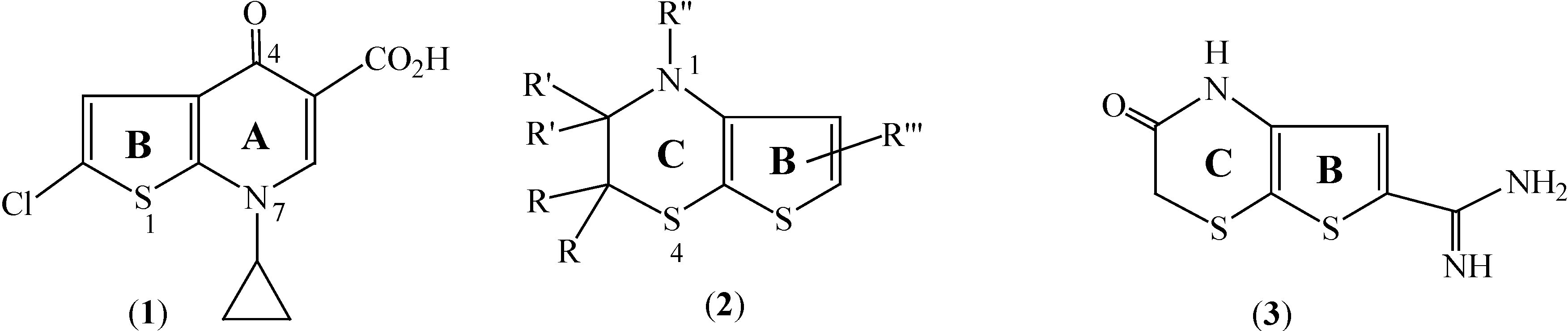
Introduction
Several substituted 4-oxothieno[2,3-
b]pyridine-5-carboxylic acids (exemplified by
1 [
1],
Figure 1) bioisosteres of fluoroquinolone antibacterials (such as ciprofloxacin), were synthesized and reported to exhibit ˝good to excellent˝ levels of antibacterial potency [
1,
2,
3,
4]. On the other hand, thieno[2,3-
b][1,4]thiazine derivatives (e.g.
2,
Figure 1) are currently of interest due to their as therapeutic properties as smooth muscle relaxants [
5] and as potassium channel-opening agents [
6] which make them potentially useful for the treatment of various diseases, while certain thieno[2,3-
b][1,4]thiazine-2-ones (e.g.
3) have been patented as urokinase inhibitors [
7].
Figure 1.
Structures of 4-oxothieno[2,3-b]pyridine (1) and thieno[2,3-b][1,4]thiazines 2, 3.
Figure 1.
Structures of 4-oxothieno[2,3-b]pyridine (1) and thieno[2,3-b][1,4]thiazines 2, 3.
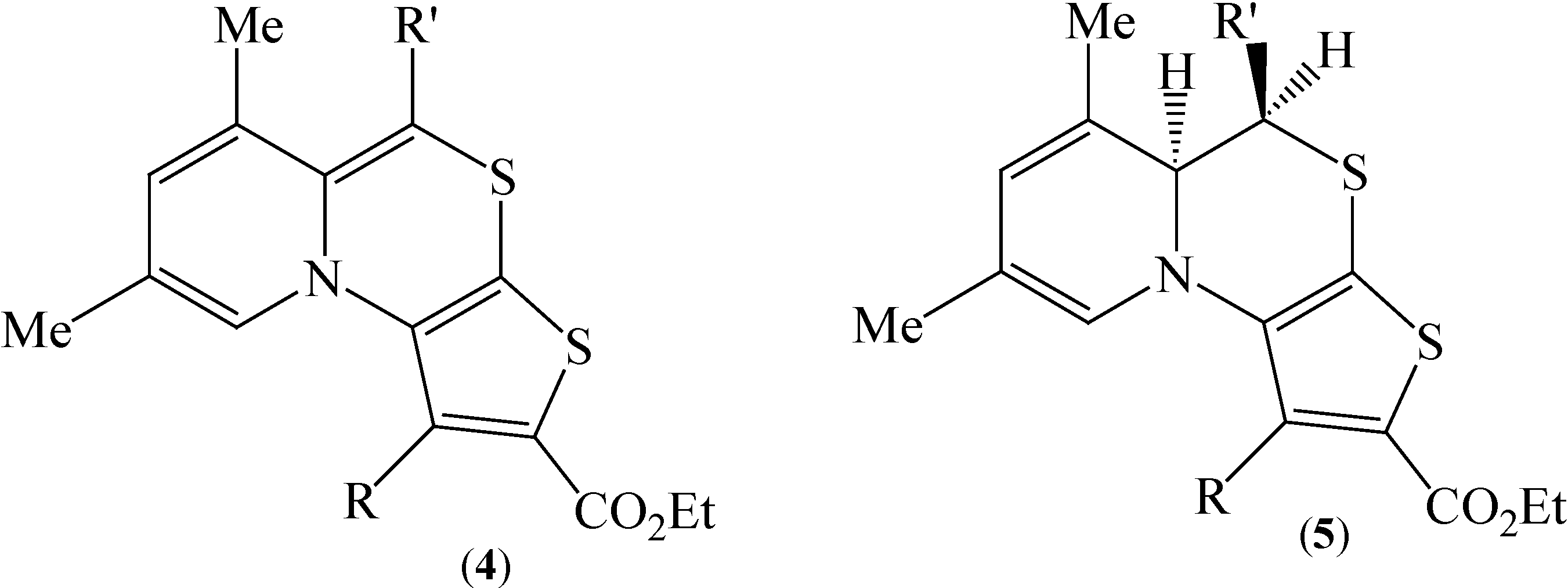
We became interested in condensed pyridothieno[1,4]thiazine tricyclic systems, for which literature data are confined to only one report [
8] describing the preparation and properties of pyrido[2,1-
c]thieno[3,2-
e][1,4]thiazines
4 and their dihydro precursors
5 (
Figure 2).
