α-Glucosidase Inhibitors from Vauquelinia corymbosa
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation of α-Glucosidase Inhibitors from V. corymbosa

| Sample | Yeast | Rat Small Intestinal | ||
|---|---|---|---|---|
| IC50 (mM) a | Maximum Inhibition (%) | IC50 (mM) a | Maximum Inhibition (%) | |
| Acarbose b | 0.50 ± 0.23 | 89.7 | 0.10 ± 0.003 | 80.2 |
| 1 | 1.60 ± 0.07 | 95.2 | ND | 44.5 d |
| 2 b | 0.30 ± 0.02 | 99.6 | ND | 15.2 d |
| 3 b | 0.40 ± 0.02 | 67.8 | 1.98 ± 0.15 | 65.1 |
| 4 b | 0.06 ± 0.005 | 99.0 | 1.63 ± 0.11 | 71.0 |
| 5 | ND c | 45.0 | 3.34 ± 0.38 | 59.7 |
| 6 | 0.03 ± 0.006 | 95.6 | 0.43 ± 0.03 | 75.8 |
| 7 | Inactive | - | 10.68 ± 0.96 | 52.6 |
| 8 | Inactive | - | ND | 39.3 d |
| 9 | 0.03 e | ND | 0.216 e | ND |
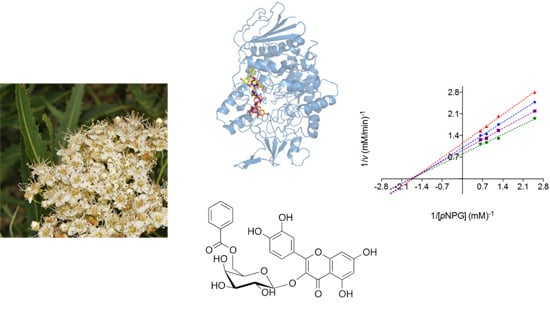
2.2. Mode of Inhibition of Yeast and Mammalian α-Glucosidases and Molecular Docking for Compound 6




2.3. Determination of Acute Toxicity Studies of Some Extracts of V. corymbosa
3. Experimental Section
3.1. General Procedures
3.2. Plant Material
3.3. Preparations of Extracts
3.4. Isolation of the Active Compounds from the Active EtOAc-Soluble Fraction
3.5. α-Glucosidase Inhibitory Assays
3.6. Kinetic Analyses
3.7. Docking Studies
3.8. Isolation of Compound 1 from OE of V. corymbosa
3.9. Chromatographic Techniques
3.10. Determination of Acute Toxicity for AE and OE
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bilous, R.; Donelly, R. Handbook of Diabetics, 4th ed.; Wiley-Blackwell: Solaris South Tower, Singapore, 2010. [Google Scholar]
- Scully, T. Diabetes in numbers. Nature 2012, 485, S2–S3. [Google Scholar] [CrossRef] [PubMed]
- International Federation of Diabetes. Diabetes Atlas IFD, 6th ed.; International Federation of Diabetes: Brussels, Belgium, 2013. [Google Scholar]
- Israili, H.Z. Advances in the treatment of type 2 diabetes mellitus. Am. J. Ther. 2011, 18, 117–152. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, N.S.; Ramos, M.; Fernandes, P. Studies on α-glucosidase inhibitors development: Magic molecules for the treatment of carbohydrate mediated diseases. Mini-Rev. Med. Chem. 2012, 12, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Asano, N. Sugar-mimicking glycosidase inhibitors: Bioactivity and application. Cell. Mol. Life Sci. 2009, 66, 1479–1492. [Google Scholar] [CrossRef] [PubMed]
- Mata, R.; Cristians, S.; Escandón-Rivera, S.; Juárez-Reyes, K.; Rivero-Cruz, I. Mexican antidiabetic herbs: Valuable source of inhibitors of α-glucosidase. J. Nat. Prod. 2013, 76, 468–483. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Angón, A.; Melgarejo, E.D.; Contreras, A.V.; González, M.A. La Biodiversidad en Guanajuato: Estudios de Estado; CONABIO: México City, Mexico, 2012. [Google Scholar]
- Rzedowski, J.; Calderón, G. Flora del Bajío y de Regiones Adyacentes; INECOL: Jalapa, Mexico, 2005. [Google Scholar]
- Trumbull, E.R.; Bianchi, E.; Eckert, D.S.; Wiedhopf, M.; Cole, J.R. Tumor inhibitory agents from Vauquelinia corymbosa (Rosaceae). J. Pharm. Sci. 1976, 65, 1407–1408. [Google Scholar] [CrossRef] [PubMed]
- Oki, T.; Matsui, T.; Osajima, Y. Inhibitory effect of α-glucosidase, inhibitors varies according to its origin. J. Agric. Food. Chem. 1999, 47, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Nicollier, F.G.; Pope, F.D.; Thompson, C.A. Biological activity of dhurrin and other compounds from Johnson grass (Sorghum halepense). J. Agric. Food Chem. 1983, 31, 744–748. [Google Scholar] [CrossRef]
