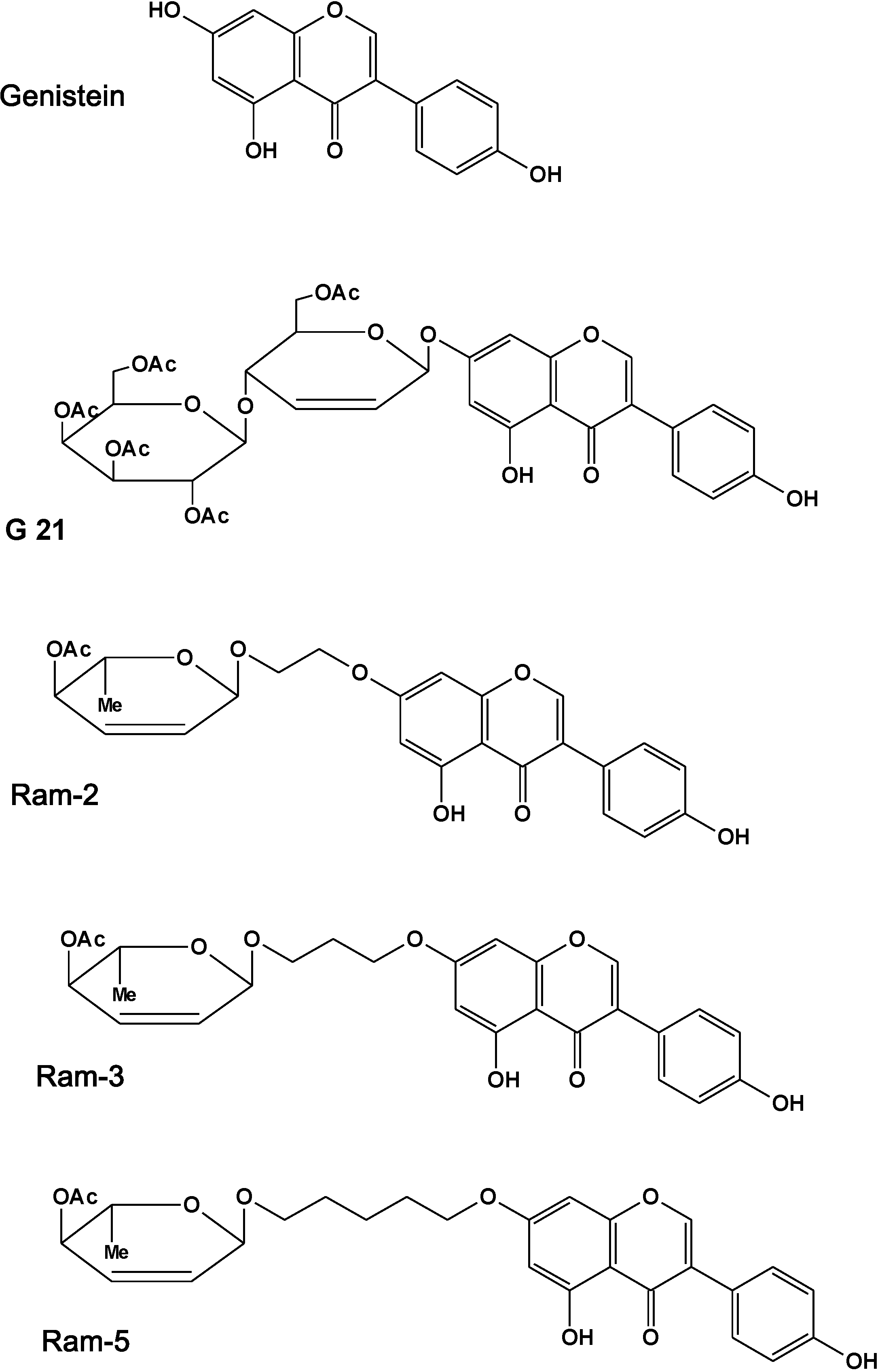
Synthetic Genistein Glycosides Inhibiting EGFR Phosphorylation Enhance the Effect of Radiation in HCT 116 Colon Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
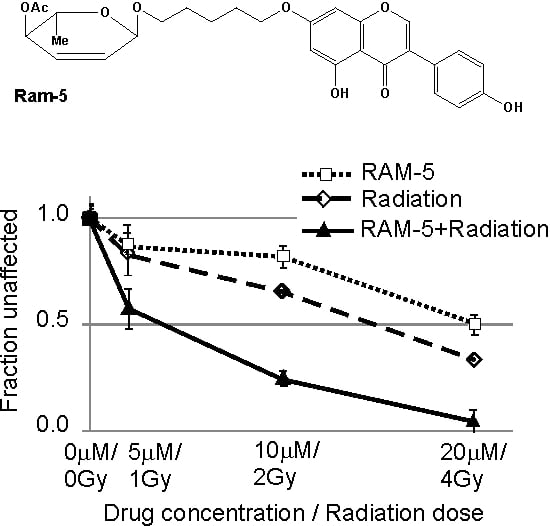
2.1. Inhibition of Clonogenic Cell Survival
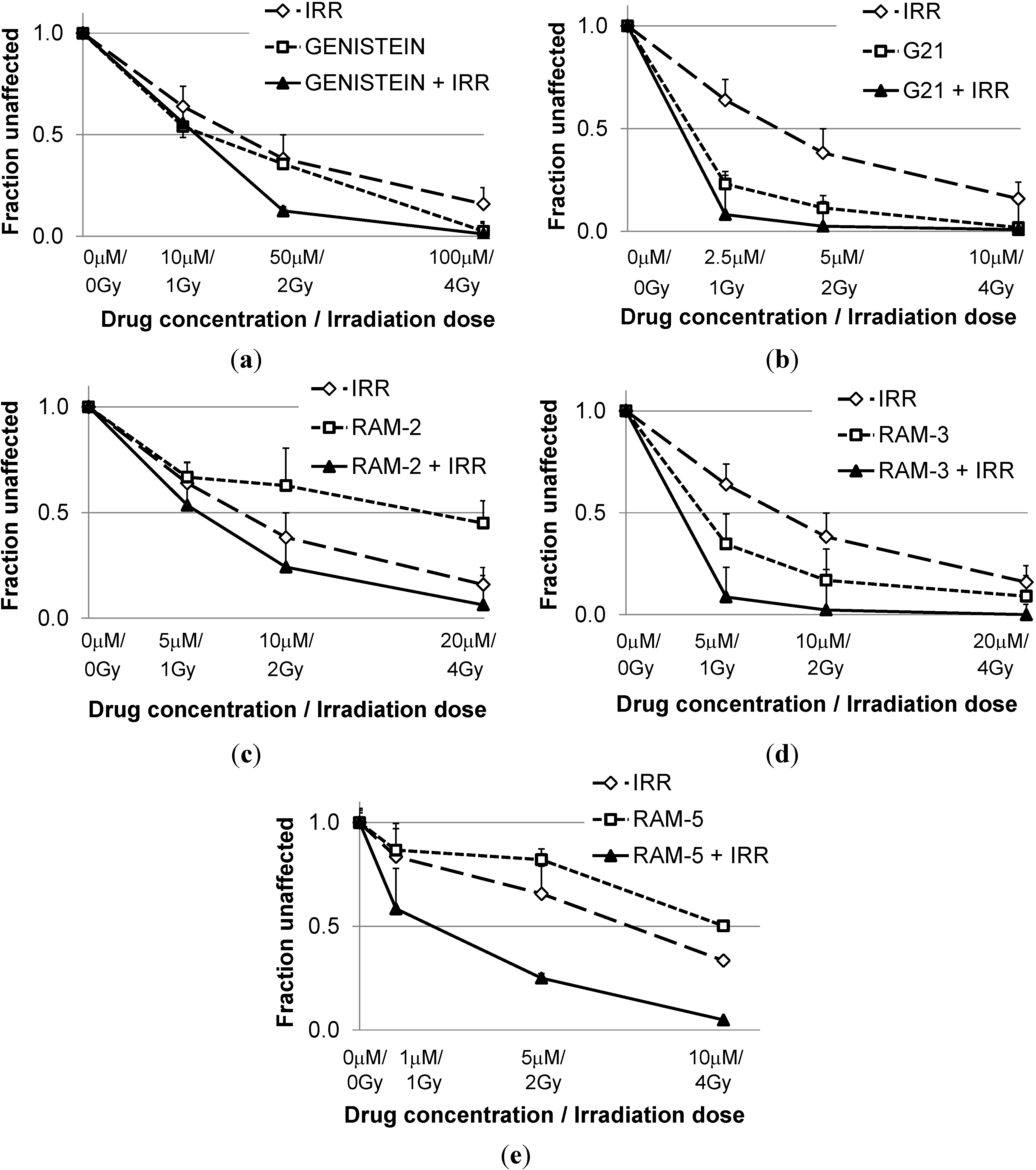
| CI * Values Calculated for Different Effects | |||
|---|---|---|---|
| ED50 | ED75 | ED90 | |
| Genist/Irr | 1.75 ± 0.25 | 1.36 ± 0.12 | 1.10 ± 0.19 |
| G21/Irr | 1.01 ± 0.54 | 0.86 ± 0.44 | 0.77 ± 0.35 |
| Ram-2/Irr | 1.14 ± 0.05 | 0.7±0.01 | 0.54±0.05 |
| Ram-3/Irr | 1.22 ± 0.16 | 0.72 ± 0.15 | 0.43 ± 0.12 |
| Ram-5/Irr | 0.63 ± 0.11 | 0.35 ± 0.08 | 0.21 ± 0.09 |
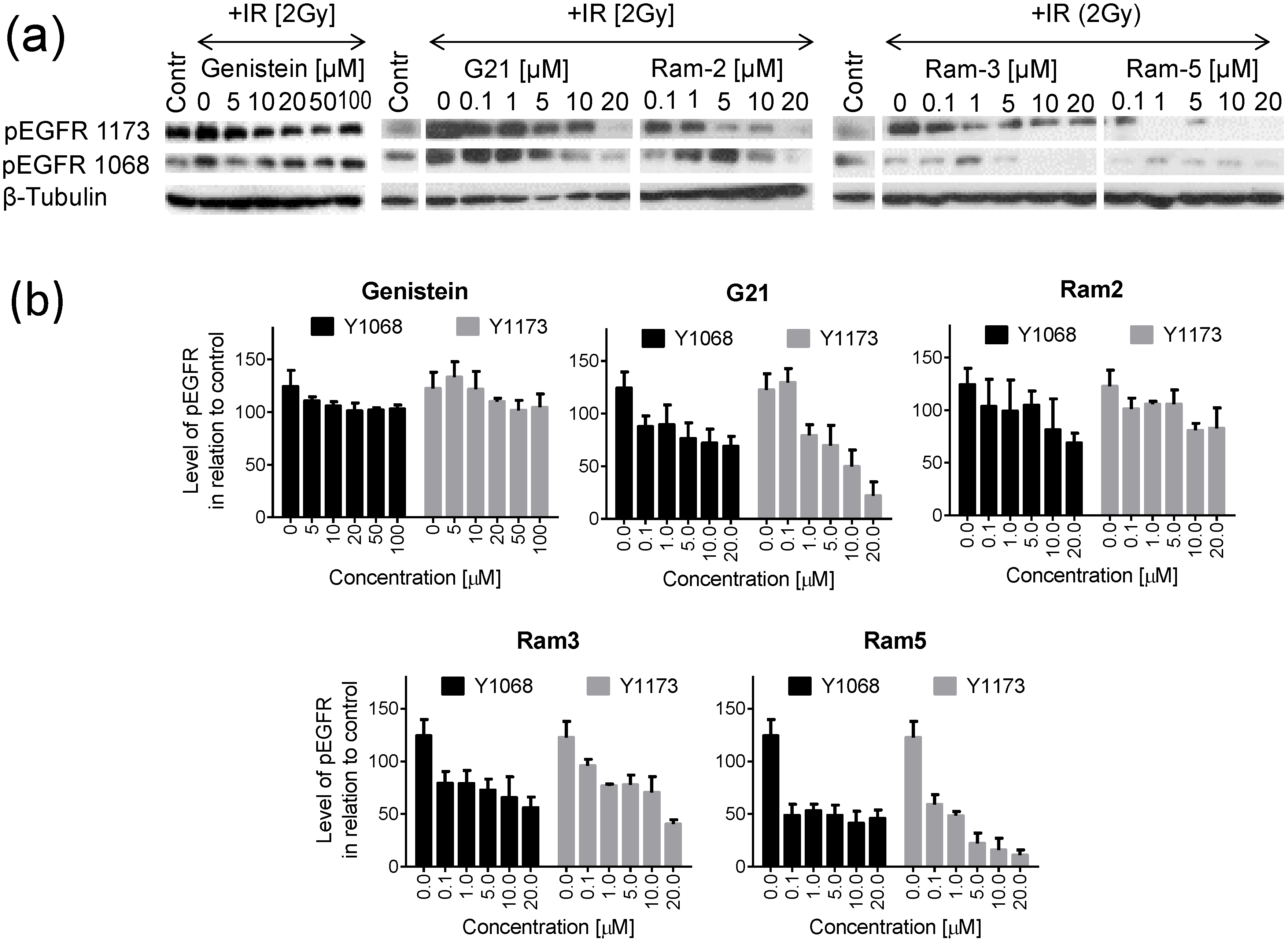
2.2. Inhibition of EGFR Phosphorylation in Cancer Cells Treated with Genistein Derivatives
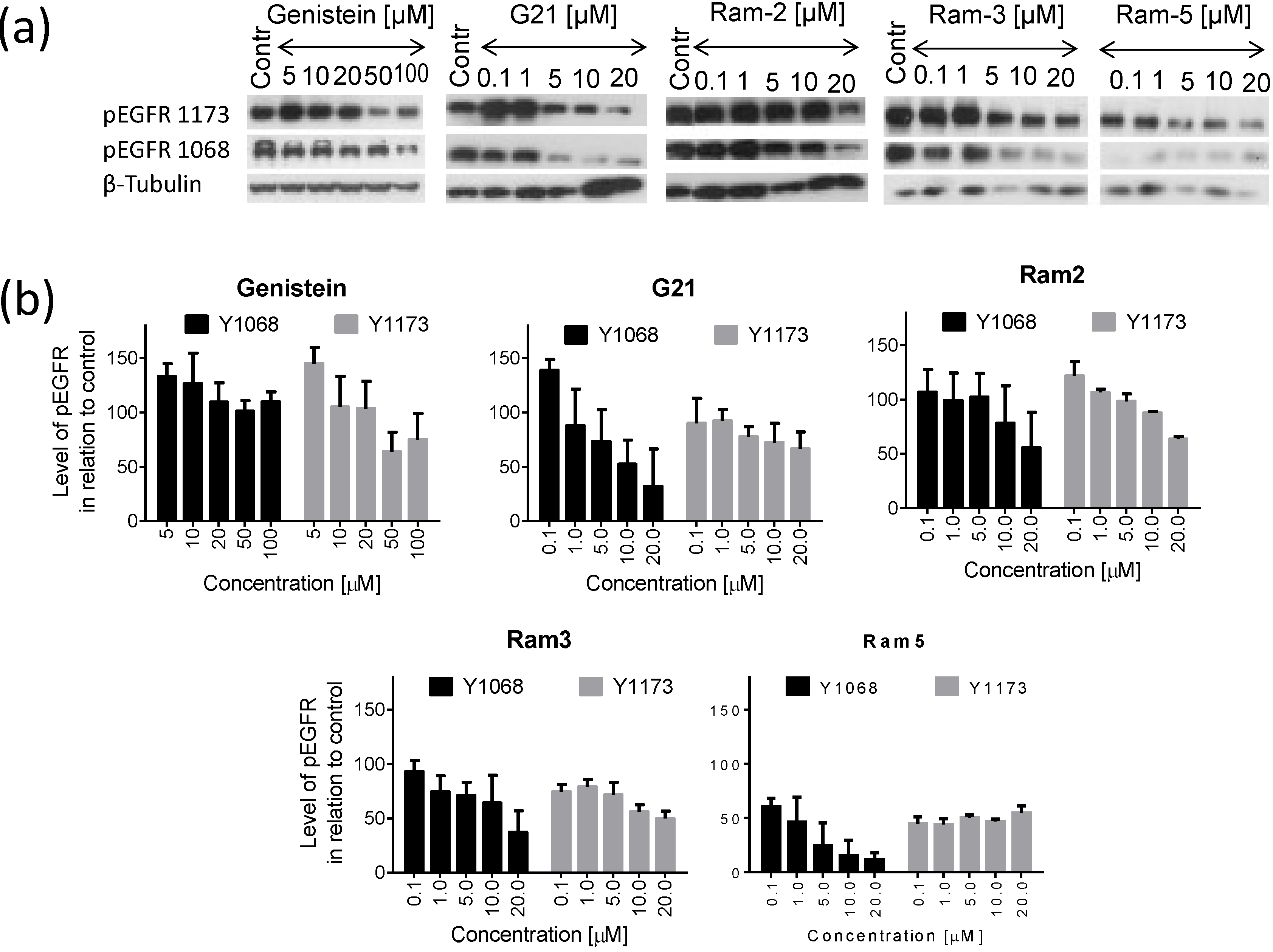
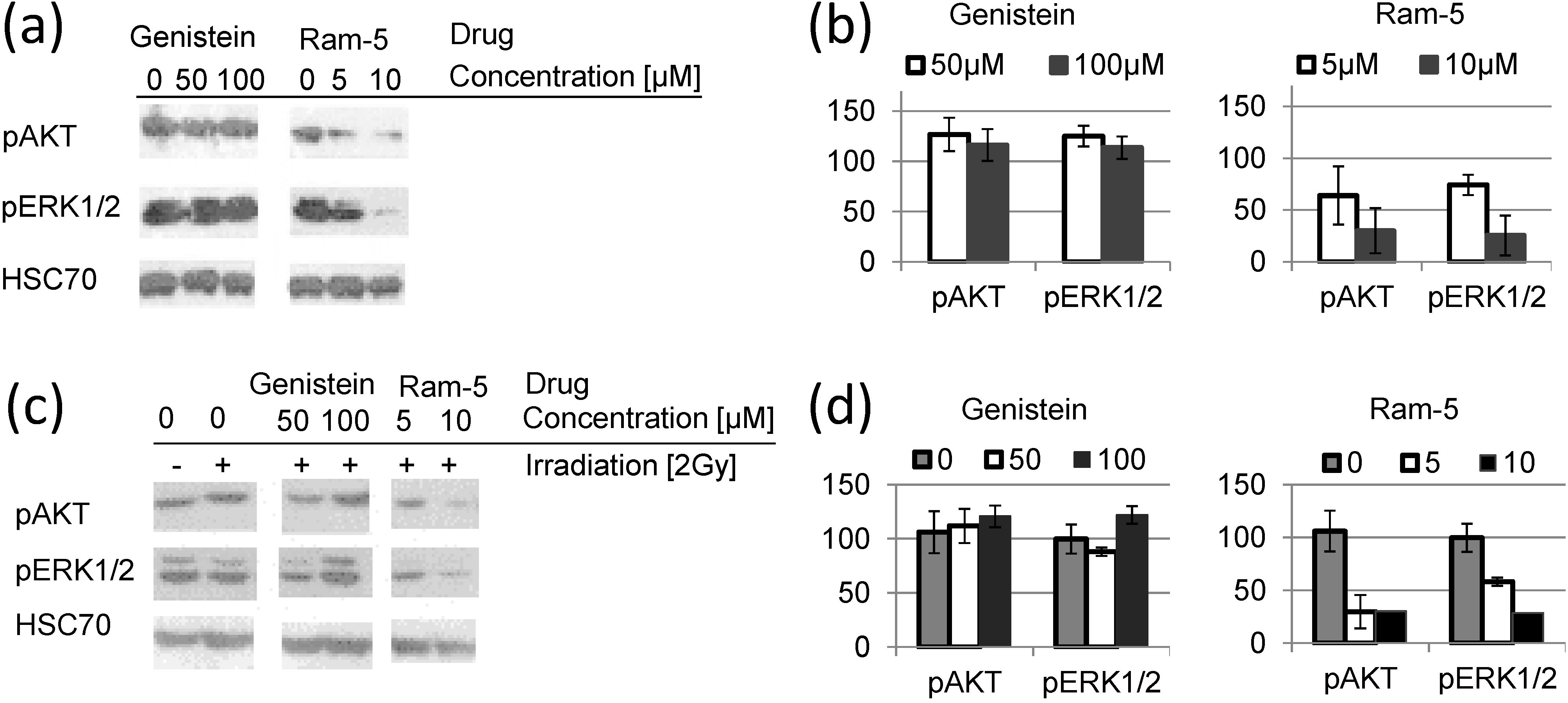
2.3. Inhibition of EGFR Phosphorylation in Cancer Cells Treated with Genistein Derivatives and Ionizing Radiation
3. Experimental Section
3.1. Chemicals
3.2. Cell Lines and Culture Conditions
3.3. Clonogenic Assay
3.4. Irradiation of Cells
3.5. CI Calculation
3.6. Western Blotting
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ono, M.; Kuwano, M. Molecular mechanisms of epidermal growth factor receptor (EGFR) activation and response to gefitinib and other EGFR-targeting drugs. Clin. Cancer Res. 2006, 12, 7242–7251. [Google Scholar] [CrossRef] [PubMed]
- Thariat, J.; Milas, L.; Ang, K.K. Integrating radiotherapy with epidermal growth factor receptor antagonists and other molecular therapeutics for the treatment of head and neck cancer. Int. J. Rad. Oncol. Biol. Phys. 2007, 69, 974–984. [Google Scholar] [CrossRef]
- Nicholson, R.I.; Gee, J.M.; Harper, M.E. EGFR and cancer prognosis. Eur. J. Cancer 2001, 37 (Suppl. 4), S9–S15. [Google Scholar] [CrossRef]
