K2CO3-Mediated Synthesis of Functionalised 4-Substituted-2-amino-3-cyano-4H-chromenes via Michael-Cyclization Reactions
Abstract
:1. Introduction
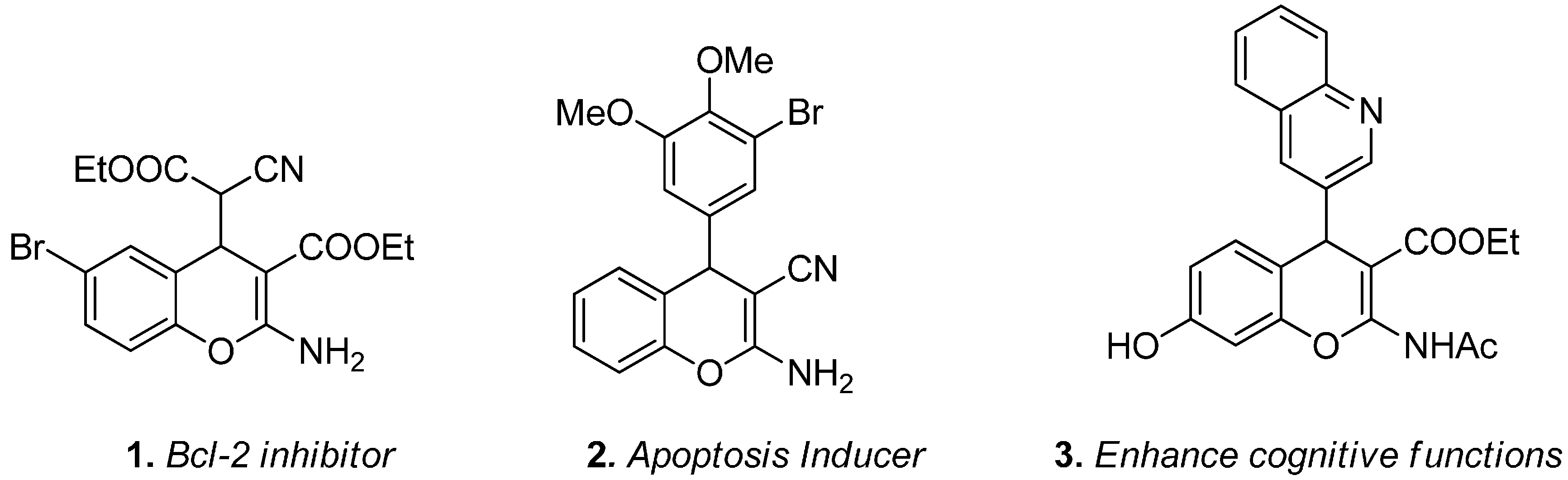
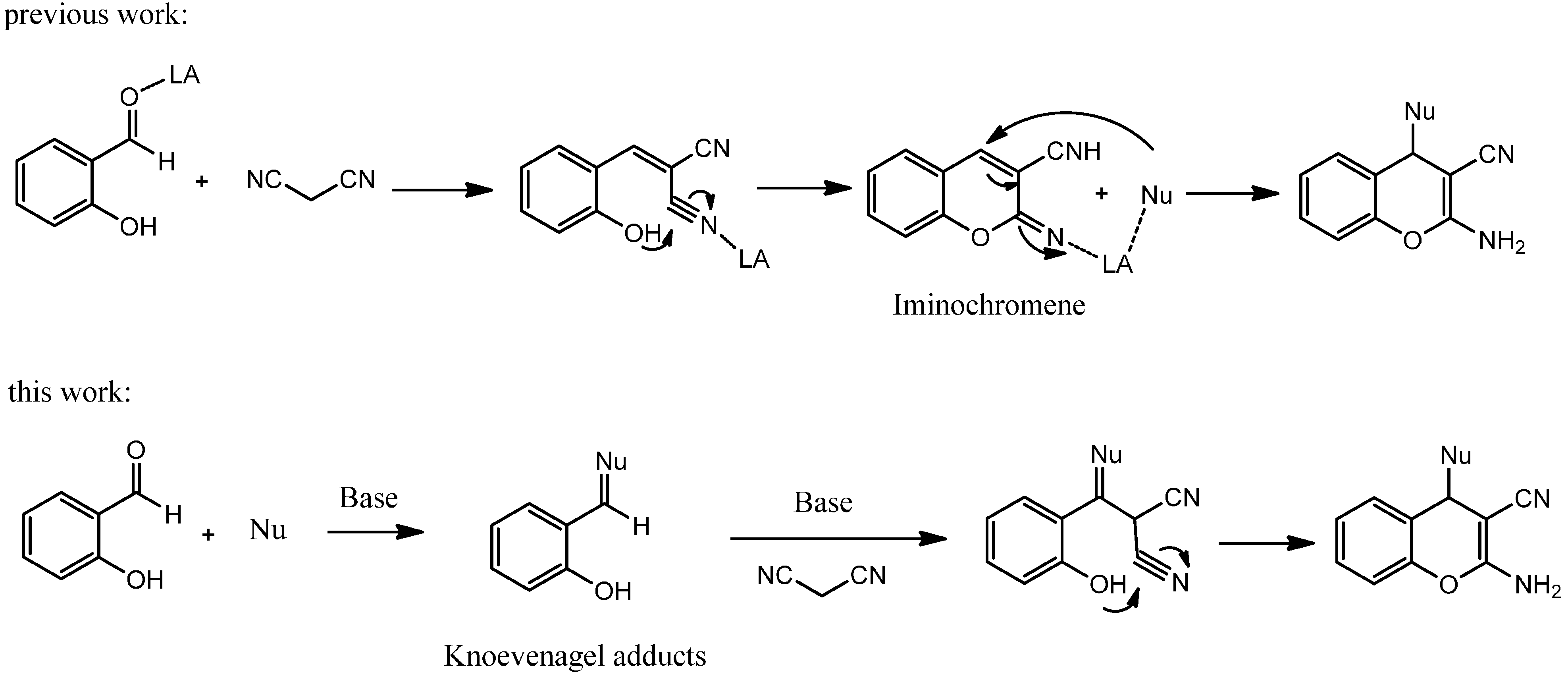
2. Results and Discussion
| Entry | Solvent | Base | Temp. | Time | Yield of 6a b,c |
|---|---|---|---|---|---|
| 1 | THF | DBU | r.t. | 2 h | 0 |
| 2 | THF | Piperidine | r.t. | 2 h | 0 |
| 3 | THF | DIPEA | r.t. | 2 h | 0 |
| 4 | THF | Et3N | r.t. | 2 h | 0 |
| 5 | THF | DMAP | r.t. | 2 h | 31% |
| 6 | THF | DABCO | r.t. | 2 h | 13% |
| 7 | THF | Na2CO3 | r.t. | 2 h | 61% |
| 8 | THF | K2CO3 | r.t. | 2 h | 95% |
| 9 | CH3OH | K2CO3 | r.t. | 2 h | 45% |
| 10 | C2H5OH | K2CO3 | r.t. | 2 h | 44% |
| 11 | CH3Cl | K2CO3 | r.t. | 2 h | 34% |
| 12 | CH2Cl2 | K2CO3 | r.t. | 2 h | 35% |
| 13 | H2O | K2CO3 | r.t. | 2 h | 0 |
| 14 | Toluene | K2CO3 | r.t. | 2 h | 40% |
| 15 | Dioxane | K2CO3 | r.t. | 2 h | 0 |
| 16 | DMF | K2CO3 | r.t. | 2 h | 0 |
| 17 | CH3CN | K2CO3 | r.t. | 2 h | 66% |
| 18 | THF | K2CO3 | 40 °C | 50 min | 95% |
| 19 | THF | K2CO3 | 60 °C | 10 min | 98% |
| 20 | CH3CN | K2CO3 | 40 °C | 35 min | 91% |
| 21 | CH3CN | K2CO3 | 60 °C | 7 min | 98% |
| Entry | R1 | R2 | R3 | R4 | Product 6 | Time | Yield b,c |
|---|---|---|---|---|---|---|---|
| 1 | H | H | H | CN |  | 10 min | 98% |
| 2 | H | 5-CH3 | H | CN |  | 8 min | 90% |
| 3 | H | 5-Cl | H | CN |  | 8 min | 95% |
| 4 | 6-Cl | H | H | CN |  | 6 min | 93% |
| 5 | H | 5-Br | H | CN |  | 6 min | 91% |
| 6 d | H | 3-F | H | CN |  | 15 h | 85% |
| 7 | H | 4-F | H | CN |  | 2 h | 83% |
| 8 | H | 5-F | H | CN |  | 10 min | 91% |
| 9 | 5-F | H | H | CN |  | 9 min | 89% |
| 10 | H | H | -ph | CN |  | 5 min | 90% |
| 11 | H | H | -CH3 | CN |  | 10 min | 87% |
| 12 e | H | H | H | -COOEt |  | 3h | 67% |
| 13 f |  |  | 70 min | 70% | |||
 |
3. Experimental Section
3.1. General Information
3.2. Experimental Procedures
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kjer, J.; Wray, V.; Edrada-Ebel, R.; Ebel, R.; Pretsch, A.; Lin, W.; Proksch, P. Xanalteric acids I and II and related phenolic compounds from an endophytic Alternaria sp. Isolated from the mangrove plant Sonneratia alba. J. Nat. Prod. 2006, 72, 2053–2057. [Google Scholar]
