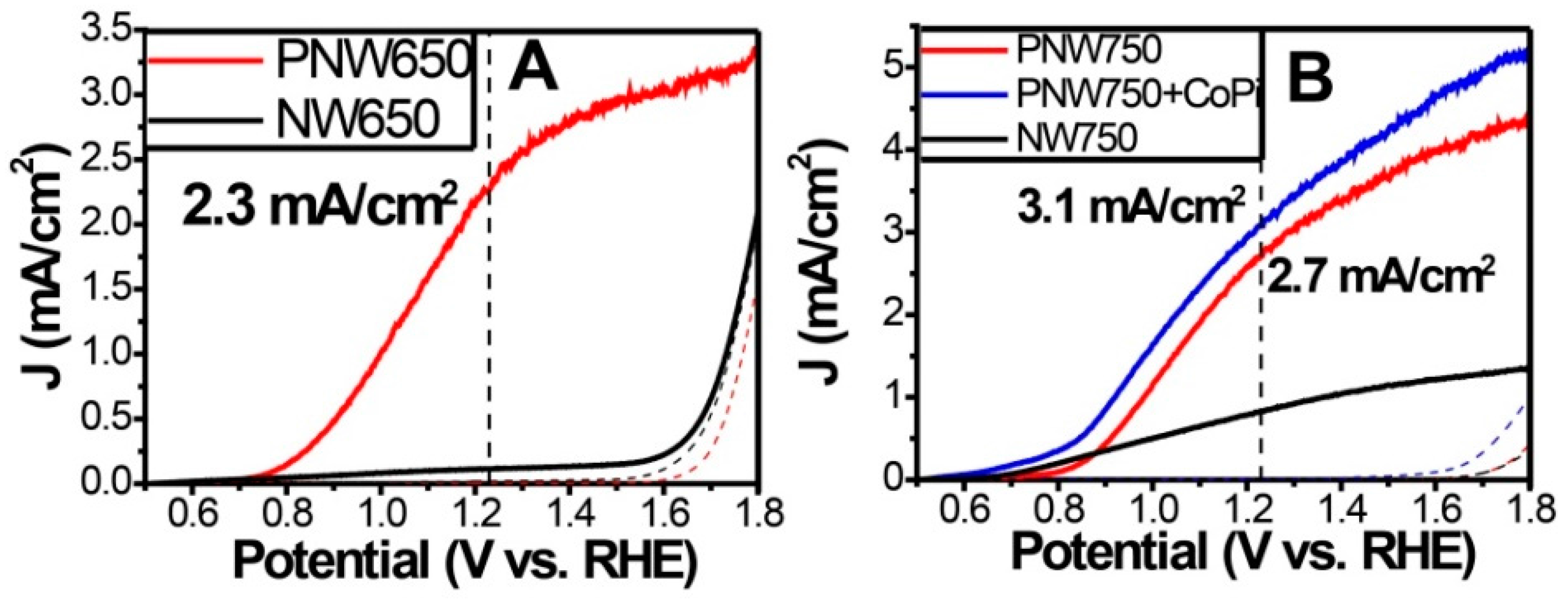
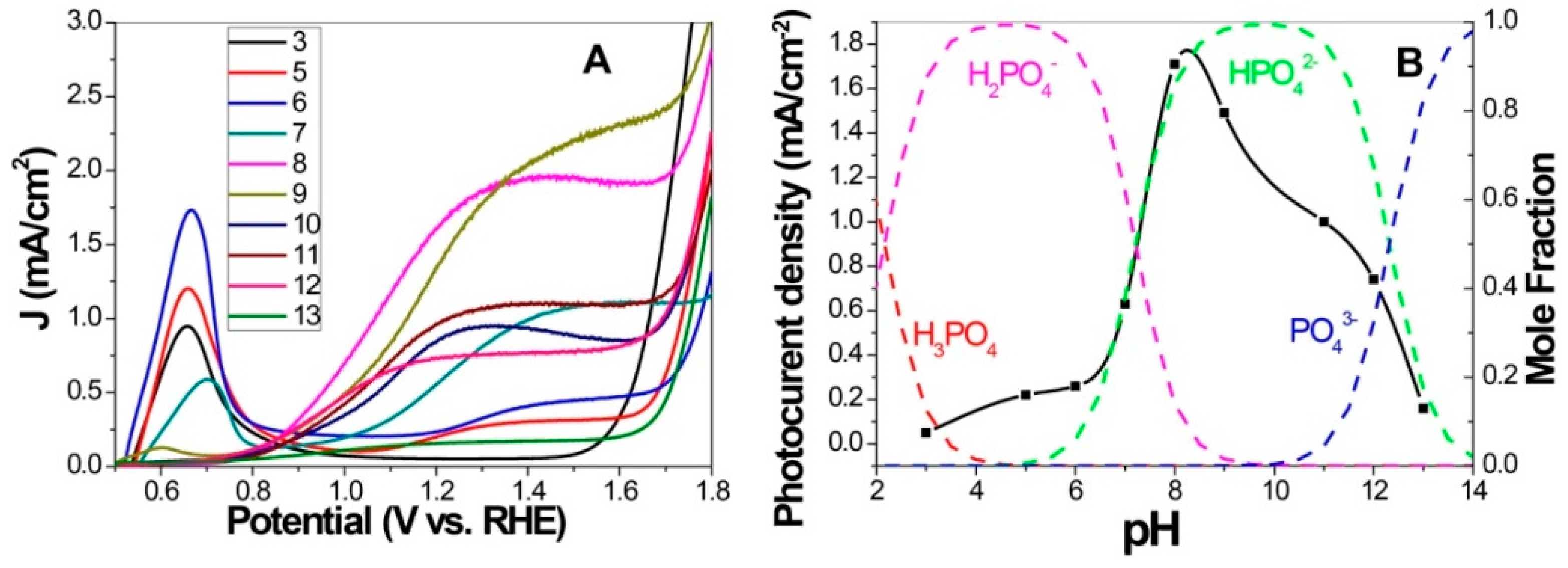
3.2. P-Doping
4. Summary and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, Y.; Zhang, H.; Ji, H.; Ma, W.; Chen, C.; Zhao, J. Pivotal role and regulation of proton transfer in water oxidation on hematite photoanodes. J. Am. Chem. Soc. 2016, 2705–2711. [Google Scholar]
- Sivula, K.; le Formal, F.; Gratzel, M. Solar water splitting: Progress using hematite (α-Fe2O3) photoelectrodes. ChemSusChem 2011, 4, 432–449. [Google Scholar] [PubMed]
- Hamann, T.W. Splitting water with rust: Hematite photoelectrochemistry. Dalton Trans 2012, 41, 7830–7834. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.; Qu, Z.W.; Kroes, G.J.; Rossmeisl, J.; Norskov, J.K. Oxidation and photo-oxidation of water on TiO2 surface. J. Phys. Chem. C 2008, 112, 9872–9879. [Google Scholar] [CrossRef]
- Chen, H.M.; Chen, C.K.; Liu, R.S.; Zhang, L.; Zhang, J.; Wilkinson, D.P. Nano-architecture and material designs for water splitting photoelectrodes. Chem. Soc. Rev. 2012, 41, 5654–5671. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.A.; Wang, G.; Ling, Y.; Li, Y.; Zhang, J.Z. Nanostructured hematite: Synthesis, characterization, charge carrier dynamics, and photoelectrochemical properties. Energy Environ. Sci. 2012, 5, 6682–6702. [Google Scholar] [CrossRef]
- Bard, A.J. Inner-sphere heterogeneous electrode reactions. Electrocatalysis and photocatalysis: The challenge. J. Am. Chem. Soc. 2010, 132, 7559–7567. [Google Scholar] [CrossRef] [PubMed]
- Dau, H.; Limberg, C.; Reier, T.; Risch, M.; Roggan, S.; Strasser, P. The mechanism of water oxidation: From electrolysis via homogeneous to biological catalysis. ChemCatChem 2010, 2, 724–761. [Google Scholar] [CrossRef]
- Lin, Y.; Yuan, G.; Sheehan, S.; Zhou, S.; Wang, D. Hematite-based solar water splitting: Challenges and opportunities. Energy Environ. Sci. 2011, 4, 4862–4869. [Google Scholar] [CrossRef]
- Sivula, K. Metal oxide photoelectrodes for solar fuel production, surface traps, and catalysis. J. Phys. Chem. Lett. 2013, 4, 1624–1633. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Hoang, S.; Berglund, S.P.; Hahn, N.T.; Bard, A.J.; Mullins, C.B. Enhancing visible light photo-oxidation of water with TiO2 nanowire arrays via cotreatment with H2 and NH3: Synergistic effects between Ti3+ and n. J. Am. Chem. Soc. 2012, 134, 3659–3662. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.J.; Jang, J.-W.; Kong, K.-J.; Kang, H.J.; Kim, J.Y.; Jun, H.; Parmar, K.P.S.; Lee, J.S. Phosphate doping into monoclinic bivo4 for enhanced photoelectrochemical water oxidation activity. Angew. Chem. Int. Ed. 2012, 51, 3147–3151. [Google Scholar] [CrossRef] [PubMed]