- Baiocchi, G.; Lopes, A.; Coudry, R.A.; Rossi, B.M.; Soares, F.A.; Aguiar, S.; Guimaraes, G.C.; Ferreira, F.O.; Nakagawa, W.T. ErbB family immunohistochemical expression in colorectal cancer patients with higher risk of recurrence after radical surgery. Int. J. Colorectal Dis. 2009, 24, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Gravalos, C.; Jimeno, A. HER2 in gastric cancer: A new prognostic factor and a novel therapeutic target. Ann. Oncol. 2008, 19, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Cappuzzo, F.; Ciuleanu, T.; Stelmakh, L.; Cicenas, S.; Szczesna, A.; Juhasz, E.; Esteban, E.; Molinier, O.; Brugger, W.; Melezinek, I.; et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: A multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010, 11, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Sun, Y.; Eberhardt, W.E.; Germonpre, P.; Saijo, N.; Zhou, C.; Wang, J.; Li, L.; Kabbinavar, F.; Ichinose, Y.; et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): A double-blind, randomised, phase 3 trial. Lancet Oncol. 2010, 11, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Gurtner, K.; Deuse, Y.; Baumann, M. Heterogeneity of tumour response to combined radiotherapy and EGFR inhibitors: differences between antibodies and TK inhibitors. Int. J. Radiat. Biol. 2009, 85, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Ishida, J.; Nakagawa, S.; Ogawara, H.; Watanabe, S.; Itoh, N.; Shibuya, M.; Fukami, Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987, 262, 5592–5595. [Google Scholar] [PubMed]
- Yan, G.R.; Xiao, C.L.; He, G.W.; Yin, X.F.; Chen, N.P.; Cao, Y.; He, Q.Y. Global phosphoproteomic effects of natural tyrosine kinase inhibitor, genistein, on signaling pathways. Proteomics 2010, 10, 976–986. [Google Scholar] [PubMed]
- Park, S.J.; Kim, M.J.; Kim, Y.K.; Kim, S.M.; Park, J.Y.; Myoung, H. Combined cetuximab and genistein treatment shows additive anti-cancer effect on oral squamous cell carcinoma. Cancer Lett. 2010, 292, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Cheng, H.; Ren, Y.; Liu, Z.G.; Zhang, Y.F.; Luo, B.D. Synergistic inhibitory effects by the combination of gefitinib and genistein on NSCLC with acquired drug-resistance in vitro and in vivo. Mol. Biol. Rep. 2012, 39, 4971–4979. [Google Scholar] [CrossRef] [PubMed]