- Isaza, J.H.; Ito, H.; Yoshida, T. A flavonol glycoside-lignan ester and accompanying acylated glucosides from Monochaetum multiflorum. Phytochemistry 2001, 58, 321–327. [Google Scholar] [CrossRef]
- Davis, A.L.; Cai, Y.; Davies, A.P. 1H- and 13C-NMR Assignments of some green tea polyphenols. Magn. Reson. Chem. 1996, 34, 887–890. [Google Scholar] [CrossRef]
- Wei, Y.; Ito, Y. Isolation of hyperoside and luteolin-glucoside from Agrimonia pilosa ledeb using stepwise elution by High-Speed Countercurrent Chromatography. J. Liq. Chromatogr. Relat. Technol. 2007, 30, 1465–1473. [Google Scholar] [CrossRef]
- Napolitano, J.G.; Lankin, D.C.; Chen, S.; Pauli, F.G. Complete 1H-NMR spectral analysis of ten chemical markers of Ginkgo biloba. Magn. Reson. Chem. 2012, 50, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Bouktaib, M.; Atmani, A.; Ronaldo, C. Regio and stereoselective synthesis of the major metabolite of quercetin, quercetin-3-O-β-d-glucuronide. Tetrahedron Lett. 2002, 43, 6263–6266. [Google Scholar] [CrossRef]
- Hasan, S.; Ahmed, I.; Mondal, S.; Uddin1, J.S.; Masud, M.M.; Sadhu, K.S.; Ishibashi, M. Antioxidant, antinociceptive activity and general toxicity study of Dendrophthoe falcate and isolation of Quercitrin as the major component. Orient. Pharm. Exp. Med. 2006, 6, 355–360. [Google Scholar]
- Vvedenskaya, I.O.; Rosen, T.R.; Guido, E.J.; Russell, J.O.; Millis, A.K.; Vorsa, N. Characterization of flavonols in cranberry (Vaccinium macrocarpon) powder. J. Agric. Food Chem. 2004, 52, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, C.; Lutterbach, R.; Stöckigt, J. Preparative biosynthesis of natural glucosides and fluorogenic substrates for β-glucosidases followed by in vivo 13C-NMR with high density plant cell cultures. Tetrahedron 1996, 52, 925–934. [Google Scholar] [CrossRef]
- Lutterbach, R.; Stockigt, J. Dynamics of the biosynthesis of methylursubinin plant cells employing in vivo 13C-NMR without labeling. Phytochemistry 1995, 40, 801–806. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, W.; Feng, F.; Zhang, Y.; Kang, W. α-Glucosidase inhibitors isolated from medicinal plants. Food Sci. Hum. Wellness 2014, 3, 136–174. [Google Scholar] [CrossRef]
- Islam, N.; Jung, A.H.; Sohn, S.H.; Kim, M.H.; Choi, S.J. Potent α-glucosidase and protein tyrosine phosphatase 1B inhibitors from Artemisia capillaris. Arch. Pharm. Res. 2013, 36, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.Y.; Zhou, C.F.; Gao, F.; Bain, S.J.; Shan, F. Comparative evaluation of quercetin, isoquercetin and rutin as inhibitors of α-glucosidase. J. Agric. Food Chem. 2009, 57, 11463–11468. [Google Scholar]
- Zhou, X.; Liu, B.; Liu, L.; Lou, Y.; Guo, J.; Ye, J. Manufacture of Vinegar Containing Eriobotrya japonica Extract. CN 101096629, 2 January 2008. [Google Scholar]
- Khayat, E. Pharmaceutical composition for alleviating excess levels of sugar in diabetic patients. U.S. Patent US2006/0257508 A1, 16 November 2006. [Google Scholar]
- Kim, S.H.; Jo, S.H.; Kwon, Y.I.; Hwang, J.K. Effects of onion (Allium cepa L.) extract administration on intestinal α-glucosidases activities and spikes in postprandial blood glucose levels in SD rats model. Int. J. Mol. Sci. 2011, 12, 3757–3769. [Google Scholar] [CrossRef] [PubMed]
