Protective Effect of 2,4′,5′-Trihydroxyl-5,2′-dibromo diphenylmethanone, a New Halophenol, against Hydrogen Peroxide-Induced EA.hy926 Cells Injury
Abstract
:1. Introduction
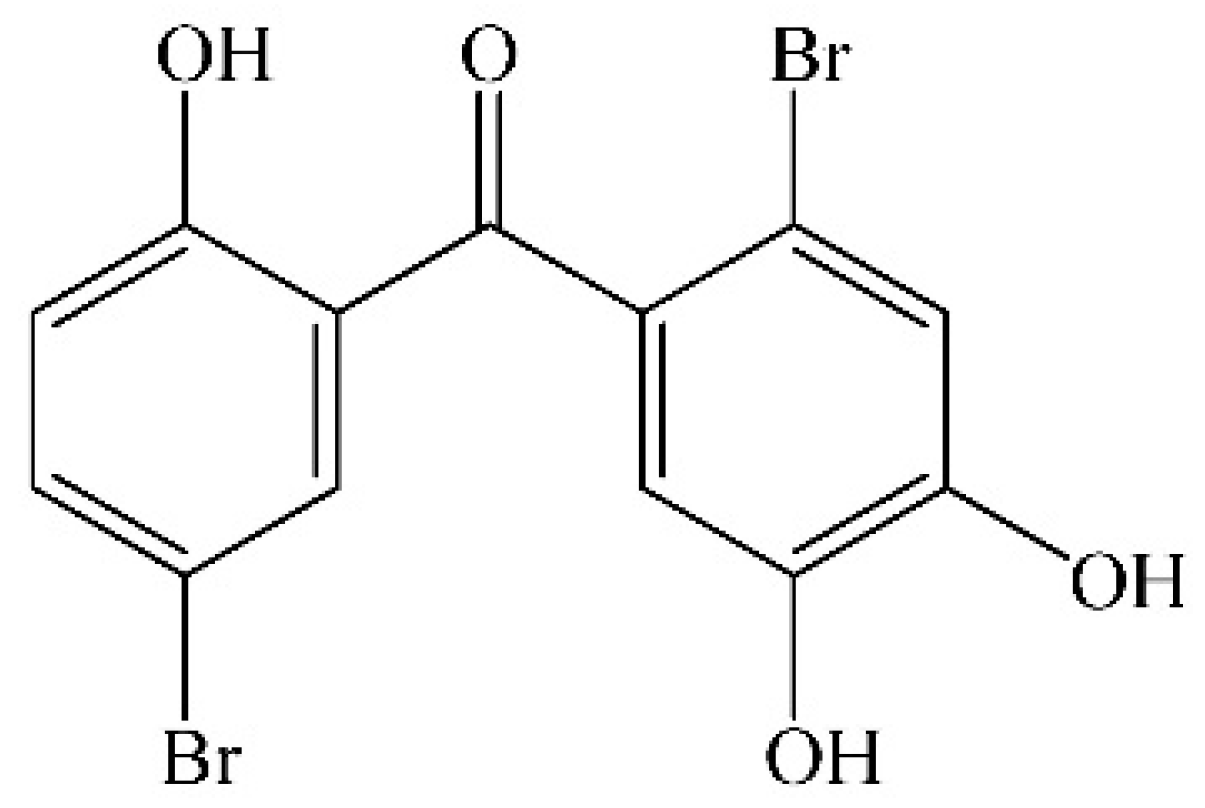
2. Results and Discussion
2.1. Cell Viability
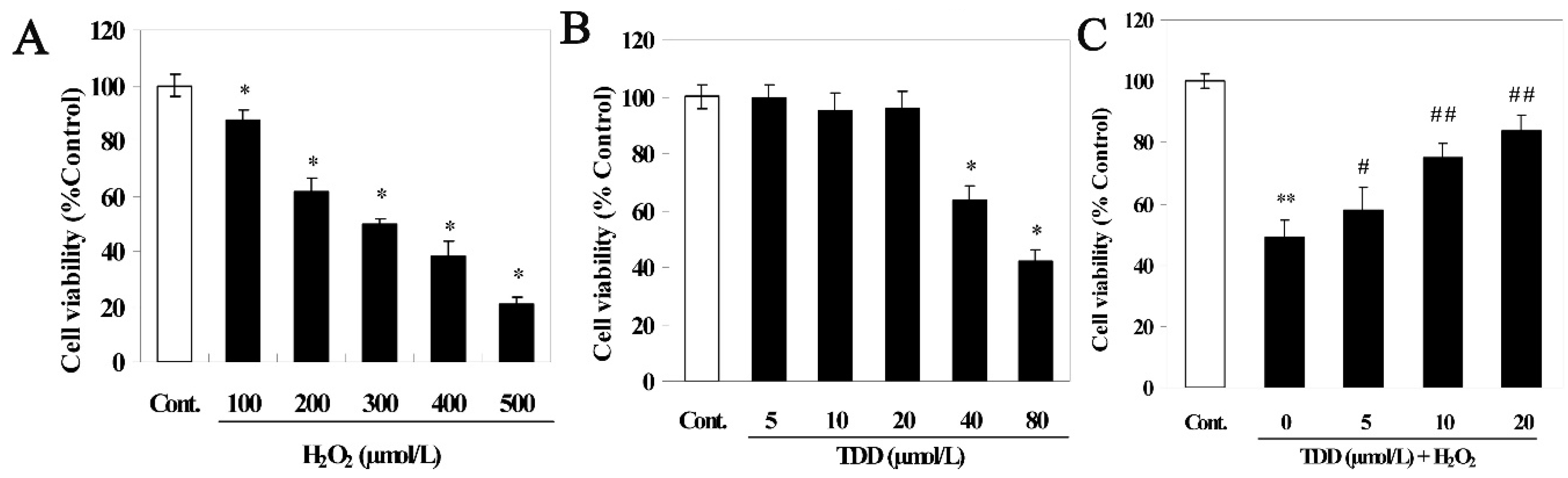
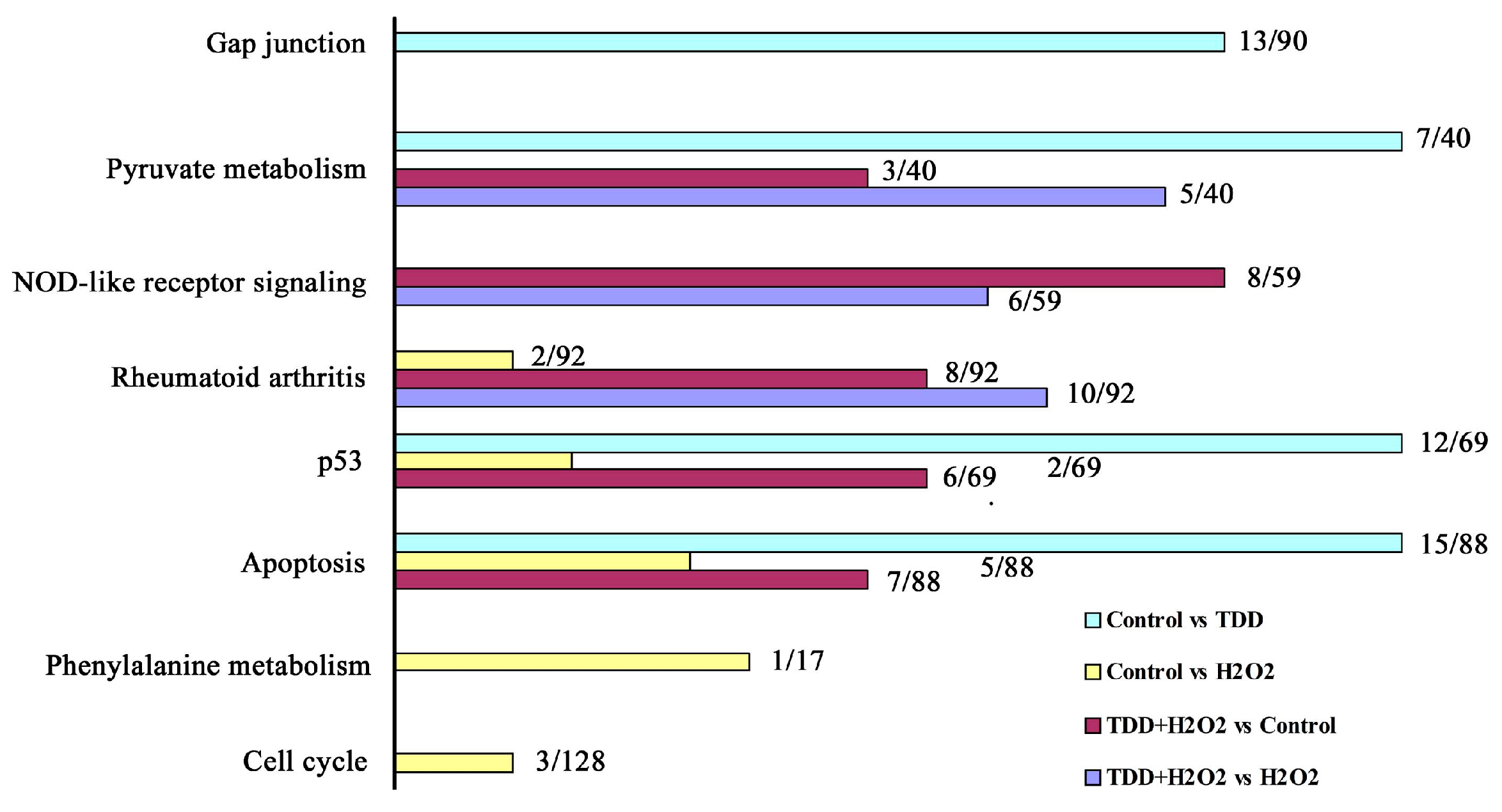
2.2. Microarray Analysis
| Biological Process | Gene Number | p Value |
|---|---|---|
| Cellular nitrogen compound metabolic process | 14 | 6.95 × 10−3 |
| Negative regulation of apoptosis | 13 | 1.33 × 10−2 |
| Regulation of small GTPase mediated signal transduction | 13 | 5.42 × 10−3 |
| Cytokine-mediated signaling pathway | 10 | 4.51 × 10−2 |
| aging | 10 | 3.17 × 10−3 |
| RNA processing | 9 | 2.08 × 10−3 |
| Reponse to lipopoly saccharide | 9 | 2.2 × 10−2 |
2.3. Flow Cytometic Analysis of Apoptosis

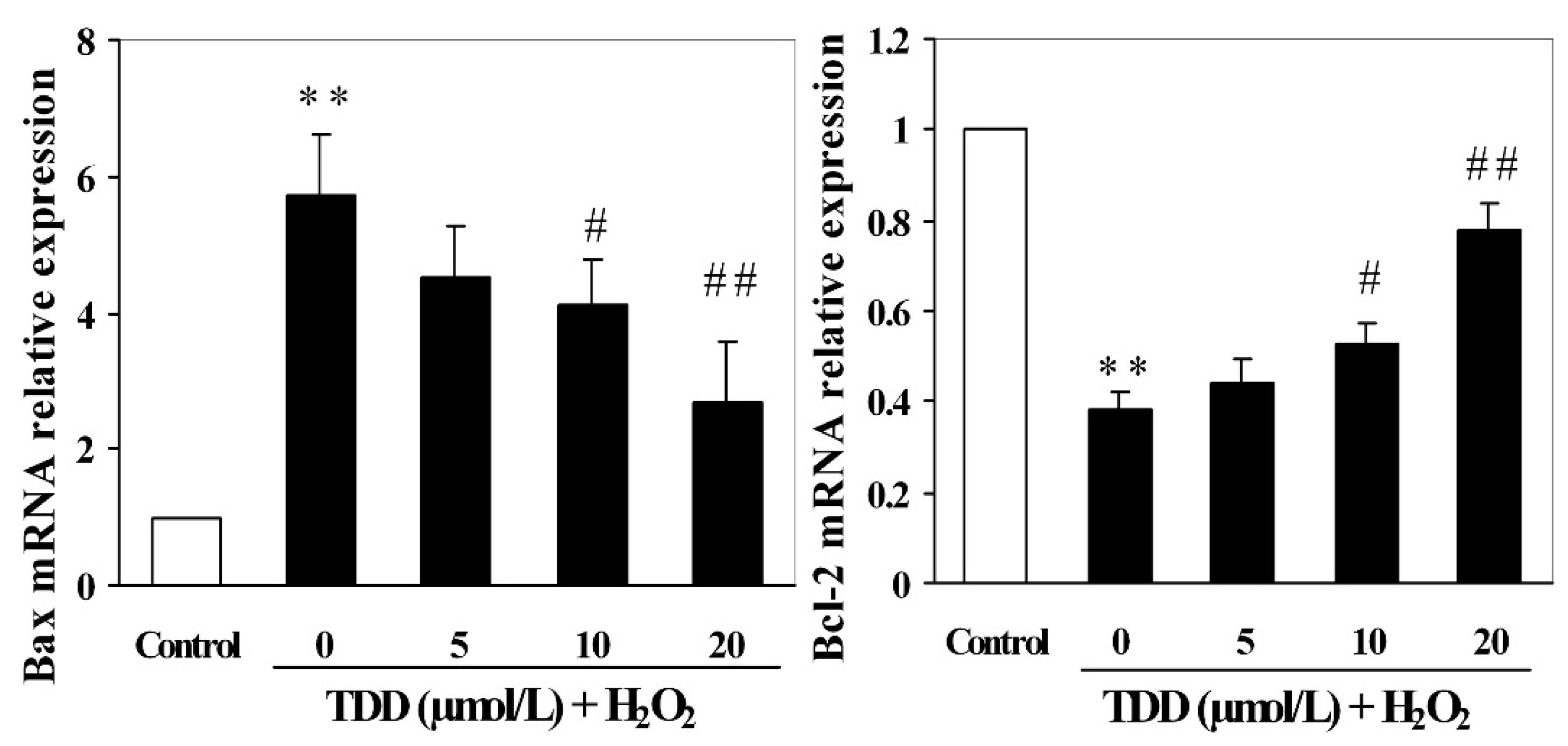
2.4. Quantitative RT-PCR Analysis
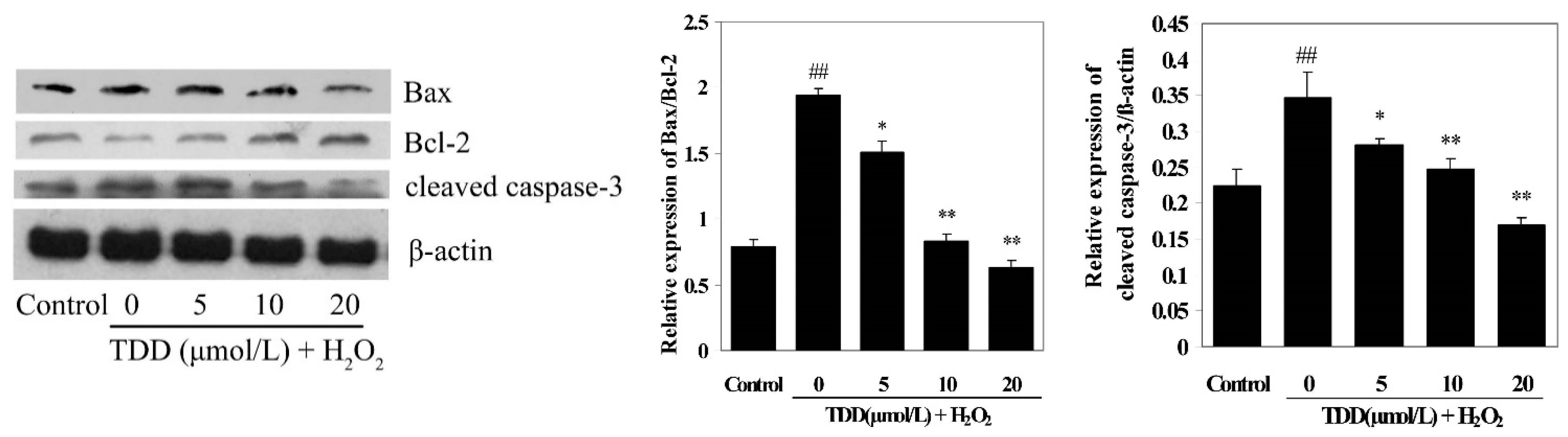
2.5. Western Blot Analysis
3. Experimental Section
3.1. Chemicals
3.2. Cell Culture and Drug Treatments
3.3. Cell Viability Assay
3.4. Preparation of Reference RNA
3.5. Microarray Analysis
3.6. Flow Cytometic Analysis of Apoptosis
3.7. RNA Reverse Transcription and Quantitative RT-PCR
3.8. Western Blot Analysis
