Allylation of Functionalized Aldehydes by Potassium Allyltrifluoroborate Catalyzed by 18-Crown-6 in Aqueous Media
Abstract
:1. Introduction
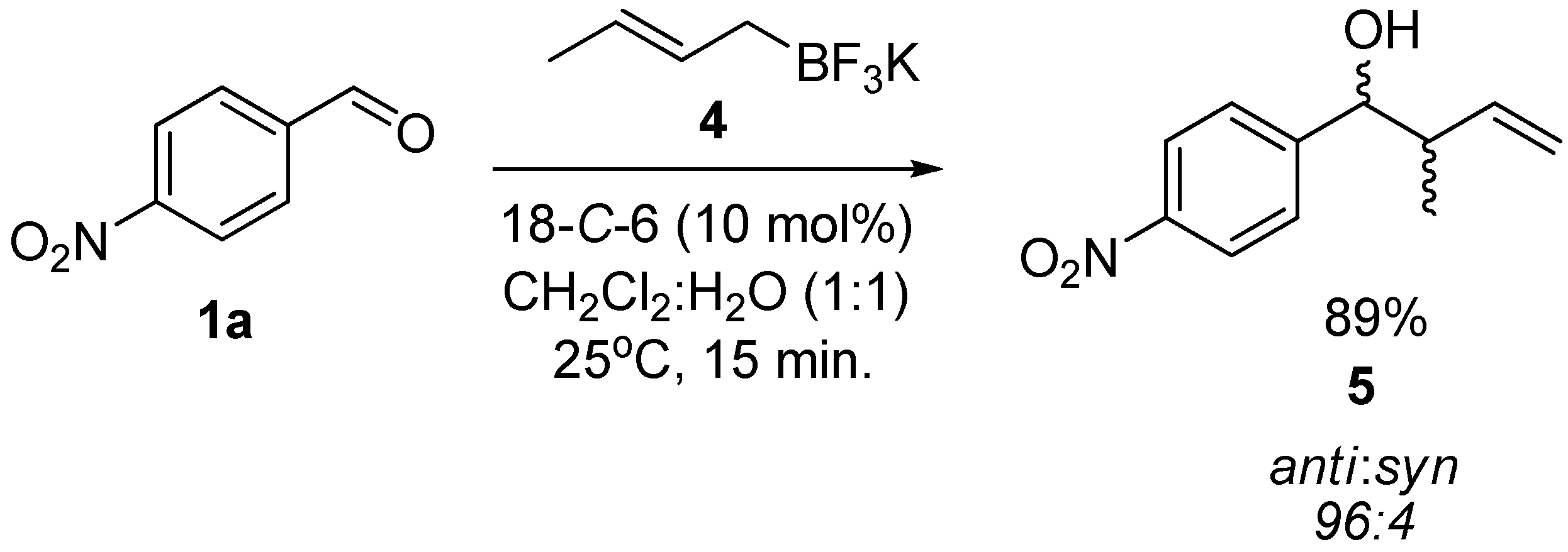
2. Results and Discussion
| Catalyst (mol%) | Solvent | 3a (%) | |
|---|---|---|---|
| 1 | 18-C-6 (1) | CH2Cl2:H2O | 20 |
| 2 | 18-C-6 (5) | CH2Cl2:H2O | 90a |
| 3 | 18-C-6 (10) | CH2Cl2:H2O | 94 |
| 4 | 18-C-6 (10) | CH2Cl2 | 15 |
| 5 | 18-C-6 (10) | H2O | 80 a |
| 6 | 15-C-5 (10) | CH2Cl2:H2O | 12 |
| 7 | - | CH2Cl2:H2O | 7 |
| RCHO | Product | Yield (%) | |||
|---|---|---|---|---|---|
| 1 |  | 1a |  | 3a | 94 |
| 2 |  | 1b |  | 3b | 82 |
| 3 |  | 1c |  | 3c | 80 |
| 4 |  | 1d |  | 3d | 87 |
| 5 |  | 1e |  | 3e | 85 |
| 6 |  | 1f |  | 3f | 86 |
| 7 |  | 1g |  | 3g | 89 |
| 8 |  | 1h |  | 3h | 90 |
| 9 |  | 1i |  | 3i | 87 |
| 10 |  | 1j |  | 3j | 88 |
| 11 |  | 1k |  | 3k | 82 |
| 12 |  | 1l |  | 3l | 89 |
| 13 |  | 1m |  | 3m | 90 |
| 14 |  | 1n |  | 3n | 91 |
| 15 |  | 1o |  | 3o | 86 |

3. Experimental
3.1. General
3.2. General Procedure for the Allylation of Aldehydes 1a–o with Potassium Allyltrifluoroborate (2) Catalyzed by 18-C-6
3.3. General Procedure for the Crotylation of 1a with Potassium (E)-crotyltrifluoroborate (4) Catalyzed by 18-C-6
3.4. General Procedure for the Allylation of Compound 6 with Potassium Allyltrifluoroborate (2) Catalyzed by 18-C-6
4. Conclusions
Supplementary Materials
Acknowledgments
References
- Denmark, S.E.; Fu, J. Catalytic Enantioselective Addition of Allylic Organometallic Reagents to Aldehydes and Ketones. Chem. Rev. 2003, 103, 2763–2793. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Asao, N. Selective reactions using allylic metals. Chem. Chem. Rev. 1993, 93, 2207. [Google Scholar] [CrossRef]
- Johnson, A.A.; Sharpless, K.B. Catalytic asymmetric epoxidation of allylic alcohols. In Comprehensive Organic Synthesis; Pergamon Press: Oxford, UK, 1990; Volume 7. [Google Scholar]
- Luche, J.L.; Damiano, J.C. Ultrasounds in organic syntheses. 1. Effect on the formation of lithium organometallic reagents. J. Am. Chem. Soc. 1980, 102, 7926–7927. [Google Scholar] [CrossRef]
- Zhang, W.-C.; Li, C.-J. Magnesium-Mediated Carbon−Carbon Bond Formation in Aqueous Media: Barbier−Grignard Allylation and Pinacol Coupling of Aldehydes. J. Org. Chem. 1999, 64, 3230–3236. [Google Scholar] [CrossRef]
- Barczak, N.T.; Jarvo, E.R. Silver-Catalyzed, Manganese-Mediated Allylation and Benzylation Reactions of Aldehydes and Ketones. Eur. J. Org. Chem. 2008, 5507–5510. [Google Scholar] [CrossRef]
- Petrier, C.; Luche, J.L. Allylzinc reagents additions in aqueous media. J. Org. Chem. 1985, 50, 910–912. [Google Scholar] [CrossRef]
- Zhou, J.-Y.; Sun, G.-F.; Zhang, M.-F.; Jia, Y.; Wu, S.-H. Lead-promoted Barbier-type reaction of aldehydes with propargyl bromides in aqueous media. Chin. J. Chem. 1997, 15, 361–365. [Google Scholar]