- Mi, Q.; Ping, Y.; Li, Y.; Cao, B.; Brunschwig, B.S.; Khalifah, P.G.; Galli, G.A.; Gray, H.B.; Lewis, N.S. Thermally stable N2-intercalated WO3 photoanodes for water oxidation. J. Am. Chem. Soc. 2012, 134, 18318–18324. [Google Scholar] [CrossRef] [PubMed]
- Cowan, A.J.; Barnett, C.J.; Pendlebury, S.R.; Barroso, M.; Sivula, K.; Gratzel, M.; Durrant, J.R.; Klug, D.R. Activation energies for the rate-limiting step in water photooxidation by nanostructured α-Fe2O3 and TiO2. J. Am. Chem. Soc. 2011, 133, 10134–10140. [Google Scholar] [CrossRef] [PubMed]
- Dotan, H.; Sivula, K.; Grätzel, M.; Rothschild, A.; Warren, S.C. Probing the photoelectrochemical properties of hematite (α-Fe2O3) electrodes using hydrogen peroxide as a hole scavenger. Energy. Environ. Sci. 2011, 4, 958. [Google Scholar] [CrossRef]
- Klahr, B.; Gimenez, S.; Fabregat-Santiago, F.; Bisquert, J.; Hamann, T.W. Electrochemical and photoelectrochemical investigation of water oxidation with hematite electrodes. Energy Environ. Sci. 2012, 5, 7626–7636. [Google Scholar] [CrossRef]
- Klahr, B.; Gimenez, S.; Fabregat-Santiago, F.; Hamann, T.; Bisquert, J. Water oxidation at hematite photoelectrodes: The role of surface states. J. Am. Chem. Soc. 2012, 134, 4294–4302. [Google Scholar] [CrossRef] [PubMed]
- Pendlebury, S.R.; Barroso, M.; Cowan, A.J.; Sivula, K.; Tang, J.; Gratzel, M.; Klug, D.; Durrant, J.R. Dynamics of photogenerated holes in nanocrystalline α-Fe2O3 electrodes for water oxidation probed by transient absorption spectroscopy. Chem. Commun. 2011, 47, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.; Keith, J.A.; Carter, E.A. Water oxidation on pure and doped hematite (0001) surfaces: Prediction of co and ni as effective dopants for electrocatalysis. J. Am. Chem. Soc. 2012, 134, 13296–13309. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Lin, Y.; Xiang, X.; Rodríguez-Córdoba, W.; McDonald, K.J.; Hagen, K.S.; Choi, K.-S.; Brunschwig, B.S.; Musaev, D.G.; Hill, C.L.; et al. In situ probe of photocarrier dynamics in water-splitting hematite (α-Fe2O3) electrodes. Energy Environ. Sci. 2012, 5, 8923–8926. [Google Scholar] [CrossRef]
- Kennedy, J.H.; Frese, K.W. Photo-oxidation of water at α-Fe2O3 electrodes. J. Electrochem. Soc. 1978, 125, 709–714. [Google Scholar] [CrossRef]
- Le Formal, F.; Pendlebury, S.R.; Cornuz, M.; Tilley, S.D.; Grätzel, M.; Durrant, J.R. Back electron–hole recombination in hematite photoanodes for water splitting. J. Am. Chem. Soc. 2014, 136, 2564–2574. [Google Scholar] [CrossRef] [PubMed]
