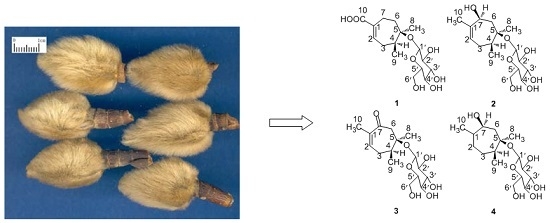
Four New Monoterpenoid Glycosides from the Flower Buds of Magnolia biondii
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Elucidation of Compounds 1–4
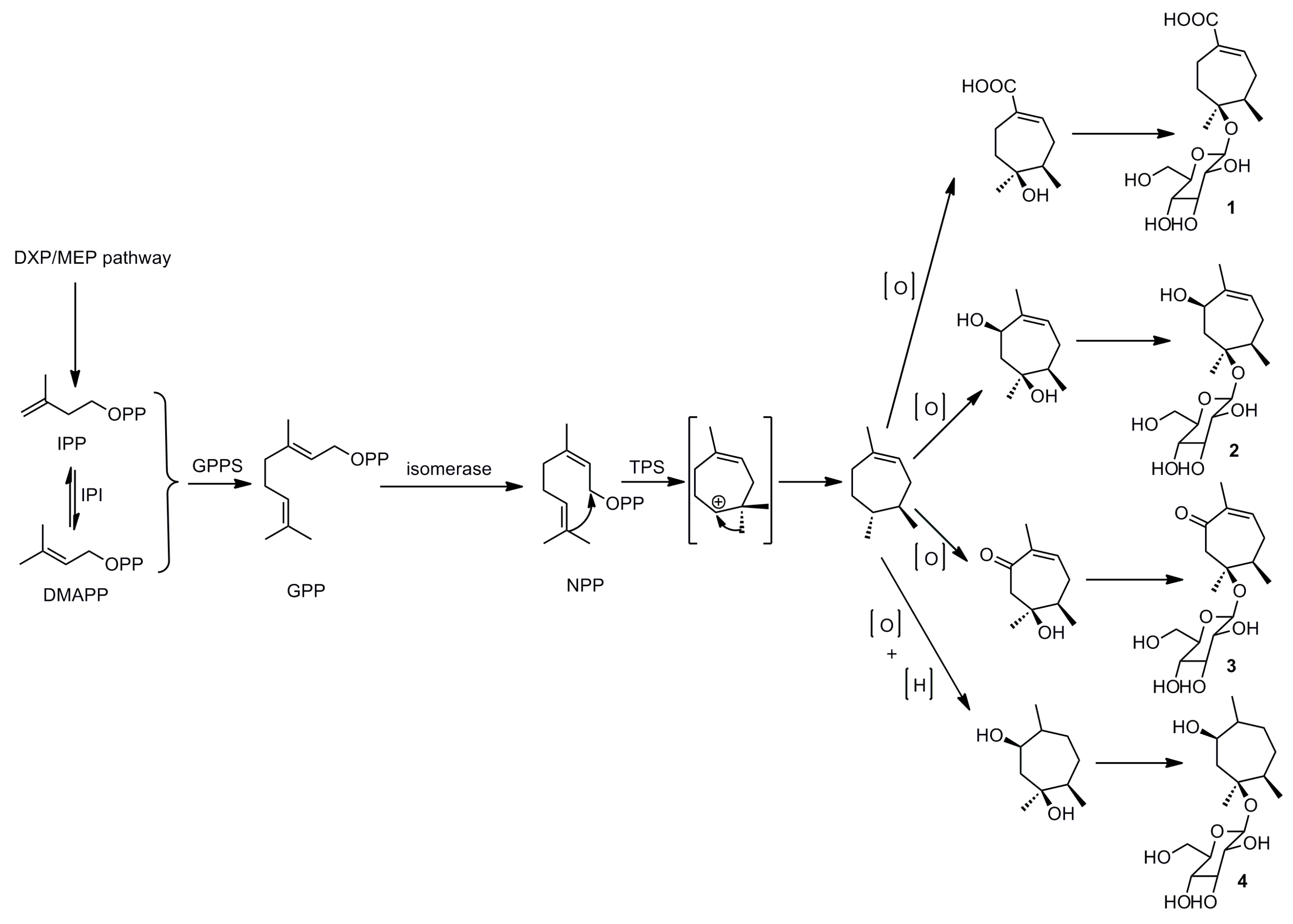
2.2. Plausible Biogenetic Pathway
2.3. Antimicrobial Activity
3. Experimental Section
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Compound characterization
3.5. Acid Hydrolysis and Sugar Analysis
3.6. Antimicrobial Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cai, H.M.; Fan, W.; Wang, X.D. Research progress on chemical constituents and pharmacological effects of Magnolia. China Pharm. 2013, 22, 3735–3737. [Google Scholar]
- Chinese Pharmacopoeia Commission. The Pharmacopoeia of the People’s Republic of China, 2015 ed.; China Medical Science Press: Beijing, China, 2015; Part I; p. 182. [Google Scholar]
- Shen, Y.; Pang, E.C.K.; Xue, C.C.L.; Zhao, Z.Z.; Lin, J.G.; Li, G.G. Inhibitions of mast cell-derived histamine release by different Flos Magnoliae species in rat peritoneal mast cells. Phytomedicine 2008, 15, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.W.; Yang, J.K.; Hu, D.W. Summarize of study on the application in medicine function and the ingredient of Magnolia liliflora. Strait Pharm. J. 2002, 14, 5–7. [Google Scholar]
- Tsuruga, T.; Ebizuka, Y.; Nakajima, J.; Chun, Y.T.; Noguchi, H.; Irtaka, Y.; Sankawa, U. Biologically active constituents of Magnolia salicifolia: Inhibitiors of induced histamine release from Rat Mast Cells. Chem. Pharm. Bull. 1991, 39, 3265–3271. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.C.; Lee, S.G.; Park, B.S.; Kim, J.Y.; Song, Y.S.; Kim, J.M.; Yoo, K.S.; Huh, G.Y.; Jeong, M.H.; Lim, Y.J.; et al. MagnoliaeFlos induces apoptosis of RBL-2H3 cells via mitochondria and caspase. Int. Arch. Allergy Immunol. 2003, 131, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Suzuki, J.; Yamada, T.; Yoshizaki, M.; Kikuchi, T.; Shigetoshi, K.; Matsuda, S. Anti-inflammatory effect of neolignans newly isolated from the Crude Drug “Shin-i” (Flos Magnoliae). Planta Med. 1985, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Kimura, I.; Kimura, M. Inhibitory effect of Magnosalin derived from Flos Magnoliae on tube formation of rat vascular endothelial cells during the angiogenic process. Biol. Pharm. Bull. 1996, 19, 1304–1306. [Google Scholar] [CrossRef] [PubMed]
- Kuroyanagi, M.; Yoshida, K.; Yamamoto, A.; Miwa, M. Bicyclo[3.2.1]octane and 6-Oxabicyclo[3.2.2]nonane type neolignans from Magnolia denudate. Chem. Pharm. Bull. 2000, 48, 832–837. [Google Scholar] [PubMed]
- Ma, Y.L.; Han, G.Q. Biologically active lignans from Magnolia biondii Pamp. China J. Chin. Mater. Med. 1995, 20, 102–104. [Google Scholar]
- Liang, Z.; Yang, E.Y. The therapeutic effects of irrigating therapy with magnolia injection on chronic maxillary sinusitis experimentally induced among rabbits. Chinese J. Otolaryngol. Integr. Tradit. West. Med. 2005, 13, 6–10. [Google Scholar]
