Evaluation of Biological Value and Appraisal of Polyphenols and Glucosinolates from Organic Baby-Leaf Salads as Antioxidants and Antimicrobials against Important Human Pathogenic Bacteria
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Phenolic Compounds
| Samples | Average levels (mg of gallic acid equivalent (GAE)·100 g−1 DW) |
|---|---|
| Green lettuce | 131.4 ± 7.9 e |
| Red lettuce | 532.0 ± 14.5 a |
| Rucola | 161.3 ± 9.0 cd |
| Watercress | 186.9 ± 3.5 c |
| Mixture 1 | 350.9 ± 12.1 b |
| Mixture 2 | 137.0 ± 3.1 de |
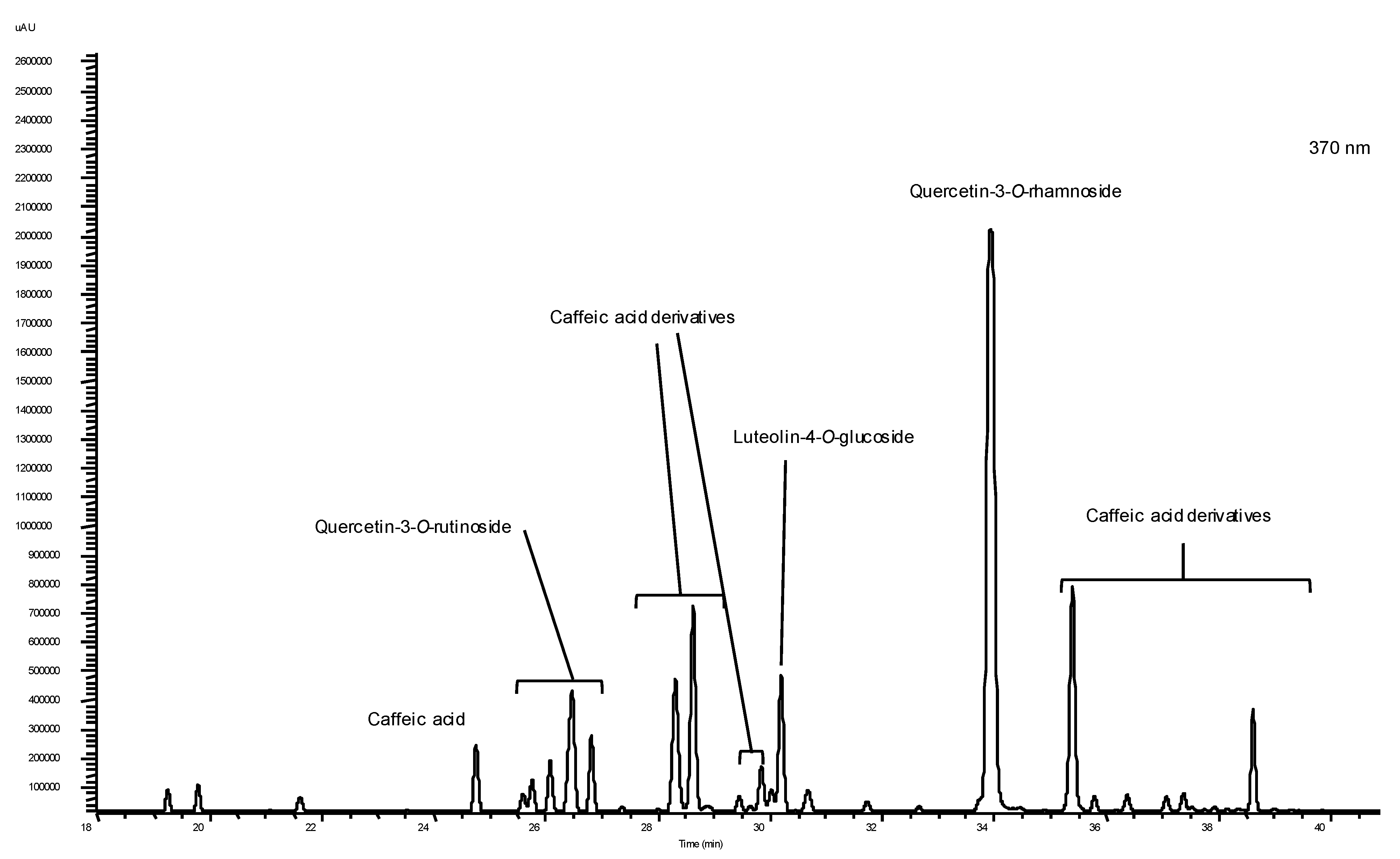
2.2. Individual Phenolics and Glucosinolates
| Polyphenols | Samples | |||||
|---|---|---|---|---|---|---|
| Green lettuce | Red lettuce | Rucola | Watercress | Mixture 1 (green lettuce + red lettuce) | Mixture 2 (green lettuce + red lettuce + watercress + rucola) | |
| Gallic acid | n.d. | n.d. | n.d. | 1.58 ± 0.18 c | n.d. | 1.15 ± 0.034 d |
| Chlorogenic acid | 2.24 ± 0.02 d | 5.97 ± 0.38 f | n.d. | 3.25 ± 0.33 b | 6.88 ± 0.17 e | 1.12 ± 0.014 d |
| Caffeic acid | 9.26 ± 0.93 c | 27.54 ± 0.51 d | 0.17 ± 0.0 d | 0.17 ± 0.0 d | 23.17 ± 0.55 c | 0.17 ± 0.0 e |
| Caffeic derivatives | 14.32 ± 0.74 b | 52.44 ± 1.25 b | n.d. | 5.48 ± 1.54 b | 59.27 ± 1.25 b | 8.22 ± 0.35 b |
| Cyanidin-3-Glucoside | 0.062 ± 0.0 e | 31.20 ± 0.74 c | 0.062 ± 0.0 d | 0.062 ± 0.0 d | 21.91 ± 1.33 c | 3.04 ± 0.069 c |
| Quercetin-3-O-rutinoside | 7.08 ± 0.39 c | 10.93 ± 0.57 e | 10.06 ± 0.22 c | 19.95 ± 0.16 a | 25.92 ± 1.42 c | 4.36 ± 0.28 c |
| Quercetin-3-O-rhamnoside | n.d. | 114.41 ± 1.86 a | 115.58 ± 2.44 a | n.d. | 128.93 ± 5.09 a | n.d. |
| Luteolin-4-O-glucoside | 6.36 ± 0.19 d | 21.68 ± 0.92 d | n.d. | n.d. | 13.64 ± 2.36 d | 0.76 ± 0.07 e |
| Isorhamnetin | 18.16 ± 0.17 a | 10.67 ± 0.65 c | 23.15 ± 0.67 b | 19.32 ± 0.11 a | 12.34 ± 1.43 d | 40.56 ± 2.23 a |
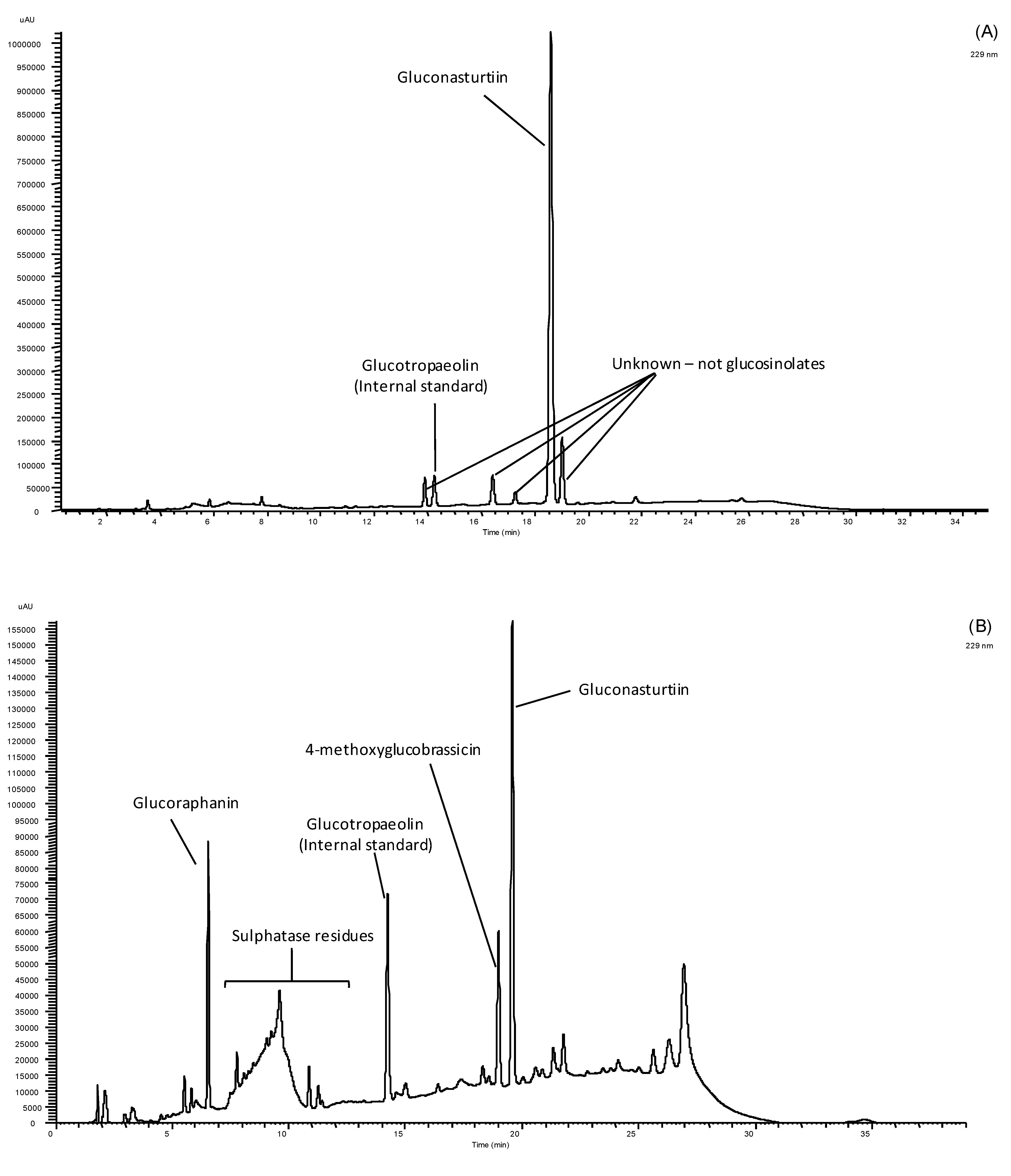
| Samples | Glucoraphanin | Gluconasturtiin | 4-Methoxyglucobrassicin | Total GSs |