- Schmidt, T.J.; Khalid, S.A.; Romanha, A.J.; Alves, T.M.; Biavatti, M.W.; Brun, R.; da Costa, F.B.; de Castro, S.L.; Ferreira, V.F.; de Lacerda, M.V.; et al. The potential of secondary metabolites from plants as drugs or leads against protozoan neglected diseases-part II. Curr. Med. Chem. 2012, 19, 2176–2228. [Google Scholar]
- Raj, T.; Bhatia, R.K.; Kapur, A.; Sharma, M.; Saxena, A.K.; Ishar, M.P. CytotoXic activity of 3-(5-phenyl-3H-[1,2,4]dithiazol-3-yl)chromen-4-ones and 4-oxo-4H-chromene-3-carbothioic acid N-phenylamides. Eur. J. Med. Chem. 2010, 45, 790–794. [Google Scholar]
- Mungra, D.C.; Patel, M.P.; Rajani, D.P.; Patel, R.G. Synthesis and identification of β-aryloxyquinolines and their pyrano[3,3-c]chromene derivatives as a new class of antimicrobial and antituberculosis agents. Eur. J. Med. Chem. 2011, 46, 4192–4200. [Google Scholar]
- Conti, C.; Proietti Monaco, L.; Desideri, N. Synthesis and anti-rhinovirus activity of novel 3-[2-(pyridinyl)vinyl]substituted-2H-chromenes and 4H-chromen-4-ones. Bioorg. Med. Chem. 2014, 22, 1201–1207. [Google Scholar]
- Mori, J.; Iwashima, M.; Takeuchi, M.; Saito, H. A synthetic study on antiviral and antioxidative chromene derivative. Chem. Pharm. Bull. 2006, 54, 391–396. [Google Scholar]
- He, Y.; Chen, Y.Y.; Shi, J.B.; Tang, W.J.; Pan, Z.X.; Dong, Z.Q.; Song, B.A.; Li, J.; Liu, X.H. New coumarin derivatives: Design, synthesis and use as inhibitors of hMAO. Bioorg. Med. Chem. 2014, 22, 3732–3738. [Google Scholar]
- Charles, J.; Michael, S.V.; Gabriel, S.W.; Amir, H.H. Zr-Catalyzed Kinetic Resolution of Allylic Ethers and Mo-Catalyzed Chromene Formation in Synthesis. Enantioselective Total Synthesis of the Antihypertensive Agent (S,R,R,R)-Nebivolol. J. Am. Chem. Soc. 1998, 120, 8340–8347. [Google Scholar]
- Rapposelli, S.; da Settiomo, F.; Digiacomo, M.; la Motta, C.; Lapucci, A.; Sartini, S.; Vanni, M. Synthesis and biological evaluation of 2'-oxo-2,3-dihydro-3'H-spiro [chromene-4,5'-[1,3]oxazolidin]-3'yl]acetis acid derivatives as aldose reductase inhibitors. Arch. Pharm. (weinheim) 2011, 344, 372–385. [Google Scholar]
- Meepagala, K.M.; Schrader, K.K.; Burandt, C.L.; Wedge, D.E.; Duke, S.O. New class of algicidal compounds and fungicidal activities derived from a chromene amide of Amyris texana. J. Agric. Food Chem. 2010, 58, 9476–9482. [Google Scholar]