- Lee, J.; Lee, D.; Jang, D.S.; Nam, J.W.; Kim, J.P.; Park, K.H.; Yang, M.S.; Seo, E.K. Two new stereoisomers of tetrahydrofuranoid lignans from the Flower Buds of Magnolia fargesii. Chem. Pharm. Bull. 2007, 55, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Seo, E.K.; Jang, D.S.; Ha, T.J.; Kim, J.P.; Nam, J.W.; Bae, G.; Lee, Y.M.; Yang, M.S.; Kim, J.S. Two new stereoisomers of neolignan and lignin from the Flower Buds of Magnolia fargesii. Chem. Pharm. Bull. 2009, 57, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Schühly, W.; Skarbina, J.; Kunert, O.; Nandi, O.I.; Bauer, R. Chemical characterization of Magnolia biondii. Nat. Prod. Commun. 2009, 4, 231–234. [Google Scholar] [PubMed]
- Chen, Y.Y.; Wang, B.; Cao, C.Y.; Qiao, L.; Han, G.Q. Study on the hydrophilic components of Magnolia biondii Pamp. Acta Pharm. Sin. 1994, 29, 506–510. [Google Scholar]
- Lee, J.; Kim, N.H.; Nam, J.W.; Lee, Y.M.; Jang, D.S.; Kim, Y.S.; Nam, S.H.; Seo, E.K.; Yang, M.S.; Kim, J.S. Scopoletin from the Flower Buds of Magnolia fargesii inhibits protein glycation, aldose reductase, and cataractogenesis ex vivo. Arch. Pharm. Res. 2010, 33, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.J.; Ma, Y.B.; Geng, C.A.; Huang, X.Y.; Xu, H.B.; Zhang, X.M.; Chen, J.J. Paeoveitols A–E from Paeonia veitchii. Fitoterapia 2015, 106, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Youn, U.J.; Lee, J.; Nam, J.W.; Lee, Y.J.; Seo, E.K. Identification of a new isomer of dihydrophaseic acid 3'-O-β-d-glucopyranoside from Nelumbo nucifera. Bull. Korean Chem. Soc. 2011, 32, 4083–4085. [Google Scholar] [CrossRef]