- Gadgeel, S.M.; Ali, S.; Philip, P.A.; Wozniak, A.; Sarkar, F.H. Genistein enhances the effect of epidermal growth factor receptor tyrosine kinase inhibitors and inhibits nuclear factor kappa B in nonsmall cell lung cancer cell lines. Cancer 2009, 115, 2165–2176. [Google Scholar] [CrossRef] [PubMed]
- Hillman, G.G.; Forman, J.D.; Kucuk, O.; Yudelev, M.; Maughan, R.L.; Rubio, J.; Layer, A.; Tekyi-Mensah, S.; Abrams, J.; Sarkar, F.H. Genistein potentiates the radiation effect on prostate carcinoma cells. Clin. Cancer Res. 2001, 7, 382–390. [Google Scholar] [PubMed]
- Raffoul, J.J.; Wang, Y.; Kucuk, O.; Forman, J.D.; Sarkar, F.H.; Hillman, G.G. Genistein inhibits radiation-induced activation of NF-kappaB in prostate cancer cells promoting apoptosis and G2/M cell cycle arrest. BMC Cancer 2006, 6. [Google Scholar] [CrossRef] [PubMed]
- Raffoul, J.J.; Banerjee, S.; Che, M.; Knoll, Z.E.; Doerge, D.R.; Abrams, J.; Kucuk, O.; Sarkar, F.H.; Hillman, G.G. Soy isoflavones enhance radiotherapy in a metastatic prostate cancer model. Int. J. Cancer 2007, 120, 2491–2498. [Google Scholar] [CrossRef] [PubMed]
- Yashar, C.M.; Spanos, W.J.; Taylor, D.D.; Gercel-Taylor, C. Potentiation of the radiation effect with genistein in cervical cancer cells. Gynecol. Oncol. 2005, 99, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Para, A.E.; Bezjak, A.; Yeung, I.W.; van Dyk, J.; Hill, R.P. Effects of genistein following fractionated lung irradiation in mice. Radiother. Oncol. 2009, 92, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Day, R.M.; Barshishat-Kupper, M.; Mog, S.R.; Mccart, E.A.; Prasanna, P.G.; Davis, T.A.; Landauer, M.R. Genistein protects against biomarkers of delayed lung sequelae in mice surviving high-dose total body irradiation. J. Radiat. Res. 2008, 49, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Grace, M.B.; Parekh, V.I.; Whitnall, M.H.; Landauer, M.R. Effects of genistein administration on cytokine induction in whole-body gamma irradiated mice. Int. Immunopharmacol. 