- Zhang, X.; Qiu, R.; Tan, N.; Yin, S.; Xia, J.; Luo, S.; Au, C.-T. Air-stable hypervalent organobismuth(III) tetrafluoroborate as effective and reusable catalyst for the allylation of aldehyde with tetraallyltin. Tetrahedron Lett. 2010, 51, 153–156. [Google Scholar] [CrossRef]
- Iamamoto, T.; Kusumoto, T.; Tawarayama, Y.; Sugiura, Y.; Mit, T. Carbon-carbon bond-forming reactions using cerium metal or organocerium(III) reagents. J. Org. Chem. 1984, 49, 3904–3912. [Google Scholar] [CrossRef]
- Suzuki, T.; Atsumi, J.-I.; Sengoku, T.; Takahashi, M.; Yoda, H. Indium-catalyzed enantioselective allylation of aldehydes with β-carbonyl allylstannanes: An efficient synthetic method for chiral α-methylene-γ-lactones. J. Organomet. Chem. 2010, 695, 128–136. [Google Scholar]
- Guimarães, R.L.; Lima, D.J.P.; Barros, M.E.S.B.; Cavalcanti, L.N.; Hallwass, F.; Navarro, M.; Bieber, L.W.; Malvestiti, I. Aqueous Barbier Allylation of Aldehydes Mediated by Tin. Molecules 2007, 12, 2089–2105. [Google Scholar] [CrossRef]
- Jiang, G.; List, B. Acid-Catalyzed α-Allylation of Aldehydes with Allylic Alcohols. Adv. Synth. Catal. 2011, 353, 1667–1670. [Google Scholar] [CrossRef]
- Bhuyan, B.K.; Borah, A.J.; Senapati, K.K.; Phukan, P. Ti-exchanged ZSM-5 as heterogeneous catalyst for allylation of aldehydes with allyltributylstannane. Tetrahedron Lett. 2011, 52, 2649–2651. [Google Scholar] [CrossRef]
- Alper, H.; Vasylyev, M. Rhodium-Catalyzed Reductive Allylation of Conjugated Aldehydes with Allyl Acetate. J. Org. Chem. 2010, 75, 2710–2713. [Google Scholar] [CrossRef]
- Deng, D.; Liu, B.; Wang, L.; Fu, W. [Cd2(tren)2(dl-alaninato)](ClO4)3: an efficient water-compatible Lewis acid catalyst for chemo-, regio-, and diastereo-selective allylation of various aldehydes. Tetrahedron Lett. 2010, 51, 5567–5570. [Google Scholar] [CrossRef]
- Bian, Y.-J.; Zhao, H.-M.; Yu, X.-G. Allylation Reactions of Aromatic Aldehydes with Antimony in Aqueous Media Under Ultrasonic Irradiation. Synth. Commun. 2009, 39, 2370–2377. [Google Scholar] [CrossRef]
- Shimizu, H.; Igarashi, T.; Miura, T.; Murakami, M. Rhodium-Catalyzed Reaction of 1-Alkenylboronates with Aldehydes Leading to Allylation Products. Angew. Chem. Int. Ed. 2011, 50, 11465–11469. [Google Scholar] [CrossRef]
- Ramadhar, T.R.; Batey, R.A. Allylation of Imines and Their Derivatives with Organoboron Reagents: Stereocontrolled Synthesis of Homoallylic Amines. Synthesis 2011, 1321–1346. [Google Scholar]
- Kennedy, J.W.J.; Hall, D.G. Recent Advances in the Activation of Boron and Silicon Reagents for Stereocontrolled Allylation Reactions. Angew. Chem. Int. Ed. 2003, 42, 4732–4739. [Google Scholar]
- Massa, A.; Acocella, M.R.; De Sio, V.; Villano, R.; Scettri, A. A catalytic asymmetric allylation of aldehydes with allyl trichlorosilane activated by a chiral tetradentate bis-sulfoxide. Tetrahedron: Asymmetry 2009, 20, 202–204. [Google Scholar]