- Smetanina, O.F.; Yurchenko, A.N.; Afiyatullov, S.S.; Kalinovsky, A.L.; Pushilin, M.A.; Khudyakova, Y.V.; Slinkina, N.N.; Ermakova, S.P.; Yurchenko, E.A. Oxirapentyns B–D produced by a marine sediment-derived fungus Isaria felina (DC.) Fr. Phytochem. Lett. 2012, 5, 165–169. [Google Scholar]
- Kumar, A.; Sharma, S.; Maurya, R.A.; Sarkar, J. Diversity oriented synthesis of benzoxanthene and benzochromene libraries via one-pot, three-component reactions and their anti-proliferative activity. J. Comb. Chem. 2010, 12, 20–24. [Google Scholar]
- Zhang, G.; Zhang, Y.; Yan, J.; Chen, R.; Wang, S.; Ma, Y,; Wang, R. One-pot enantioselective synthesis of functionalized pyranocoumarins and 2-amino-4H-chromens: Discovery of a type of potent antibacterial agent. J. Org. Chem. 2012, 77, 878–888. [Google Scholar]
- Kemnitzer, W.; Drewe, J.; Jiang, S.; Zhang, H.; Zhao, J.; Crogan-Grundy, C.; Xu, L.; Lamothe, S.; Gourdeau, H.; Denis, R.; et al. Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell- and caspase-based high throughput screening assay. 3. Structure-activity relationships of fused rings at the 7,8-positions. J. Med. Chem. 2007, 50, 2858–2864. [Google Scholar]
- Kemnitzer, W.; Drewe, J.; Jiang, S.; Zhang, H.; Crogan-Grundy, C.; Labreque, D.; Bubenick, M.; Attardo, G.; Denis, R.; Lamothe, S.; et al. Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell- and caspase-based high throughput screening assay. 4. Structure-activity relationships of N-alkyl substituted pyrrole fused at the 7,8-positions. J. Med. Chem. 2008, 51, 417–423. [Google Scholar]
- Albiston, A.L.; DiWakarla, S.; Fernando, R.N.; Mountford, S.J.; Yeatman, H.R.; Morgan, B.; Pham, V.; Holien, J.K.; Parker, M.W.; Thompson, P.E.; et al. Identification and development of specific inhibitors for insulin-regulated aminopeptidase as a new class of cognitive enhancers. Br. J. Pharmacol. 2011, 164, 37–47. [Google Scholar]
- Gao, S.J.; Tsai, C.H.; Tseng, C.; Yao, C.F. Fluoride ion catalyzed multicomponent reactions for efficient synthesis of 4H-chromene and N-arylquinoline derivatives in aqueous media. Tetrahedron 2008, 64, 9143–9149. [Google Scholar]
- Kandhasamy, K.; Gnanasambandam, V. Four-component catalyst-free reaction in water: Combinatorial library synthesis of novel 2-amino-4-(5-hydroxy-3-methyl-1H-pyazol-4-yl)-4H-chromene-3-carbonitrile derivatives. Green Chem. 2009, 11, 1945–1947. [Google Scholar]