2009, 9, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Booth, C.; Hargreaves, D.F.; Hadfield, J.A.; Mcgown, A.T.; Potten, C.S. Isoflavones inhibit intestinal epithelial cell proliferation and induce apoptosis in vitro. Br. J. Cancer 1999, 80, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Gargala, G.; Baishanbo, A.; Favennec, L.; Francois, A.; Ballet, J.J.; Rossignol, J.F. Inhibitory activities of epidermal growth factor receptor tyrosine kinase-targeted dihydroxyisoflavone and trihydroxydeoxybenzoin derivatives on Sarcocystis neurona, Neospora caninum, and Cryptosporidium parvum development. Antimicrob. Agents Chemother. 2005, 49, 4628–4634. [Google Scholar] [CrossRef] [PubMed]
- Rusin, A.; Krawczyk, Z.; Grynkiewicz, G.; Gogler, A.; Zawisza-Puchalka, J.; Szeja, W. Synthetic derivatives of genistein, their properties and possible applications. Acta Biochem. Pol. 2010, 57, 23–34. [Google Scholar]
- Rusin, A.; Gogler, A.; Glowala-Kosinska, M.; Bochenek, D.; Gruca, A.; Grynkiewicz, G.; Zawisza, J.; Szeja, W.; Krawczyk, Z. Unsaturated genistein disaccharide glycoside as a novel agent affecting microtubules. Bioorg. Med. Chem. Lett. 2009, 19, 4939–4943. [Google Scholar] [CrossRef] [PubMed]
- Rusin, A.; Zawisza-Puchalka, J.; Kujawa, K.; Gogler-Piglowska, A.; Wietrzyk, J.; Switalska, M.; Glowala-Kosinska, M.; Gruca, A.; Szeja, W.; Krawczyk, Z.; et al. Synthetic conjugates of genistein affecting proliferation and mitosis of cancer cells. Bioorg. Med. Chem. 2011, 19, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.A.; Goldsmith, J.; Fokt, I.; Le, X.F.; Krzysko, K.A.; Lesyng, B.; Bast, R.C.; Priebe, W. A genistein derivative, ITB-301, induces microtubule depolymerization and mitotic arrest in multidrug-resistant ovarian cancer. Cancer Chemother. Pharm. 2011, 68, 1033–1044. [Google Scholar] [CrossRef]