- Souza, R.F.M.; Areias, M.C.C.; Bieber, L.W.; Navarro, M. Electrochemical allylation of aldehydes in a solvent-free cavity cell with a graphite powder cathode. Green Chem. 2011, 13, 1118. [Google Scholar] [CrossRef]
- Preite, M.D.; Jorquera-Geroldi, H.A.; Pérez-Carvajal, A. Barbier allylation of aldehydes and ketones with aluminium and catalytic indium metal: an economical alternative. ARKIVOC 2011, 7, 380–388. [Google Scholar]
- Dam, J.H.; Fristrup, P.; Madsen, R. Combined Experimental and Theoretical Mechanistic Investigation of the Barbier Allylation in Aqueous Media. J. Org. Chem. 2008, 73, 3228–3235. [Google Scholar] [CrossRef]
- Oliveira, R.A. Organotrifluoroborate Salts. Synlett 2009, 505–506. [Google Scholar] [CrossRef]
- Darses, S.; Genet, J.-P. Potassium Organotrifluoroborates: New Perspectives in Organic Synthesis. Chem. Rev. 2008, 108, 288. [Google Scholar] [CrossRef]
- Stefani, H.A.; Cella, R.; Vieira, A.S. Recent advances in organotrifluoroborates chemistry. Tetrahedron 2007, 63, 3623–3658. [Google Scholar] [CrossRef]
- Molander, G.A.; Figueroa, R. Organotrifluoroborates: Expanding organoboron chemistry. Aldrichim. Acta 2005, 38, 49–56. [Google Scholar]
- Nowrouzi, F.; Thadani, A.N.; Batey, R.A. Allylation and Crotylation of Ketones and Aldehydes Using Potassium Organotrifluoroborate Salts under Lewis Acid and Montmorillonite K10 Catalyzed Conditions. Org. Lett. 2009, 11, 2631–2634. [Google Scholar] [CrossRef]
- Matsuoka, H.; Kondo, K. General and convenient TsOH-induced allylboration of ketones. Tetrahedron Lett. 2009, 50, 2320–2321. [Google Scholar] [CrossRef]
- Thadani, A.N.; Batey, R.A. A Mild Protocol for Allylation and Highly Diastereoselective Syn or Anti Crotylation of Aldehydes in Biphasic and Aqueous Media Utilizing Potassium Allyl- and Crotyltrifluoroborates. Org. Lett. 2002, 4, 3827–3830. [Google Scholar] [CrossRef]
- Nakamura, H.; Shimizu, K. Catalytic reactions of bis-π-allylpalladium generated from allyltrifluoroborate. Tetrahedron Lett. 2011, 52, 426–429. [Google Scholar] [CrossRef]
- Shaghafi, M.B.; Kohn, B.L.; Jarvo, E.R. Palladium-Catalyzed Conjugate Allylation Reactions of α,β-Unsaturated N-Acylpyrroles. Org. Lett. 2008, 10, 4743–4746. [Google Scholar] [CrossRef]
- Batey, R.A.; Thadani, A.N.; Smil, D.V. Potassium allyl- and crotyltrifluoroborates: Stable and efficient agents for allylation and crotylation. Tetrahedron Lett. 1999, 40, 4289–4292. [Google Scholar] [CrossRef]
- Horváth, I.T.; Anatas, P.T. Innovations and Green Chemistry. Chem. Rev. 2007, 107, 2169–2173. [Google Scholar] [CrossRef]
- Hutchings, G.J. A golden future for green chemistry. Catal. Today 2007, 122, 196–200. [Google Scholar] [CrossRef]
- Hancock, R.D.; Martell, A.E. Ligand design for selective complexation of metal ions in aqueous solution. Chem. Rev. 1989, 89, 1875–1914. [Google Scholar] [CrossRef]