- Shaabani, A.; Ghadari, R.; Sarvary, A.; Rezavan, A.H. Synthesis of highly functionalized bis(4H-benzo[g]chromene dervatives via an isocyanide-based pseudo-five-component reaction. J. Org. Chem. 2009, 74, 4372–4374. [Google Scholar]
- Thelagathoti, H.B.; Paramasivan, T. A simple Method for the Synthesis of Functionalised Chromenes via Vinylogous Michael Addition of α,α-Dicyanoalkenes on iminocoumarin Derivatives. Synlett 2011, 341–344. [Google Scholar]
- Neelakandan, V.L.; Selvarangam, E.; Kiruthika, T.; Paramasivan, T. A Rapid and Efficient Access to 4-Substituted 2-Amino-4H-chromenes Catalyzed by InCl3. Synlett 2011, 1389–1394. [Google Scholar]
- Chen, W.; Cai, Y.; Fu, X.; Liu, X.; Lin, X.; Feng, X. Enantioselective one-pot synthesis of 2-amino-4-(indol-3-yl)-4H-chromenes. Org. Lett. 2011, 13, 4910–4913. [Google Scholar]
- Dong, A.H.; Liu, X.H.; Feng, J.H.; Wang, M.; Lin, L.L.; Feng, X.M. Efficient Asymmetric Synthesis of 4H-Chromene Derivatives through a Tandem Michael Addition-Cyclization Reaction Catalyzed by a Salen-Cobalt(II)complex. Eur. J. Org. Chem. 2011, 2011, 137–142. [Google Scholar]
- Elinson, M.N.; Iiovaisky, A.I.; Merkulova, V.M.; Belyakov, P.A.; Chizhov, A.O.; Nikishin, G.I. Solvent-Free Cascade Reaction: Diect Multicomponent Assembling of 2-amino-4H-chromene Scaffold from Salicyaldehyde, Malononitrile or Cyanoacetate and Nitroalkanes. Tetrahedron 2010, 66, 4043–4048. [Google Scholar]
- Naimi-Jamal, M.R.; Mashkouri, S.; Sharifi, A. An efficient, multicomponet approach for solvent-free synthesis of 2-amino-4H-chromene scaffold. Mol. Divers. 2010, 14, 473–477. [Google Scholar]
- Kulakarni, M.A.; Pandurangi, V.R.; Desai, U.V.; Wadgaonkar, P.P. A practical and highly efficient protocol for multicomponent synthesis of β-phosphonomalononitriles and 2-amino-4H- chromen-4-ylphosphonates using diethylamine as a novel organocatalyst. C. R. Chim. 2012, 15, 745–752. [Google Scholar]
- Kolla, S.R.; Lee, Y.R. Efficient one-pot synthesis of β-phosphono malonates and 2-amino-4H- chromen-4-ylphosphonate derivatives by ethylenediamine diacetate-catalyzed three-component reactions. Tetrahedron 2012, 68, 226–237. [Google Scholar]
- Rajasekhar, M.; Rao, K.U.M.; Sundar, C.S.; Reddy, N.B.; Nayak, S.K.; Reddy, C.S. Green Synthesis and Bioactivity of 2-Amino-4H-chromen-4-yl-phosphonates. Chem. Pharm. Bull. 2012, 60, 854–858. [Google Scholar]