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ryter, S.W.; Kim, H.P.; Hoetzel, A.; Park, J.W.; Nakahira, K.; Wang, X.; Choi, A.M. Mechanisms of cell death in oxidative stress. Antioxid. Redox Signal. 2007, 9, 49–89. [Google Scholar] [CrossRef] [PubMed]
- Donepudi, M.; Grütter, M.G. Structure and zymogen activation of caspases. Biophys. Chem. 2002, 101, 145–153. [Google Scholar] [CrossRef]
- Harrison, D.; Griendling, K.K.; Landmesser, U.; Hornig, B.; Drexler, H. Role of oxidative stress in atherosclerosis. Am. J. Cardiol. 2003, 91, 7–11. [Google Scholar] [CrossRef]
- Pollock, D.M.; Pollock, J.S. Endothelin and oxidative stress in the vascular system. Curr. Vasc. Pharmacol. 2005, 3, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Fortuno, A.; José, G.S.; Moreno, M.U.; Díez, J.; Zalba, G. Oxidative stress and vascular remodeling. Exp. Physiol. 2005, 90, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Harrison, D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Dhalla, N.S.; Temsah, R.M.; Netticadan, T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000, 18, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis: An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Karsan, A.; Harlan, J.M. Modulation of endothelial cell apoptosis: Mechanism and pathophysiological roles. J. Atheroscler. Thromb. 1996, 3, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, S.J.; Su, H.; Han, L.J.; Shi, D.Y. Total synthesis of bis-(2,3-dibromo-4,5-dihydroxyphenyl)-methane as potent PTP1B inhibitor. Chin. Chem. Lett. 2008, 19, 1290–1292. [Google Scholar] [CrossRef]