- Gogler-Piglowska, A.; Rusin, A.; Bochenek, D.; Krawczyk, Z. Aneugenic effects of the genistein glycosidic derivative substituted at C7 with the unsaturated disaccharide. Cell Biol. Toxicol. 2012, 28, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Rusin, A.; Chrubasik, M.; Papaj, K.; Grynkiewicz, G.; Szeja, W. C-Glycosidic genistein conjugates and their antiproliferative activity. J. Chem. 2013, 2013. [Google Scholar] [CrossRef]
- Sawhney, R.S.; Zhou, G.H.K.; Humphrey, L.E.; Ghosh, P.; Kreisberg, J.I.; Brattain, M.G. Differences in sensitivity of biological functions mediated by epidermal growth factor receptor activation with respect to endogenous and exogenous ligands. J. Biol. Chem. 2002, 277, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, T.; Nonaka, T.; Ishikawa, H.; Sakurai, H.; Saitoh, J.; Takahashi, T.; Mitsuhashi, N. Genistein, a tyrosine kinase inhibitor, enhanced radiosensitivity in human esophageal cancer cell lines in vitro: Possible involvement of inhibition of survival signal transduction pathways. Int. J. Radiat. Oncol. 2001, 50, 195–201. [Google Scholar] [CrossRef]
- Toillon, R.A.; Magne, N.; Laios, I.; Lacroix, M.; Duvillier, H.; Lagneaux, L.; Devriendt, D.; van Houtte, P.; Leclercq, G. Interaction between estrogen receptor alpha, ionizing radiation and (anti-) estrogens in breast cancer cells. Breast Cancer Res. Treat. 2005, 93, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Merajver Sd, R.D.; van Wassenhove, L. PKC and breast cancer. In Protein Kinase C in Cancer Signaling and Therapy, 1st ed.; Springer: New York, NY, USA, 2010; p. 494. [Google Scholar]
- Donnem, T.; Al-Shibli, K.; Al-Saad, S.; Busund, L.T.; Bremnes, R.M. Prognostic impact of fibroblast growth factor 2 in non-small cell lung cancer coexpression with VEGFR-3 and PDGF-B predicts poor survival. J. Thorac. Oncol. 2009, 4, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Jakobkiewicz-Banecka, J.; Piotrowska, E.; Narajczyk, M.; Baranska, S.; Wegrzyn, G. Genistein-mediated inhibition of glycosaminoglycan synthesis, which corrects storage in cells of patients suffering from mucopolysaccharidoses, acts by influencing an epidermal growth factor-dependent pathway. J. Biomed. Sci. 2009, 2, 16–26. [Google Scholar]