|---|---|---|---|---|
| Watercress | n.d. | 3143.19 ± 202.76 a | n.d. | 3143.19 ± 202.76 a |
| Rucola | 238.28 ± 43.64 | 144.95 ± 3.95 b | 396.59 ± 43.95 | 779.81 ± 11.36 b |
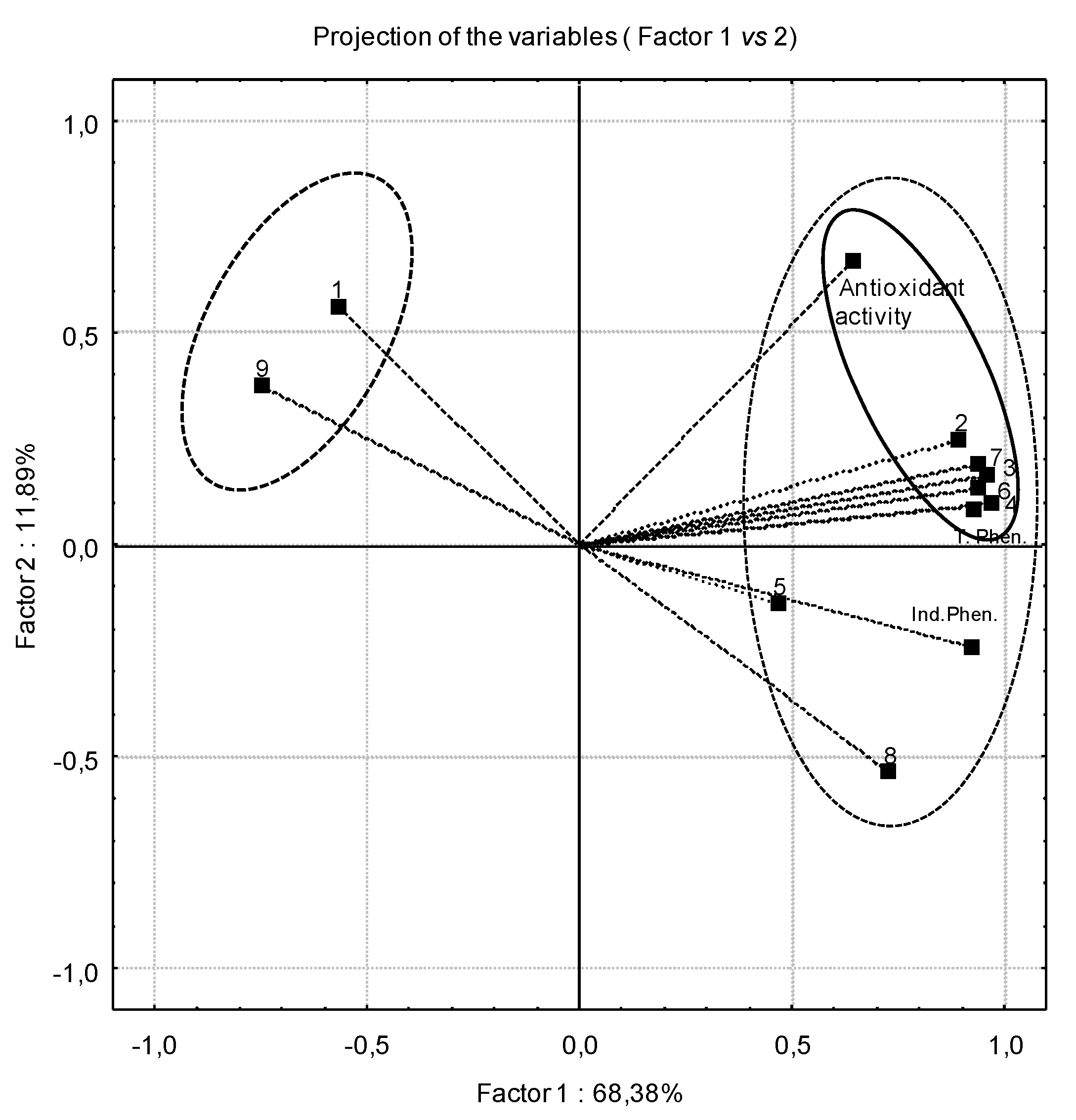
2.3. Antioxidant Activity
| Samples | % DPPH• scavenging activity | IC50(mg·mL−1) |
|---|---|---|
| Green lettuce | 41.0 ± 6.66 d | 1.43 ± 0.03 bc |
| Red lettuce | 79.7 ± 1.38 b | 0.23 ± 0.03 d |
| Rucola | 8.0 ± 3.53 f | 3.03 ± 0.58 a |
| Watercress | 25.4 ± 2.46 e | 1.87 ± 0.03 b |
| Mixture 1 (green lettuce + red lettuce) | 81.7 ± 1.22 b | 0.37 ± 0.03 d |
| Mixture 2 (green lettuce + red lettuce + watercress + rucola) | 73.8 ± 0.12 c | 0.70 ± 0.00 cd |
| Trolox (positive control) | 83.3 ± 0.00 a |
| Total phenolic content | Total individual phenolics | % DPPH• (at 1 mg·mL−1) | |
|---|---|---|---|
| Total phenolic content | 1.000 | 0.855 ** | 0.599 ** |
| Gallic acid | −0.394 | −0.641 | −0.133 |
| Chlorogenic acid | 0.816 ** | 0.724 ** | 0.669 ** |
| Caffeic acid | 0.922 ** | 0.844 ** | 0.687 ** |
| Caffeic acid derivatives | 0.812 ** | 0.772 ** | 0.739 ** |
| Cyanidine-3-glucoside | 0.971 ** | 0.889 ** | 0.736 ** |
| Quercitin-3-O-rutinoside | 0.338 | 0.463 | 0.111 |
| Luteolin-7-O-glucoside | 0.913 ** | 0.799 ** | 0.680 ** |
| Quercitin-3-O-rhamnoside | 0.663 ** | 0.923 ** | 0.194 |
| Isorhamnetin | −0.658 | −0.638 | −0.079 |
| Total individual phenolics | 0.855 ** | 1.000 | 0.495 |
| % DPPH at 1 mg·mL−1 | 0.599 ** | 0.495 | 1.000 |
2.4. Antimicrobial Activity
| Isolates | Source | Green lettuce | Red lettuce | Watercress | Rucola | Mixture 1 | Mixture 2 | DMSO 4 | Commercial antibiotic 5 |
|---|---|---|---|---|---|---|---|---|---|
| Escherichia coli MJS260 | Clinical | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | 15.7 ± 0.3 |
| Escherichia coli 006 AZ | Food | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | 17.0 ± 0.3 |
| Escherichia coli | ATCC 25992 | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | 17.0 ± 0.3 |