- Rahman, M.M.; Gray, A.I. A benzoisofuranone derivative and carbazole alkaloids from Murraya koenigii and their antimicrobial activity. Phytochemistry 2005, 66, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1–4 are available from the authors.
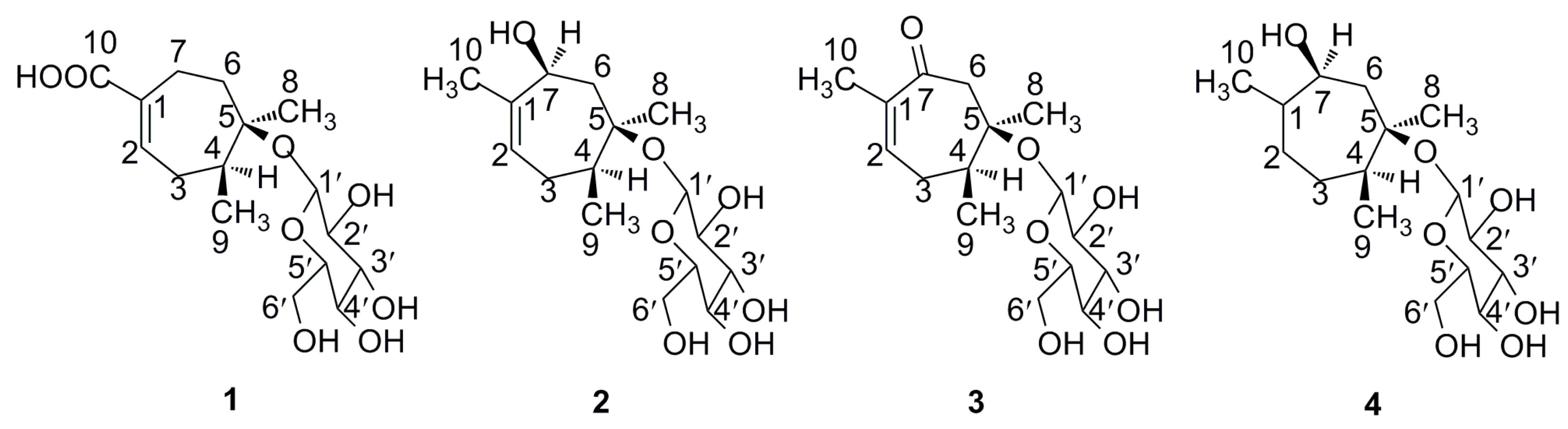

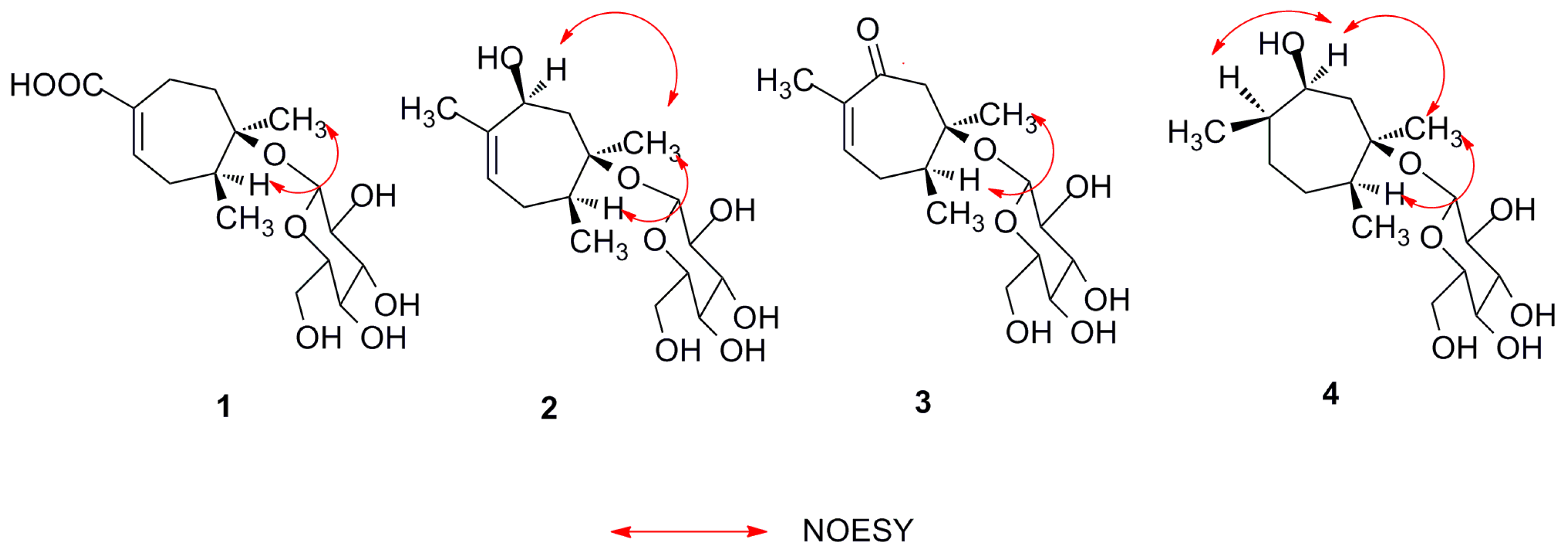
| Position | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | δH | δC | |
| 1 | 131.5 (s) | 138.2 (s) | 135.8 (s) | 1.40 (1H, m) | 37.9 (d) | |||
| 2 | 6.98 (1H, t, 2.2, 4.9) | 141.3 (d) | 5.44 (1H, d, 4.2) | 124.6 (d) | 6.87 (1H, d, 5.4) | 148.3 (d) | 1.40 (2H, m) | 29.6 (t) |
| 3 | 2.02 (1H, m) | 28.6 (t) | 1.84 (1H, m) | 28.3 (t) | 2.28 (1H, m) | 28.6 (t) | 1.01 (1H, m) | 28.3 (t) |
| 2.41 (1H, m) | 2.05 (1H, m) | 2.48 (1H, m) | 1.76 (1H, m) | |||||
| 4 | 1.72 (1H, m) | 44.5 (d) | 1.76 (1H, m) | 44.6 (d) | 2.16 (1H, m) | 46.7 (d) | 1.93 (1H, m) | 41.3 (d) |
| 5 | 80.5 (s) | 80.3 (s) | 79.3 (s) | 81.1 (s) | ||||
| 6 | 2.15 (2H, m) | 24.5 (t) | 1.21 (1H, m) | 35.1 (t) | 2.28 (1H, m) | 40.4 (t) | 1.23 (1H, m) | 35.1 (t) |
| 2.27 (1H, dd, 5.7, 11.6) | 2.66 (1H, m) | 2.17 (1H, m) | ||||||
| 7 | 2.45 (2H, m) | 26.3 (t) | 4.08 (1H, br. s) | 71.9 (d) | 203.5 (s) | 3.84 (1H, m) | 71.9 (d) | |
| 8 | 1.25 (3H, s) | 24.8 (q) | 1.21 (3H, s) | 24.1 (q) | 1.28 (3H, s) | 24.9 (q) | 1.23 (3H, s) | 24.1 (q) |
| 9 | 1.23 (3H, s) | 23.3 (q) | 1.21 (3H, s) | 23.6 (q) | 1.24 (3H, s) | 23.5 (q) | 1.14 (3H, s) | 23.6 (q) |
| 10 | 170.9 (s) | 1.70 (3H, s) | 19.2 (q) | 1.72 (3H, s) | 15.6 (q) | 0.91 (3H, d, 6.2) | 18.8 (q) | |