- Nikolovska-Coleska, Z.; Suturkova, L.; Dorevski, K.; Krbavcic, A.; Solmajer, T. Quantitative structure-activity relationship of flavonoid inhibitors of p56lck protein tyrosine kinase: A classical/quantum chemical approach. Quant. Struct.-Act. Relat. 1998, 17, 7–13. [Google Scholar] [CrossRef]
- Oblak, M.; Randic, M.; Solmajer, T. Quantitative structure-activity relationship of flavonoid analogues. 3. Inhibition of p56lck protein tyrosine kinase. J. Chem. Inf. Comput. Sci. 2000, 40, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Ogawara, H.; Akiyama, T.; Watanabe, S.; Ito, N.; Kobori, M.; Seoda, Y. Inhibition of tyrosine protein-kinase activity by synthetic isoflavones and flavones. J. Antibiot. 1989, 42, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Batzer, A.G.; Rotin, D.; Urena, J.M.; Skolnik, E.Y.; Schlessinger, J. Hierarchy of binding-sites for Grb2 and Shc on the epidermal growth-factor receptor. Mol. Cell. Biol. 1994, 14, 5192–5201. [Google Scholar] [PubMed]
- Rojas, M.; Yao, S.Y.; Lin, Y.Z. Controlling epidermal growth factor (EGF)-stimulated ras activation in intact cells by a cell-permeable peptide mimicking phosphorylated EGF receptor. J. Biol. Chem. 1996, 271, 27456–27461. [Google Scholar] [CrossRef] [PubMed]
- Blenis, J. Signal-transduction via the MAP kinases: Proceed at your own Rsk. Proc. Natl. Acad. Sci. USA 1993, 90, 5889–5892. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, K.S. Signaling via Shc family adapter proteins. Oncogene 2001, 20, 6322–6330. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, A.; Miller, A.; Caron, R.W.; Qiao, L.; Curiel, D.A.; Fisher, P.B.; Hagan, M.P.; Grant, S.; Dent, P. Radiotherapy-induced signal transduction. Endocr.-Related Cancer 2006, 13, S99–S114. [Google Scholar] [CrossRef]
- Tai, C.J.; Chang, C.C.; Jiang, M.C.; Yeh, C.M.; Su, T.C.; Wu, P.R.; Chen, C.J.; Yeh, K.T.; Lin, S.H.; Chen, H.C. Clinical-pathological correlation of K-Ras mutation and ERK phosphorylation in colorectal cancer. Pol. J. Pathol. 2012, 63, 93–100. [Google Scholar] [PubMed]