| Escherichia coli 1 | Animal | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | 18.0 ± 0.4 |
| Pseudomonas aeruginosa | ATCC 10145 | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | 19.5 ± 0.5 |
| Pseudomonas aeruginosa MJ323 | Clinical | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | 16.7 ± 0.3 |
| Enterococcus spp. A1Sa | Food | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | 20.0 ± 0.3 |
| Enterococcus faecalis MJS257 | Clinical | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | 14.7 ± 0.3 |
| Enterococcus faecalis | ATCC 29212 | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | 14.7 ± 0.3 |
| Enterococcus faecalis 49 | Animal | 8.0 ± 0.0 (+) | 9.0 ± 0.3 (+) | 11.0 ± 0.3 (+) | 13.0 ± 0.3 (+) | 9.0 ± 0.3 (+) | 11.0 ± 0.3 (+) | n.i. | 16.0 ± 0.3 |
| Staphylococcus aureus | ATCC 13565 | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | 16.7 ± 0.6 |
| Staphylococcus aureus MJS241 | Clinical | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | 16.3 ± 0.3 |
| Isolates | Source | Gallic acid | Chlorogenic acid | Caffeic acid | Quercitin-3-O-rutinoside | Quercitin-3-O-rhamnoside | PEITC |
|---|---|---|---|---|---|---|---|
| Escherichia coli MJS260 | Clinical | n.i. | n.i. | n.i. | n.i. | n.i. | 9.7 ± 0.7 (+) |
| Escherichia coli 006 AZ | Food | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| Escherichia coli | ATCC 25992 | n.i. | n.i. | n.i. | n.i. | n.i. | 10.0 ± 0.0 (+) |
| Escherichia coli 1 | Animal | n.i. | n.i. | n.i. | n.i. | n.i. | 11.0 ± 0.0 (+) |
| Pseudomonas aeruginosa | ATCC 10145 | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| Pseudomonas aeruginosa MJ323 | Clinical | n.i. | n.i. | n.i. | n.i. | n.i. | 7.3 ± 0.3 (+) |
| Enterococcus spp. A1Sa | Food | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| Enterococcus faecalis MJS257 | Clinical | n.i. | n.i. | n.i. | n.i. | n.i. | 14.3 ± 0.3 (+) |