- Bloch, R. Additions of Organometallic Reagents to C=N Bonds: Reactivity and Selectivity. Chem. Rev. 1998, 98, 1407–1438. [Google Scholar] [CrossRef]
- Rüdel, H. Case study: Bioavailability of tin and tin compounds. Ecotoxicol. Environ. Saf. 2003, 56, 180–189. [Google Scholar] [CrossRef]
- Oliveira, R.A.; Silva, R.O.; Molander, G.A.; Menezes, P.H. 1H, 13C, 19F and 11B NMR spectral reference data of some potassium organotrifluoroborates. Magn. Res. Chem. 2009, 47, 873–878. [Google Scholar] [CrossRef]
- Petrillo, D.E.; Kohli, R.K.; Molander, G.A. Accurate mass determination of organotrifluoroborates. J. Am. Soc. Mass Spectrom. 2007, 18, 404–405. [Google Scholar] [CrossRef]
- Oliveira, R.A.; Savegnago, L.; Jesse, C.R.; Menezes, P.H.; Molander, G.A.; Nogueira, C.W. Toxicological investigation and antinociceptive property of potassium thiophene-3-trifluoroborate. Basic Clin. Pharmacol. Toxicol. 2009, 104, 448–454. [Google Scholar] [CrossRef]
- Jaruchoktaweechai, C.; Suwanborirux, K.; Tanasupawatt, S.; Kittakoop, P.; Menasveta, P. New Macrolactins from a Marine Bacillus sp. Sc026. J. Nat. Prod. 2000, 63, 984–986. [Google Scholar] [CrossRef]
- Oliveira, R.A.; Oliveira, J.M.; Rahmeier, L.H.S.; Comasseto, J.V.; Marino, J.P.; Menezes, P.H. Synthesis of the C7–C24 fragment of (−)-Macrolactin F. Tetrahedron Lett. 2008, 49, 5759–5761. [Google Scholar]
- Molander, G.A.; Figueroa, R. cis-Dihydroxylation of Unsaturated Potassium Alkyl- and Aryltrifluoroborates. Org. Lett. 2006, 8, 75–78. [Google Scholar] [CrossRef]
- Denmark, S.E.; Nguyen, S.T. Catalytic, Nucleophilic Allylation of Aldehydes with Allyl Acetate. Org. Lett. 2009, 11, 781–784. [Google Scholar] [CrossRef]
- Li, G.-L.; Zhao, G. Allylation of Aldehydes and Imines: Promoted by Reuseable Polymer-Supported Sulfonamide of N-Glycine. Org. Lett. 2006, 8, 633–636. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 2, 3a–o, 4–7 are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Barbosa, F.C.G.; Freitas, J.C.R.; Melo, C.F.; Menezes, P.H.; Oliveira, R.A. Allylation of Functionalized Aldehydes by Potassium Allyltrifluoroborate Catalyzed by 18-Crown-6 in Aqueous Media. Molecules 2012, 17, 14099-14110. https://doi.org/10.3390/molecules171214099
Barbosa FCG, Freitas JCR, Melo CF, Menezes PH, Oliveira RA. Allylation of Functionalized Aldehydes by Potassium Allyltrifluoroborate Catalyzed by 18-Crown-6 in Aqueous Media. Molecules. 2012; 17(12):14099-14110. https://doi.org/10.3390/molecules171214099
Chicago/Turabian StyleBarbosa, Fernanda C. G., Juliano C. R. Freitas, Caio F. Melo, Paulo H. Menezes, and Roberta A. Oliveira. 2012. "Allylation of Functionalized Aldehydes by Potassium Allyltrifluoroborate Catalyzed by 18-Crown-6 in Aqueous Media" Molecules 17, no. 12: 14099-14110. https://doi.org/10.3390/molecules171214099