- Raponi, M.; Winkler, H.; Dracopoli, N.C. KRAS mutations predict response to EGFR inhibitors. Curr. Opin. Pharmacol. 2008, 8, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Linardou, H.; Dahabreh, I.J.; Kanaloupiti, D.; Siannis, F.; Bafaloukos, D.; Kosmidis, P.; Papadimitriou, C.A.; Murray, S. Assessment of somatic k-RAS mutations as a mechanism associated with resistance to EGFR-targeted agents: a systematic review and meta-analysis of studies in advanced non-small-cell lung cancer and metastatic colorectal cancer. Lancet Oncol. 2008, 9, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Jutten, B.; Dubois, L.; Li, Y.N.; Aerts, H.; Wouters, B.G.; Lambin, P.; Theys, J.; Lammering, G. Binding of cetuximab to the EGFRvIII deletion mutant and its biological consequences in malignant glioma cells. Radiother. Oncol. 2009, 92, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.E.; Suzuki, S.; Thomas, S.M.; Sen, M.; Leeman-Neill, R.J.; Chiosea, S.I.; Kuan, C.T.; Bigner, D.D.; Gooding, W.E.; Lai, S.Y.; et al. Epidermal growth factor receptor variant III mediates head and neck cancer cell invasion via STAT3 activation. Oncogene 2010, 29, 5135–5145. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Armstrong, E.A.; Benavente, S.; Chinnaiyan, P.; Harari, P.M. Dual-agent molecular targeting of the epidermal growth factor receptor (EGFR): Combining anti-EGFR antibody with tyrosine kinase inhibitor. Cancer Res. 2004, 64, 5355–5362. [Google Scholar] [CrossRef] [PubMed]
- Huether, A.; Hopfner, M.; Baradari, V.; Schuppan, D.; Scherubl, H. EGFR blockade by cetuximab alone or as combination therapy for growth control of hepatocellular cancer. Biochem. Pharmacol. 