| Enterococcus faecalis | ATCC 29212 | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| Enterococcus faecalis 49 | Animal | 8.0 ± 0.0 (+) | 9.0 ± 0.3 (+) | 11.0 ± 0.3 (+) | 13.0 ± 0.3 (+) | 9.0 ± 0.3 (+) | 11.0 ± 0.3 (+) |
| Staphylococcus aureus | ATCC 13565 | n.i. | n.i. | n.i. | n.i. | n.i. | t.i (+++) |
| Staphylococcus aureus MJS241 | Clinical | n.i. | n.i. | n.i. | n.i. | n.i. | 44.0 ± 0.0 (+++) |
3. Experimental
3.1. Plant Material
3.2. Methods of Sample Preparation for Assessing the Phytochemical Composition, Antioxidant and Antimicrobial Bioassays
3.2.1. Methanol Extraction for Total and Individual Phenolics Determination
3.2.2. Determination of Total Phenolic Content
3.2.3. Determination of Individual Phenolics
3.2.4. Determination and Quantification of Individual and Total Glucosinolates
3.3. Antioxidant Activity Assays
Scavenging of 2,2-Diphenyl-2-picrylhydrazyl (DPPH) Radicals
3.4. Antimicrobial Activity in Vitro Assays
3.4.1. Microorganisms and Culture Conditions
| Bacterial strain | Source | Class |
|---|---|---|
| Escherichia coli MJS260 | Clinical | Gram-negative |
| Escherichia coli 006 AZ | Food | |
| Escherichia coli | ATCC 25992 | |
| Escherichia coli 1 | Animal | |
| Pseudomonas aeruginosa | ATCC 10145 | |
| Pseudomonas aeruginosa MJS323 | Clinical | |
| Enterococcus spp. A1Sa | Food | Gram-positive |
| Enterococcus faecalis MJS257 | Clinical | |
| Enterococcus faecalis | ATCC 29212 | |
| Enterococcus faecalis 49 | Animal | |
| Staphylococcus aureus | ATCC 13565 | |
| Staphylococcus aureus MJS241 | Clinical |
3.4.1.1. Antibacterial Susceptibility Tests
3.4.1.2. Antibacterial Classification
3.5. Statistical Analysis and Quality Assurance of the Results
4. Conclusions
Acknowledgments
References
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Ag. 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Zhang, Y.; Seeram, N.P.; Lee, R.; Feng, L.; Heber, D. Isolation and identification of strawberry phenolics with antioxidant and human cancer cell antiproliferative properties. J. Agric. Food Chem. 2008, 56, 670–675. [Google Scholar] [CrossRef]