- Khayat, E.; Sinai, Y.M. Pharmaceutical Composition for Alleviating Excess Levels of Sugar in Diabetic Patients. U.S. Patent US2006/025750 A1, 16 February 2006. [Google Scholar]
- Xu, H. Inhibition kinetics of flavonoids on y east α-glucosidase merged whit docking simulations. Prot. Pep. Lett. 2010, 17, 1270–1279. [Google Scholar] [CrossRef]
- Priscilla, D.H.; Roy, D.; Suresh, A.; Kumar, V.; Thirumurugan, K. Naringenin inhibits α-glucosidase activity: A promising strategy for the regulation of postprandial hyperglycemia in high fat diet fed streptozotocin induced diabetic rats. Chem. Biol. Interact. 2014, 210, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Tadera, K.; Minami, Y.; Takamutsu, K.; Matsuoka, T. Inhibition of α-glucosidase and α-amylase by flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Lorke, D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983, 54, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Johansen, H.; Rasmussen, H.L.; Olsen, E.C.; Hansen, B. Rate of hydrolysis and degradation of the cyanogenic glycoside-dhurrin-in soil. Chemosphere 2007, 67, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Zagrobelny, M.; Bak, S.; Lindberg, M.B. Cyanogenesis in plants and arthropods. Phytochemistry 2008, 69, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Copeland, R.A. Enzymes: A Practical Introduction to Structure, Mechanism, and Data Analysis, 2nd ed.; Wiley-VCH: New York, NY, USA, 2000. [Google Scholar]
- Segel, I.H. Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems; John Wiley & Sons: New York, NY, USA, 1993. [Google Scholar]
- Dallakyan, S. MGL Tools. Available online: http://mgl-tools.scripps.edu (accessed on 14 March 2015).
- Morris, G.G.; Goodsell, D.S.; Huey, R.; Lindstroom, W.; Hart, W.E.; Kurowski, S.; Halliday, S.; Belew, R.; Olson, A.J. AutoDock. Available online: http://autodock.scripps.edu (accessed on 14 March 2015).
- Rivera-Chávez, J.; González-Andrade, M.; Gonzáles, M.C.; Glenn, A.; Mata, R. Thielavins A, J and K: α-Glucosidase inhibitors from MEXU 27095, an endophytic fungus from Hintonia latiflora. Phytochemistry 2013, 94, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Kurakane, S.; Yamada, N.; Sato, H.; Igarashi, K. Anti-diabetic effects of Actinidia arguta polyphenols on rats and KK-A y mice. Food Sci. Technol. Res. 2011, 17, 93–102. [Google Scholar] [CrossRef]
- Babujanarthanam, R.; Kavitha, P.; Mahadeva-Rao, U.S.; Pandian, M.R. Quercitrin a bioflavonoid improves the antioxidant status in streptozotocin: Induced diabetic rat tissues. Mol. Cell. Biochem. 2011, 358, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1–8 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Bocanegra, L.; Pérez-Vásquez, A.; Torres-Piedra, M.; Bye, R.; Linares, E.; Mata, R. α-Glucosidase Inhibitors from Vauquelinia corymbosa. Molecules 2015, 20, 15330-15342. https://doi.org/10.3390/molecules200815330
Flores-Bocanegra L, Pérez-Vásquez A, Torres-Piedra M, Bye R, Linares E, Mata R. α-Glucosidase Inhibitors from Vauquelinia corymbosa. Molecules. 2015; 20(8):15330-15342. https://doi.org/10.3390/molecules200815330
Chicago/Turabian StyleFlores-Bocanegra, Laura, Araceli Pérez-Vásquez, Mariana Torres-Piedra, Robert Bye, Edelmira Linares, and Rachel Mata. 2015. "α-Glucosidase Inhibitors from Vauquelinia corymbosa" Molecules 20, no. 8: 15330-15342. https://doi.org/10.3390/molecules200815330