- Li, K.; Li, X.M.; Ji, N.Y.; Gloer, J.B.; Wang, B.G. Urceolatin, a structurally unique bromophenol from Polysiphonia urceolata. Bioorg. Med. Chem. 2007, 15, 6627–6631. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.J.; Li, X.M.; Wang, B.G. Highly brominated mono- and bis-phenols from the marine red alga Symphyocladia latiuscula with radical-scavenging activity. J. Nat. Prod. 2007, 70, 1210–1213. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Li, X.; Li, J.; Guo, S.; Su, H.; Fan, X. Antithrombotic effect of bromophenol, the alga-derived thrombin inhibitor. Chin. J. Oceanol. Limnol. 2010, 28, 96–98. [Google Scholar] [CrossRef]
- Popplewell, W.L.; Northcote, P.T. Colensolide A: A new nitrogenous bromophenol from the New Zealand marine red alga Osmundaria colensoi. Tetrahedron Lett. 2009, 50, 6814–6817. [Google Scholar] [CrossRef]
- Oh, K.B.; Lee, J.H.; Lee, J.W.; Yoon, K.M.; Chung, S.C.; Jeon, H.B.; Shin, J.; Lee, H.S. Synthesis and antimicrobial activities of halogenated bis(hydroxyphenyl)methanes. Bioorg. Med. Chem. Lett. 2009, 19, 945–948. [Google Scholar] [CrossRef] [PubMed]