- Le Formal, F.; Pastor, E.; Tilley, S.D.; Mesa, C.A.; Pendlebury, S.R.; Grätzel, M.; Durrant, J.R. Rate law analysis of water oxidation on a hematite surface. J. Am. Chem. Soc. 2015, 137, 6629–6637. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.T.; Du, C.; Wang, D. Hematite/si nanowire dual-absorber system for photoelectrochemical water splitting at low applied potentials. J. Am. Chem. Soc. 2012, 134, 12406–12409. [Google Scholar] [CrossRef] [PubMed]
- Le Formal, F.; Tétreault, N.; Cornuz, M.; Moehl, T.; Grätzel, M.; Sivula, K. Passivating surface states on water splitting hematite photoanodes with alumina overlayers. Chem. Sci. 2011, 2, 737–743. [Google Scholar] [CrossRef]
- Hou, Y.; Zuo, F.; Dagg, A.; Feng, P. Visible light-driven α-Fe2O3 nanorod/graphene/BiVO4 core/shell heterojunction array for efficient photoelectrochemical water splitting. Nano Lett. 2012, 12, 6464–6473. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Jang, J.-W.; Youn, D.H.; Magesh, G.; Lee, J.S. A stable and efficient hematite photoanode in a neutral electrolyte for solar water splitting: Towards stability engineering. Adv. Energy Mater. 2014, 4, 1400476. [Google Scholar] [CrossRef]
- Zhong, D.K.; Sun, J.; Inumaru, H.; Gamelin, D.R. Solar water oxidation by composite catalyst/α-Fe2O3 photoanodes. J. Am. Chem. Soc. 2009, 131, 6086–6087. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.K.; Gamelin, D.R. Photoelectrochemical water oxidation by cobalt catalyst ("Co-Pi")/α-Fe2O3 composite photoanodes: Oxygen evolution and resolution of a kinetic bottleneck. J. Am. Chem. Soc. 2010, 132, 4202–4207. [Google Scholar] [CrossRef] [PubMed]
- Tilley, S.D.; Cornuz, M.; Sivula, K.; Gratzel, M. Light-induced water splitting with hematite: Improved nanostructure and iridium oxide catalysis. Angew. Chem. Int. Ed. 2010, 49, 6405–6408. [Google Scholar] [CrossRef] [PubMed]
- Pendlebury, S.R.; Wang, X.; le Formal, F.; Cornuz, M.; Kafizas, A.; Tilley, S.D.; Grätzel, M.; Durrant, J.R. Ultrafast charge carrier recombination and trapping in hematite photoanodes under applied bias. J. Am. Chem. Soc. 2014, 136, 9854–9857. [Google Scholar] [CrossRef] [PubMed]
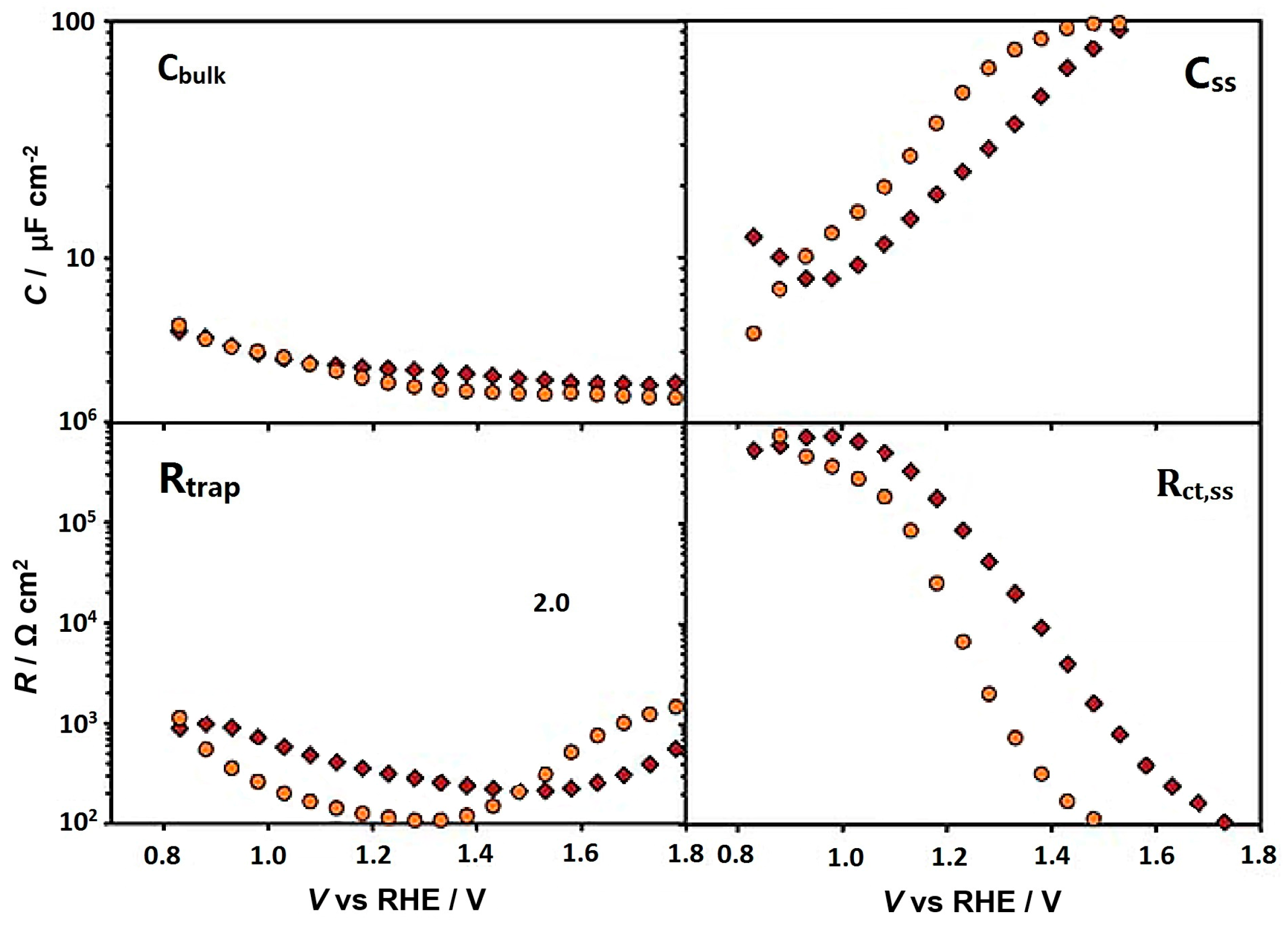
- Zhang, Y.; Jiang, S.; Song, W.; Zhou, P.; Ji, H.; Ma, W.; Hao, W.; Chen, C.; Zhao, J. Nonmetal p-doped hematite photoanode with enhanced electron mobility and high water oxidation activity. Energy Environ. Sci. 2015, 8, 1231–1236. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Z.; Chen, C.; Che, Y.; Ji, H.; Ma, W.; Zhang, J.; Song, D.; Zhao, J. Gradient FeOx(PO4)y layer on hematite photoanodes: Novel structure for efficient light-driven water oxidation. ACS Appl. Mater. Interfaces 2014, 6, 12844–12851. [Google Scholar] [CrossRef] [PubMed]