2005, 70, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Takamori, S.; Fujii, T.; Ono, M.; Yamana, H.; Kuwano, M.; Shirouzu, K. Cooperative cell-growth inhibition by combination treatment with ZD1839 (Iressa) and trastuzumab (Herceptin) in non-small-cell lung cancer. Cancer Lett. 2005, 230, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.H.; Allred, C.D.; Allred, K.F.; Karko, K.L.; Doerge, D.R.; Helferich, W.G. Physiological concentrations of dietary genistein dose-dependently stimulate growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in athymic nude mice. J. Nutr. 2001, 131, 2957–2962. [Google Scholar] [PubMed]
- Nakamura, H.; Wang, Y.W.; Kurita, T.; Adomat, H.; Cunha, G.R.; Wang, Y.Z. Genistein increases epidermal growth factor receptor signaling and promotes tumor progression in advanced human prostate cancer. PLoS One 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.T.; Weber, C.R.; Wasland, K.; Savkovic, S.D. Genistein inhibits proliferation of colon cancer cells by attenuating a negative effect of epidermal growth factor on tumor suppressor FOXO3 activity. BMC Cancer 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Talalay, P. Analysis of combined drug effects: A new look at a very old problem. Trends Pharmacol. Sci. 1983, 4, 450–454. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds G21, Ram-2, Ram-3 and Ram-5 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruca, A.; Krawczyk, Z.; Szeja, W.; Grynkiewicz, G.; Rusin, A. Synthetic Genistein Glycosides Inhibiting EGFR Phosphorylation Enhance the Effect of Radiation in HCT 116 Colon Cancer Cells. Molecules 2014, 19, 18558-18573. https://doi.org/10.3390/molecules191118558
Gruca A, Krawczyk Z, Szeja W, Grynkiewicz G, Rusin A. Synthetic Genistein Glycosides Inhibiting EGFR Phosphorylation Enhance the Effect of Radiation in HCT 116 Colon Cancer Cells. Molecules. 2014; 19(11):18558-18573. https://doi.org/10.3390/molecules191118558
Chicago/Turabian StyleGruca, Aleksandra, Zdzisław Krawczyk, Wiesław Szeja, Grzegorz Grynkiewicz, and Aleksandra Rusin. 2014. "Synthetic Genistein Glycosides Inhibiting EGFR Phosphorylation Enhance the Effect of Radiation in HCT 116 Colon Cancer Cells" Molecules 19, no. 11: 18558-18573. https://doi.org/10.3390/molecules191118558