- Wiemer, D.F.; Idler, D.D.; Fenical, W. Vidalols A and B, new anti-inflammatory bromophenols from the Caribbean marine alga Vidalia obtusiloba. Experientia 1991, 47, 851–853. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Okada, Y.; Shi, H.; Wang, Y.; Okuyama, T. Structures and aldose reductase inhibitory effects of bromophenols from the red alga Symphyocladia latiuscula. J. Nat. Prod. 2005, 68, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Song, F.; Wang, S.; Li, S.; Xiao, F.; Zhao, J.; Yang, Y.; Shang, S.; Yang, L.; Shi, G. Dibenzyl bromophenols with diverse dimerization patterns from the brown alga Leathesia nana. J. Nat. Prod. 2004, 67, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Kurata, K.; Taniguchii, K.; Takashima, K.; Hayashi, I.; Suzuki, M. Feeding-deterrent bromophenols from Odonthalia corymbifera. Phytochemistry 1997, 45, 485–487. [Google Scholar] [CrossRef]
- Shi, D.; Li, J.; Guo, S.; Su, H.; Fan, X. The antitumor effect of bromophenol derivatives in vitro and Leathesia nana extract in vivo. Chin. J. Oceanol. Limnol. 2009, 27, 277–282. [Google Scholar] [CrossRef]
- Feng, X.E.; Zhao, W.Y.; Ban, S.R.; Zhao, C.X.; Li, Q.S.; Lin, W.H. Structure–Activity relationship of halophenols as a new class of protein tyrosine kinase inhibitors. Int. J. Mol. Sci. 2011, 12, 6104–6115. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Y.; Feng, X.E.; Ban, S.R.; Lin, W.; Li, Q. Synthesis and biological activity of halophenols as potent antioxidant and cytoprotective agents. Bioorg. Med. Chem. Lett. 2010, 20, 4132–4134. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.L.; Ban, S.R.; Feng, X.E.; Zhao, C.X.; Lin, W.; Li, Q.S. Synthesis and in vitro protein tyrosine kinase inhibitory activity of furan-2-yl (phenyl) methanone derivatives. Molecules 2011, 16, 4897–4911. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, G.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Shaw, M.H.; Kim, Y.G.; Nuñez, G. NOD-like receptors: Role in innate immunity and inflammatory disease. Annu. Rev. Pathol. Mech. Dis. 2009, 4, 365–398. [Google Scholar] [CrossRef] [PubMed]
- Plantinga, T.S.; Fransen, J.; Knevel, R.; Netea, M.G.; Zwerina, J.; Helsen, M.M.A.; van der Meer, J.W.M.; van Riel, P.L.; Schett, G.; van der Helm-van Mil, A.H.M.; et al. Role of NOD1 polymorphism in susceptibility and clinical progression of rheumatoid arthritis. Rheumatology 2013, 52, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Dejana, E.; Corada, M.; Lampugnani, M.G. Endothelial cell-to-cell junctions. FASEB J. 1995, 9, 910–918. [Google Scholar] [PubMed]