- Iandolo, B.; Hellman, A. The role of surface states in the oxygen evolution reaction on hematite. Angew. Chem. Int. Ed. 2014, 53, 13404–13408. [Google Scholar] [CrossRef] [PubMed]
- Peerakiatkhajohn, P.; Yun, J.-H.; Chen, H.; Lyu, M.; Butburee, T.; Wang, L. Stable hematite nanosheet photoanodes for enhanced photoelectrochemical water splitting. Adv. Mater. 2016. [Google Scholar] [CrossRef] [PubMed]
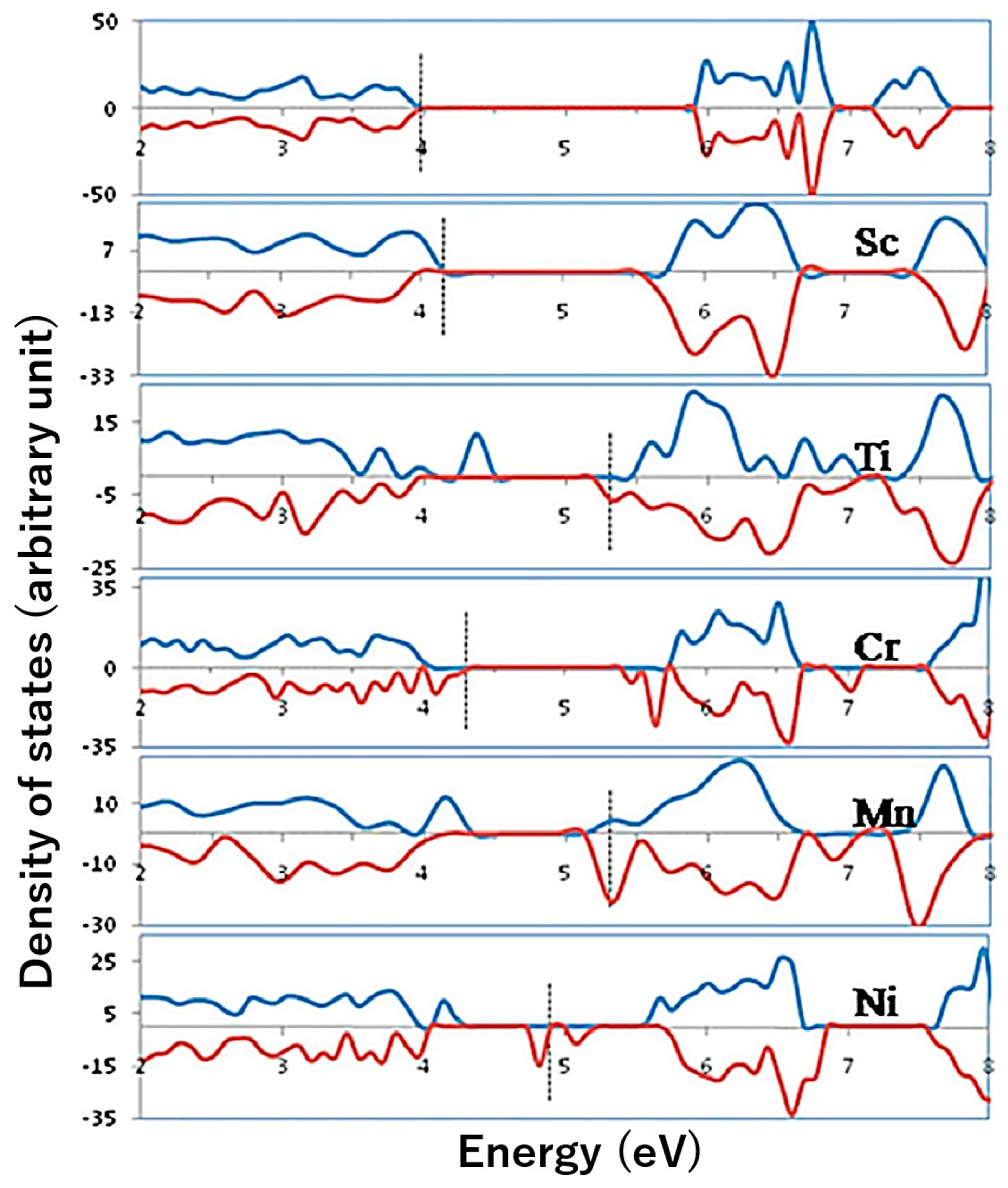
- Huda, M.N.; Walsh, A.; Yan, Y.; Wei, S.-H.; Al-Jassim, M.M. Electronic, structural, and magnetic effects of 3d transition metals in hematite. J. Appl. Phys. 2010, 107, 123712. [Google Scholar] [CrossRef]
- Velev, J.; Bandyopadhyay, A.; Butler, W.H.; Sarker, S. Electronic and magnetic structure of transition-metal-doped alpha-hematite. Phys. Rev. B 2005, 71, 205208. [Google Scholar] [CrossRef]
- Wang, G.; Ling, Y.; Wheeler, D.A.; George, K.E.; Horsley, K.; Heske, C.; Zhang, J.Z.; Li, Y. Facile synthesis of highly photoactive α-Fe2O3-based films for water oxidation. Nano Lett. 2011, 11, 3503–3509. [Google Scholar] [CrossRef] [PubMed]
- Morin, F.J. Electrical properties of α-Fe2O3 and α-Fe2O3 containing titanium. Phys. Rev. 1951, 83, 1005–1010. [Google Scholar] [CrossRef]
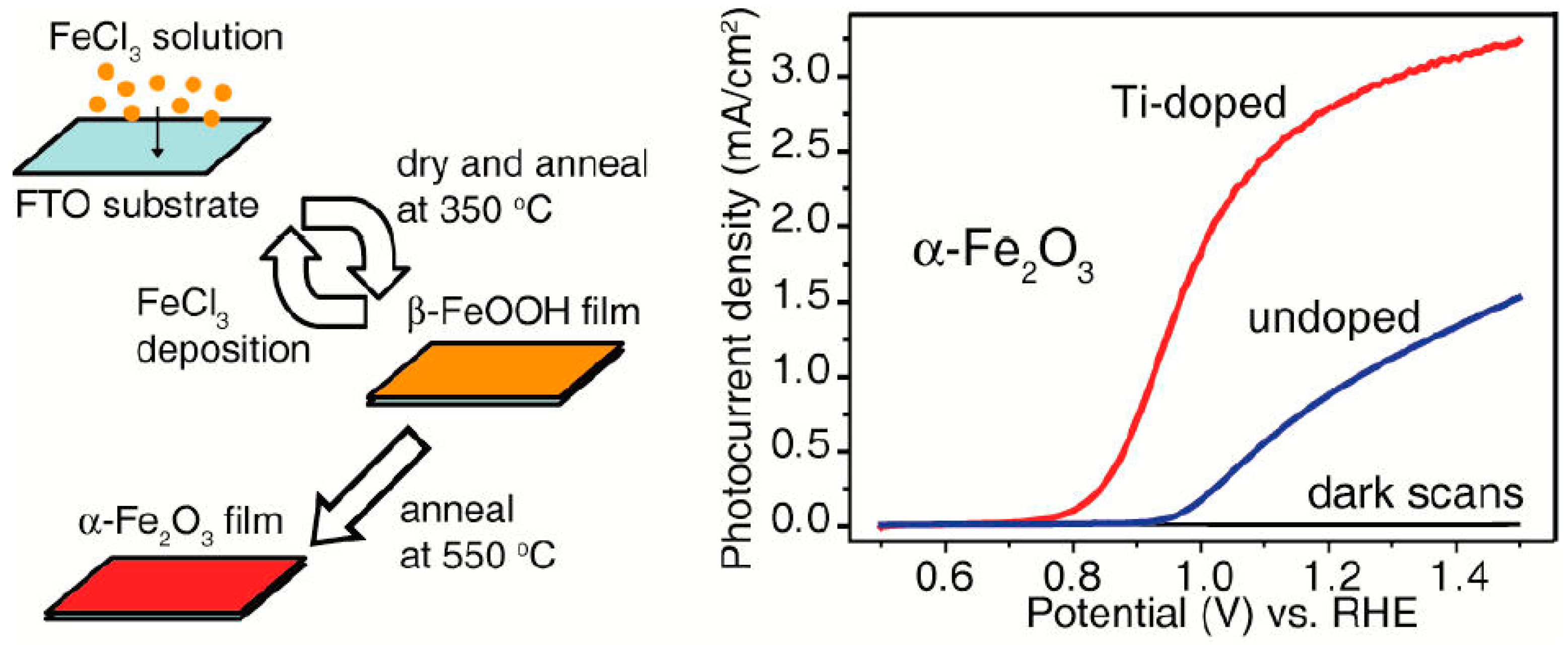
- Zandi, O.; Klahr, B.M.; Hamann, T.W. Highly photoactive Ti-doped α-Fe2O3 thin film electrodes: Resurrection of the dead layer. Energy Environ. Sci. 2013, 6, 634–642. [Google Scholar] [CrossRef]
- Rioult, M.; Magnan, H.; Stanescu, D.; Barbier, A. Single crystalline hematite films for solar water splitting: Ti-doping and thickness effects. J. Phys. Chem. C 2014, 118, 3007–3014. [Google Scholar] [CrossRef]
- Kronawitter, C.X.; Zegkinoglou, I.; Shen, S.H.; Liao, P.; Cho, I.S.; Zandi, O.; Liu, Y.S.; Lashgari, K.; Westin, G.; Guo, J.H.; et al. Titanium incorporation into hematite photoelectrodes: Theoretical considerations and experimental observations. Energy Environ. Sci. 2014, 7, 3100–3121. [Google Scholar] [CrossRef]
- Pu, A.; Deng, J.; Li, M.; Gao, J.; Zhang, H.; Hao, Y.; Zhong, J.; Sun, X. Coupling ti-doping and oxygen vacancies in hematite nanostructures for solar water oxidation with high efficiency. J. Mater. Chem. A 2014, 2, 2491. [Google Scholar] [CrossRef]
- Deng, J.; Zhong, J.; Pu, A.; Zhang, D.; Li, M.; Sun, X.; Lee, S.-T. Ti-doped hematite nanostructures for solar water splitting with high efficiency. J. Appl. Phys. 2012, 112, 084312. [Google Scholar] [CrossRef]