- Talalay, P.; Fahey, J.W. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J. Nutr. 2001, 131 (Suppl. 11), S3027–S3033. [Google Scholar]
- Kunyanga, C.N.; Imungi, J.K.; Okoth, M.W.; Biesalski, H.K.; Vadivel, V. Flavonoid content in ethanolic extracts of selected raw and traditionally processed indigenous foods consumed by vulnerable groups of Kenya: antioxidant and type II diabetes-related functional properties. Int. J. Food Sci. Nutr. 2011, 62, 465–473. [Google Scholar]
- Shahidi, F.; Liyana-Pathirana, C.M.; Wall, D.S. Antioxidant activity of white and black sesame seeds and their hull fractions. Food Chem. 2006, 99, 478–483. [Google Scholar] [CrossRef]
- Abadias, M.; Usall, J.; Anguera, M.; Solsona, C.; Viñas, I. Microbiological quality of fresh, minimally-processed fruit and vegetables, and sprouts from retail establishment. Int. J. Food Microbiol. 2008, 123, 121–129. [Google Scholar] [CrossRef]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. J. Nutr. 2010, 9, 2–11. [Google Scholar] [CrossRef]
- Rodríguez-Hidalgo, S.; Artés-Hernández, F.; Gómez, P.A.; Fernández, J.A.; Artés, F. Quality of fresh-cut baby spinach grown under a floating trays system as affected by nitrogen fertilization and innovative packaging treatments. J. Sci. Food Agric. 2010, 90, 1089–1097. [Google Scholar]
- Tomás-Callejas, A.; López-Velasco, G.; Artés, F.; Artés-Hernández, F. Acidified sodium chlorite optimization assessment to improve quality of fresh-cut tatsoi baby leaves. J. Sci. Food Agric. 2012, 92, 877–885. [Google Scholar] [CrossRef]
- Conte, A.; Scrocco, C.; Lecce, L.; Mastromatteo, M.; Del Nobile, M.A. Ready-to-eat sweet cherries: Study on different packaging systems. Innov. Food Sci. Emerg. 2009, 10, 564–571. [Google Scholar] [CrossRef]
- Kou, L.; Luo, Y.; Wu, D.; Liu, X. Effects of mild heat treatment on microbial growth and product quality of packaged fresh-cut table grapes. J. Food Sci. 2007, 72, 567–573. [Google Scholar] [CrossRef]