- Youle, R.J.; Strasser, A. The Bcl-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell. Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Budihardjo, I.; Oliver, H.; Lutter, M.; Luo, X.; Wang, X.D. Biochemical pathway of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 1999, 15, 269–290. [Google Scholar] [CrossRef] [PubMed]
- Riedl, S.J.; Shi, Y.G. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 2004, 5, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of halophenols including 2,4′,5′-trihydroxyl-5,2′-dibromo diphenylmethanone (TDD) are available from professor Qingshan Li.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Feng, X.; Ge, R.; Li, J.; Li, Q. Protective Effect of 2,4′,5′-Trihydroxyl-5,2′-dibromo diphenylmethanone, a New Halophenol, against Hydrogen Peroxide-Induced EA.hy926 Cells Injury. Molecules 2015, 20, 14254-14264. https://doi.org/10.3390/molecules200814254
Li J, Feng X, Ge R, Li J, Li Q. Protective Effect of 2,4′,5′-Trihydroxyl-5,2′-dibromo diphenylmethanone, a New Halophenol, against Hydrogen Peroxide-Induced EA.hy926 Cells Injury. Molecules. 2015; 20(8):14254-14264. https://doi.org/10.3390/molecules200814254
Chicago/Turabian StyleLi, Jianguo, Xiue Feng, Rui Ge, Jiankuan Li, and Qingshan Li. 2015. "Protective Effect of 2,4′,5′-Trihydroxyl-5,2′-dibromo diphenylmethanone, a New Halophenol, against Hydrogen Peroxide-Induced EA.hy926 Cells Injury" Molecules 20, no. 8: 14254-14264. https://doi.org/10.3390/molecules200814254