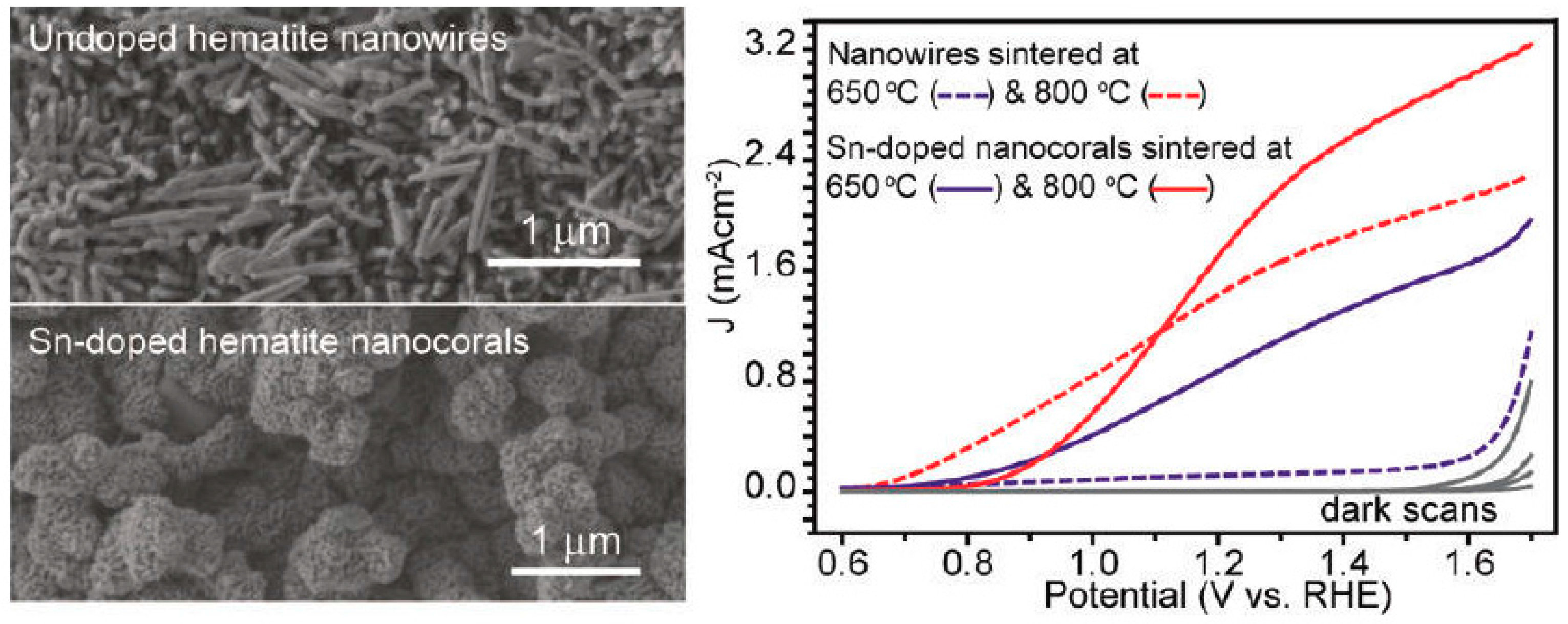
- Ling, Y.; Wang, G.; Wheeler, D.A.; Zhang, J.Z.; Li, Y. Sn-doped hematite nanostructures for photoelectrochemical water splitting. Nano Lett. 2011, 11, 2119–2125. [Google Scholar] [CrossRef] [PubMed]
- Bohn, C.D.; Agrawal, A.K.; Walter, E.C.; Vaudin, M.D.; Herzing, A.A.; Haney, P.M.; Talin, A.A.; Szalai, V.A. Effect of tin doping on α-Fe2O3 photoanodes for water splitting. J. Phys. Chem. C 2012, 116, 15290–15296. [Google Scholar] [CrossRef]
- Frydrych, J.; Machala, L.; Tucek, J.; Siskova, K.; Filip, J.; Pechousek, J.; Safarova, K.; Vondracek, M.; Seo, J.H.; Schneeweiss, O.; et al. Facile fabrication of tin-doped hematite photoelectrodes—Effect of doping on magnetic properties and performance for light-induced water splitting. J. Mater. Chem. 2012, 22, 23232. [Google Scholar] [CrossRef]
- Dunn, H.K.; Feckl, J.M.; Muller, A.; Fattakhova-Rohlfing, D.; Morehead, S.G.; Roos, J.; Peter, L.M.; Scheu, C.; Bein, T. Tin doping speeds up hole transfer during light-driven water oxidation at hematite photoanodes. Phys. Chem. Chem. Phys. 2014, 16, 24610–24620. [Google Scholar] [CrossRef] [PubMed]
- Shinde, P.S.; Choi, S.H.; Kim, Y.; Ryu, J.; Jang, J.S. Onset potential behavior in α-Fe2O3 photoanodes: The influence of surface and diffusion sn doping on the surface states. Phys. Chem. Chem. Phys. 2016, 18, 2495–2509. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, A.; Lee, H.H.; Choi, S.H.; Lee, S.Y.; Gracia-Espino, E.; Subramanian, A.; Park, J.; Kong, K.J.; Jang, J.S. Sn/Be sequentially co-doped hematite photoanodes for enhanced photoelectrochemical water oxidation: Effect of Be2+ as co-dopant. Sci. Rep. 2016, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Guo, P.; Wheeler, D.A.; Jiang, J.; Lindley, S.A.; Kronawitter, C.X.; Zhang, J.Z.; Guo, L.; Mao, S.S. Physical and photoelectrochemical properties of Zr-doped hematite nanorod arrays. Nanoscale 2013, 5, 9867–9874. [Google Scholar] [CrossRef] [PubMed]
- Franking, R.; Li, L.; Lukowski, M.A.; Meng, F.; Tan, Y.; Hamers, R.J.; Jin, S. Facile post-growth doping of nanostructured hematite photoanodes for enhanced photoelectrochemical water oxidation. Energy Environ. Sci. 2013, 6, 500–512. [Google Scholar] [CrossRef]
- Neufeld, O.; Toroker, M.C. Platinum-doped α-Fe2O3 for enhanced water splitting efficiency: A dft+ustudy. J. Phys. Chem. C 2015, 119, 5836–5847. [Google Scholar] [CrossRef]