- Das, B.; Balasubramnyam, P.; Reddy, G.C.; Salvanna, N. Simple, Efficient, and Catalyst-Free Synthesis of (2-Amino-4H-1-benzopyran-4-yl)phosphonates in Polyethylene Glycol. Helv. Chim. Acta 2011, 94, 1347–1350. [Google Scholar]
- Murthy, S.N.; Madhav, B.; Reddy, V.P.; Nageswar, Y.V.D. One-pot synthesis of 2-amino-4H-chromen-4-yl phosphonate derivatives using β-cyclodextrin as reusable catalyst in water. Tetrahedron Lett. 2010, 51, 3649–3653. [Google Scholar]
- Jayashree, P.; Shanthi, G.; Perumal, P.T. Indium Trichloride Catalyzed One-Pot Synthesis of New (2-Amino-3-cyano-4H-chromen-4-yl) Phosphonic Acid Diethyl Ester. Synlett 2009, 6, 917–920. [Google Scholar]
- Kalla, R.M.N.; Byeon, S.J.; Heo, M.S.; Kim, I. Synthesis of 2-amino-3-cyano-4H-chromen-4-ylphosphontes and 2-amino-4H-chromenes catalyzed by tetramethylguanidine. Tetrahedron 2013, 69, 10544–10551. [Google Scholar]
- Safari, J.; Zarnegar, Z. Ultrasonic activated efficient synthesis of chromenes using amino-silane modified Fe3O4 nanoparticles: A versatile integration of high catalytic activity and facile recovery. J. Mol. Struct. 2014, 1072, 53–60. [Google Scholar]
- Sobhani, S.; Honarmand, M. Silica-Bonded 2-Hydroxyethylammonium Acetate as an Efficient and Recyclable Catalyst for the Synthesis of 2-Amino-4H-chromen-4-yl Phosphonates and β-Phosphonomalonates. Catal. Lett. 2013, 143, 476–485. [Google Scholar]
- Gogoi, S.; Zhao, C.G. Organocatalyzed enantioselective synthesis of 6-amino-5-cyanodihydropyrano[2,3-c]pyrazoles. Treahedron Lett. 2009, 50, 2252–2255. [Google Scholar]
- Ding, D.; Zhao, C.G. Organocatalyzed synthesis of 2-amino-8-oxo-5,6,7,8-tretrahydro-4H-chromene-3-carbonitriles. Treahedron Lett. 2010, 51, 1322–1325. [Google Scholar]
- Wang, X.S.; Yang, G.S.; Zhao, G. Enantioselective synthesis of naphthopyran derivatives catalyzed by bifunctional thiourea-tertiary amines. Tetrahedron 2008, 19, 709–714. [Google Scholar]
- Li, W.J.; Huang, J.Y.; Wang, J. Organocatalytic conjugate addition promoted by multi-hydrogen-bond cooperation: Access to chiral 2-amino-3-nitrile-chromenes. Org. Biomol. Chem. 2013, 11, 400–406. [Google Scholar]
- Gao, Y.G.; Du, D.M. Facile synthesis of chiral 2-amino-4-(indol-3-yl)-4H-chromene derivatives using thiourea as the catalyst. Tetrahedron 2013, 24, 1312–1317. [Google Scholar]
- Rao, H.S.P.; Rao, A.V.B. Copper-Catalyzed C(sp3)-C(sp2) Cross-Coupling: Synthesis of 4-Aryl-2-alkylamino-3-nitro-4H-chromenes. Eur. Org. Chem. 2014, 17, 3646–3655. [Google Scholar]