- Caponigro, V.; Ventura, M.; Chianconea, I.; Amatoa, L.; Parente, E.; Piro, F. Variation of microbial load and visual quality of ready-to-eat salads by vegetable type, season, processor and retailer. Food Microbiol. 2010, 27, 1071–1077. [Google Scholar] [CrossRef]
- Brooks, J.D.; Paton, V.G.; Vidanes, G. Potent induction of phase 2 enzymes in human prostate cells by sulforaphane. Cancer Epidem. Biom. 2001, 10, 949–954. [Google Scholar]
- Bacon, J.R.; Williamson, G.; Garner, R.C.; Lappin, G.; Langouet, S.; Bao, Y. Sulforaphane and quercetin modulate PhIP–DNA adduct formation in human HepG2 cells and hepatocytes. Carcinogenesis 2003, 24, 1903–1911. [Google Scholar] [CrossRef]
- Ismail, A.; Marjan, Z.M.; Foong, C.W. Total antioxidant activity and phenolic content in selected vegetables. Food Chem. 2004, 87, 581–586. [Google Scholar] [CrossRef]
- Pinela, J.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Nutritional composition and antioxidant activity of four tomato (Lycopersicon esculentum L.) farmer’ varieties in Northeastern Portugal homegardens. Food Chem. Toxicol. 2012, 50, 829–834. [Google Scholar] [CrossRef]
- Papi, A.; Orlandi, M.N.; Bartolini, G.; Barillari, J.; Iori, R.; Paolini, M.; Ferroni, F.; Fumo, M.G.; Pedulli, G.F.; Valgimigli, L. Cytotoxic and antioxidant activity of 4-methylthio-3-butenyl isothiocyanate from Raphanus sativus L. (Kaiware Daikon) sprouts. J. Agric. Food Chem. 2008, 56, 875–883. [Google Scholar] [CrossRef]
- Jakobek, L.; Seruga, M.; Seruga, B.; Novak, I.; Medvidovic-Kosanovic, M. Phenolic compound composition and antioxidant activity of fruits of Rubus and Prunus species from Croatia. Int. J. Food Sci. Tech. 2009, 44, 860–868. [Google Scholar] [CrossRef]
- Chambers, H.F.; Deleo, F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nature Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef]