- Hu, Y.-S.; Kleiman-Shwarsctein, A.; Forman, A.J.; Hazen, D.; Park, J.-N.; McFarland, E.W. Pt-doped α-Fe2O3 thin films active for photoelectrochemical water splitting. Chem. Mater. 2008, 20, 3803–3805. [Google Scholar] [CrossRef]
- Kim, J.Y.; Magesh, G.; Youn, D.H.; Jang, J.-W.; Kubota, J.; Domen, K.; Lee, J.S. Single-crystalline, wormlike hematite photoanodes for efficient solar water splitting. Sci. Rep. 2013, 3, 2681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Xu, Y.; Mayer, M.T.; Simpson, Z.I.; McMahon, G.; Zhou, S.; Wang, D. Growth of p-type hematite by atomic layer deposition and its utilization for improved solar water splitting. J. Am. Chem. Soc. 2012, 134, 5508–5511. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zuo, F.; Dagg, A.; Feng, P. A three-dimensional branched cobalt-doped α-Fe2O3 nanorod/MgFe2O4 heterojunction array as a flexible photoanode for efficient photoelectrochemical water oxidation. Angew. Chem. Int. Ed. 2013, 52, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Wen, X.; Yang, S.; Lu, J.G. Controlled p- and n-type doping of Fe2O3 nanobelt field effect transistors. Appl. Phys. Lett. 2005, 87, 013113. [Google Scholar] [CrossRef]
- Qi, X.; She, G.; Wang, M.; Mu, L.; Shi, W. Electrochemical synthesis of p-type Zn-doped α-Fe2O3 nanotube arrays for photoelectrochemical water splitting. Chem. Commun. 2013, 49, 5742–5744. [Google Scholar] [CrossRef] [PubMed]
- Mirbagheri, N.; Wang, D.; Peng, C.; Wang, J.; Huang, Q.; Fan, C.; Ferapontova, E.E. Visible light driven photoelectrochemical water oxidation by Zn- and Ti-doped hematite nanostructures. Acs Catal. 2014, 4, 2006–2015. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H.; Jang, J.-W.; Kim, J.Y.; Choi, S.H.; Magesh, G.; Lee, J.; Lee, J.S. Awakening solar water-splitting activity of ZnFe2O4 nanorods by hybrid microwave annealing. Adv. Energy Mater. 2015, 5, 1401933. [Google Scholar] [CrossRef]
- Ingler, W.B.; Khan, S.U.M. A self-driven p/n-Fe2O3 tandem photoelectrochemical cell for water splitting. Electrochem. Solid State. Lett. 2006, 9, G144. [Google Scholar] [CrossRef]
- Shen, S.; Zhou, J.; Dong, C.L.; Hu, Y.; Tseng, E.N.; Guo, P.; Guo, L.; Mao, S.S. Surface engineered doping of hematite nanorod arrays for improved photoelectrochemical water splitting. Sci. Rep. 2014, 4, 6627. [Google Scholar] [CrossRef] [PubMed]
- Inglerjr, W.; Khan, S. Photoresponse of spray pyrolytically synthesized copper-doped p- thin film electrodes in water splitting. Int. J. Hydrogen Energy 2005, 30, 821–827. [Google Scholar] [CrossRef]