- Kidwai, M.; Saxena, S.; Khan, M.K.R.; Thukral, S.S. Aqua mediated synthesis of substituted 2-amino-4H-chromenes and in vitro study as antibacterial agents. Bioorg. Med. Chem. Lett. 2005, 15, 4295–4298. [Google Scholar]
- Shanthi, G.; Perumal, P.T. Indium-Mediated One-Pot Synthesis of New 4-Allyl-2-amino-4H-chromenes in Water. Synlett 2008, 2791–2794. [Google Scholar]
- Yin, G.D.; Lai, T.T.; Yan, Z.S.; Chen, H.; Zheng, J.; Tao, Q. Catalyst-free C-S/C-O bond formation: Synthesis of novel 4-thio-substituted 2-aryl-4H-chromenes from easily available 2-hydroxychalcones. Tetrahedron 2013, 69, 2430–2435. [Google Scholar]
- Massimo, C.; Francesco, E.; Stefano, C.; Francesca, M.; Morena, N.; Ornelio, R. Potassium exchanged layered zirconium phosphate as catalyst in the preparation of 4H-chromenes. Tetrahedron Lett. 2005, 46, 3497–3499. [Google Scholar]
- Yu, N.F.; Aramini, J.M.; Germann, M.W.; Huang, Z.W. Reactions of salicylaldehydes with alkyl cyanoacetates on the surface of solid catalysts: Syntheses of 4H-chromene derivatives. Tetrahedron Lett. 2000, 41, 6993–6996. [Google Scholar]
- Fujimoto, A.; Sakurai, A. A New Selective Preparation of 4H-Chromenes by Reaction of alkyl Cyanoacetate with 3,5-Dibromosalicylaldehyde in the Presence of Ammonium Acetate. Synthesis 1977, 12, 872–872. [Google Scholar]
- Roudier, J.F.; Foucaud, A. A Convenient Synthesis of 4H-Chromenes. Synthesis 1984, 1984, 159–160. [Google Scholar]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Hu, R.; Tong, R.; Li, F.; Shi, J.; Zhang, M. K2CO3-Mediated Synthesis of Functionalised 4-Substituted-2-amino-3-cyano-4H-chromenes via Michael-Cyclization Reactions. Molecules 2014, 19, 19253-19268. https://doi.org/10.3390/molecules191219253
He Y, Hu R, Tong R, Li F, Shi J, Zhang M. K2CO3-Mediated Synthesis of Functionalised 4-Substituted-2-amino-3-cyano-4H-chromenes via Michael-Cyclization Reactions. Molecules. 2014; 19(12):19253-19268. https://doi.org/10.3390/molecules191219253
Chicago/Turabian StyleHe, Yanyang, Rong Hu, Rongsheng Tong, Fengqiong Li, Jianyou Shi, and Mei Zhang. 2014. "K2CO3-Mediated Synthesis of Functionalised 4-Substituted-2-amino-3-cyano-4H-chromenes via Michael-Cyclization Reactions" Molecules 19, no. 12: 19253-19268. https://doi.org/10.3390/molecules191219253