| 1′ | 4.46 (1H, d, 7.8) | 98.5 (d) | 4.45 (1H, d, 7.8) | 98.4 (d) | 4.45 (1H, d, 7.8) | 98.5 (d) | 4.45 (1H, d, 7.7) | 98.6 (d) |
| 2′ | 3.11 (1H, m) | 75.3 (d) | 3.12 (1H, m) | 75.2 (d) | 3.12 (1H, m) | 75.2 (d) | 3.10 (1H, m) | 75.4 (d) |
| 3′ | 3.21 (1H, m) | 77.5 (d) | 3.20 (1H, m) | 77.5 (d) | 3.20 (1H, m) | 77.5 (d) | 3.23 (1H, m) | 77.8 (d) |
| 4′ | 3.26 (1H, m) | 71.8 (d) | 3.23 (1H, m) | 71.7 (d) | 3.26 (1H, m) | 71.6 (d) | 3.16 (1H, m) | 72.2 (d) |
| 5′ | 3.35 (1H, m) | 78.3 (d) | 3.34 (1H, m) | 78.3 (d) | 3.33 (1H, m) | 78.2 (d) | 3.33 (1H, m) | 78.2 (d) |
| 6′ | 3.63 (1H, m) | 62.9 (t) | 3.62 (1H, dd, 5.6, 11.9) | 62.8 (t) | 3.61 (1H, m) | 62.8 (t) | 3.56 (1H, m) | 63.2 (t) |
| 3.80 (1H, m) | 3.78 (1H, dd, 2.1, 11.9) | 3.78 (1H, m) | 3.81 (1H, m) |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, W.-S.; He, Y.-H.; Zheng, X.-K.; Wang, J.-C.; Cao, Y.-G.; Zhang, Y.-L.; Song, K. Four New Monoterpenoid Glycosides from the Flower Buds of Magnolia biondii. Molecules 2016, 21, 728. https://doi.org/10.3390/molecules21060728
Feng W-S, He Y-H, Zheng X-K, Wang J-C, Cao Y-G, Zhang Y-L, Song K. Four New Monoterpenoid Glycosides from the Flower Buds of Magnolia biondii. Molecules. 2016; 21(6):728. https://doi.org/10.3390/molecules21060728
Chicago/Turabian StyleFeng, Wei-Sheng, Yu-Huan He, Xiao-Ke Zheng, Jian-Chao Wang, Yan-Gang Cao, Yan-Li Zhang, and Kai Song. 2016. "Four New Monoterpenoid Glycosides from the Flower Buds of Magnolia biondii" Molecules 21, no. 6: 728. https://doi.org/10.3390/molecules21060728