- Kleiman-Shwarsctein, A.; Huda, M.N.; Walsh, A.; Yan, Y.; Stucky, G.D.; Hu, Y.-S.; Al-Jassim, M.M.; McFarland, E.W. Electrodeposited aluminum-doped α-Fe2O3 photoelectrodes: Experiment and theory. Chem. Mater. 2010, 22, 510–517. [Google Scholar] [CrossRef]
- Cesar, I.; Kay, A.; Martinez, J.A.G.; Gratzel, M. Translucent thin film Fe2O3 photoanodes for efficient water splitting by sunlight: Nanostructure-directing effect of si-doping. J. Am. Chem. Soc. 2006, 128, 4582–4583. [Google Scholar] [CrossRef] [PubMed]
- Cesar, I.; Sivula, K.; Kay, A.; Zboril, R.; Graetzel, M. Influence of feature size, film thickness, and silicon doping on the performance of nanostructured hematite photoanodes for solar water splitting. J. Phys. Chem. C 2009, 113, 772–782. [Google Scholar] [CrossRef]
- Kay, A.; Cesar, I.; Graetzel, M. New benchmark for water photooxidation by nanostructured α-Fe2O3 films. J. Am. Chem. Soc. 2006, 128, 15714–15721. [Google Scholar] [CrossRef] [PubMed]
- Saremi-Yarahmadi, S.; Wijayantha, K.G.U.; Tahir, A.A.; Vaidhyanathan, B. Nanostructured α-Fe2O3 electrodes for solar driven water splitting: Effect of doping agents on preparation and performance. J. Phys. Chem. C 2009, 113, 4768–4778. [Google Scholar] [CrossRef]
- Zhang, M.; Luo, W.; Li, Z.; Yu, T.; Zou, Z. Improved photoelectrochemical responses of si and ti codoped α-fe2o3 photoanode films. Appl. Phys. Lett. 2010, 97, 042105. [Google Scholar] [CrossRef]
- Chemelewski, W.D.; Mabayoje, O.; Tang, D.; Rettie, A.J.E.; Buddie Mullins, C. Bandgap engineering of Fe2O3 with Cr—Application to photoelectrochemical oxidation. Phys. Chem. Chem. Phys. 2016, 18, 1644–1648. [Google Scholar] [CrossRef] [PubMed]
- Kleiman-Shwarsctein, A.; Hu, Y.-S.; Forman, A.J.; Stucky, G.D.; McFarland, E.W. Electrodeposition of α-Fe2O3 doped with mo or cr as photoanodes for photocatalytic water splitting. J. Phys. Chem. C 2008, 112, 15900–15907. [Google Scholar] [CrossRef]
- Braun, A.; Sivula, K.; Bora, D.K.; Zhu, J.; Zhang, L.; Grätzel, M.; Guo, J.; Constable, E.C. Direct observation of two electron holes in a hematite photoanode during photoelectrochemical water splitting. J. Phys. Chem. C 2012, 116, 16870–16875. [Google Scholar] [CrossRef]
- Kim, D.W.; Riha, S.C.; DeMarco, E.J.; Martinson, A.B.F.; Farha, O.K.; Hupp, J.T. Greenlighting photoelectrochemical oxidation of water by iron oxide. Acs Nano 2014, 8, 12199–12207. [Google Scholar] [CrossRef] [PubMed]
- Monllor-Satoca, D.; Bartsch, M.; Fabrega, C.; Genc, A.; Reinhard, S.; Andreu, T.; Arbiol, J.; Niederberger, M.; Morante, J.R. What do you do, titanium? Insight into the role of titanium oxide as a water oxidation promoter in hematite-based photoanodes. Energy Environ. Sci. 2015, 8, 3242–3254. [Google Scholar] [CrossRef]