- Javanmardi, J.; Stushnoff, C.; Locke, E.; Vivanco, J.M. Antioxidant capacity and total phenolic content of Iranian Ocimum acessions. Food Chem. 2003, 83, 547–550. [Google Scholar] [CrossRef]
- Pereira, F.M.V.; Rosa, E.; Fahey, J.W.; Stephenson, K.K.; Carvalho, R.; Aires, A. Influence of temperature and ontogeny on the levels of glucosinolates in broccoli (Brassica oleracea var italica) sprouts and their effect on the induction of mammalian phase 2 enzymes. J. Agric. Food Chem. 2002, 50, 6239–6244. [Google Scholar]
- Siddhraju, P.; Becker, K. Antioxidant properties of various solvents extracts of total phenolic constituents from three different agro climatic origins of drumstick tree (Moringa oleifera Lam) leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI), Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, In Approved Standard—Eighth Edition, CLSI Document M07-A8; CLSI: Wayne, PA, USA, 2009; p. 182.
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Aires, A.; Marques, E.; Carvalho, R.; Rosa, E.A.S.; Saavedra, M.J. Evaluation of Biological Value and Appraisal of Polyphenols and Glucosinolates from Organic Baby-Leaf Salads as Antioxidants and Antimicrobials against Important Human Pathogenic Bacteria. Molecules 2013, 18, 4651-4668. https://doi.org/10.3390/molecules18044651
Aires A, Marques E, Carvalho R, Rosa EAS, Saavedra MJ. Evaluation of Biological Value and Appraisal of Polyphenols and Glucosinolates from Organic Baby-Leaf Salads as Antioxidants and Antimicrobials against Important Human Pathogenic Bacteria. Molecules. 2013; 18(4):4651-4668. https://doi.org/10.3390/molecules18044651
Chicago/Turabian StyleAires, Alfredo, Esperança Marques, Rosa Carvalho, Eduardo A. S. Rosa, and Maria J. Saavedra. 2013. "Evaluation of Biological Value and Appraisal of Polyphenols and Glucosinolates from Organic Baby-Leaf Salads as Antioxidants and Antimicrobials against Important Human Pathogenic Bacteria" Molecules 18, no. 4: 4651-4668. https://doi.org/10.3390/molecules18044651