- Bassi, P.S.; Chiam, S.Y.; Gurudayal; Barber, J.; Wong, L.H. Hydrothermal grown nanoporous iron based titanate, Fe2TiO5 for light driven water splitting. ACS Appl. Mater. Interfaces 2014, 6, 22490–22495. [Google Scholar] [CrossRef] [PubMed]
- Bassi, P.S.; Antony, R.P.; Boix, P.P.; Fang, Y.; Barber, J.; Wong, L.H. Crystalline Fe2O3/ Fe2TiO5 heterojunction nanorods with efficient charge separation and hole injection as photoanode for solar water oxidation. Nano Energy 2016, 22, 310–318. [Google Scholar] [CrossRef]
- Ruoko, T.-P.; Kaunisto, K.; Bärtsch, M.; Pohjola, J.; Hiltunen, A.; Niederberger, M.; Tkachenko, N.V.; Lemmetyinen, H. Subpicosecond to second time-scale charge carrier kinetics in hematite–titania nanocomposite photoanodes. J. Phys. Chem. Lett. 2015, 6, 2859–2864. [Google Scholar] [CrossRef] [PubMed]
- Sivula, K.; Zboril, R.; Le Formal, F.; Robert, R.; Weidenkaff, A.; Tucek, J.; Frydrych, J.; Grätzel, M. Photoelectrochemical water splitting with mesoporous hematite prepared by a solution-based colloidal approach. J. Am. Chem. Soc. 2010, 132, 7436–7444. [Google Scholar] [CrossRef] [PubMed]
- Tamirat, A.G.; Su, W.-N.; Dubale, A.A.; Chen, H.-M.; Hwang, B.-J. Photoelectrochemical water splitting at low applied potential using a niooh coated codoped (Sn, Zr) α-Fe2O3 photoanode. J. Mater. Chem. A 2015, 3, 5949–5961. [Google Scholar] [CrossRef]
- Liao, P.; Toroker, M.C.; Carter, E.A. Electron transport in pure and doped hematite. Nano Lett. 2011, 11, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Huo, P.; Guo, L.; Prezhdo, O.V. Understanding hematite doping with group iv elements: A DFT+U study. J. Phys. Chem. C 2015, 119, 26303–26310. [Google Scholar] [CrossRef]
- Ling, Y.; Wang, G.; Reddy, J.; Wang, C.; Zhang, J.Z.; Li, Y. The influence of oxygen content on the thermal activation of hematite nanowires. Angew. Chem. Int. Ed. 2012, 51, 4074–4079. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Ji, H.; Ma, W.; Chen, C.; Song, W.; Zhao, J. Doping-Promoted Solar Water Oxidation on Hematite Photoanodes. Molecules 2016, 21, 868. https://doi.org/10.3390/molecules21070868
Zhang Y, Ji H, Ma W, Chen C, Song W, Zhao J. Doping-Promoted Solar Water Oxidation on Hematite Photoanodes. Molecules. 2016; 21(7):868. https://doi.org/10.3390/molecules21070868
Chicago/Turabian StyleZhang, Yuchao, Hongwei Ji, Wanhong Ma, Chuncheng Chen, Wenjing Song, and Jincai Zhao. 2016. "Doping-Promoted Solar Water Oxidation on Hematite Photoanodes" Molecules 21, no. 7: 868. https://doi.org/10.3390/molecules21070868