Figure 2.
Structures of pyrido[2,1-c]thieno[3,2-e][1,4]thiazines 4, 5.
Figure 2.
Structures of pyrido[2,1-c]thieno[3,2-e][1,4]thiazines 4, 5.
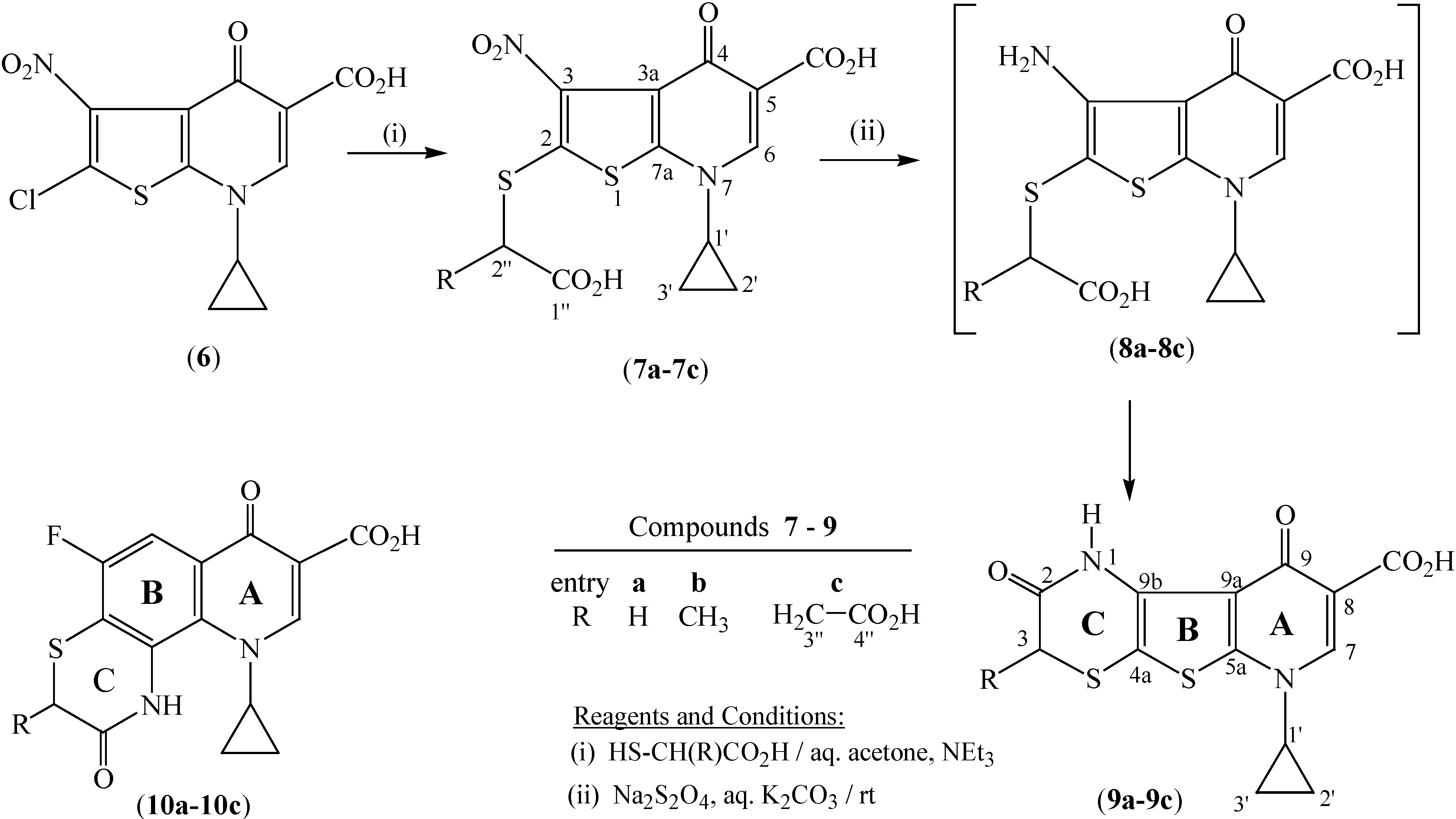
In particular, we envisaged that the hitherto undescribed tetrahydro-1
H-pyrido[3',2':4,5]thieno[2,3-
b][1,4]thiazine-8-carboxylic acids
9a-
9c (
Scheme 1), representing a tricyclic hybrid of
1 and
3, might exhibit interesting bioproperties such as antimicrobial and antitumor activity. Hence, the present work deals with the synthesis and properties of
9a-
9c, as outlined in
Scheme 1 and detailed in the Experimental section. These novel heterocyclics
9a-
9c are potential bioisosteres of the recently described [1,4]thiazino-[2,3-
h]quinoline-8-carboxylic acids
10a-
10c shown in
Scheme 1 [
9], in which the benzene nucleus (
B) is replaced by a thiophene ring.
Scheme 1.
Synthesis of pyrido[3', 2': 4, 5]thieno[2,3-b][1,4]thiazines 9a-9c.
Scheme 1.
Synthesis of pyrido[3', 2': 4, 5]thieno[2,3-b][1,4]thiazines 9a-9c.
Experimental
General
2,5-Dichlorothiophene, ethyl 3-(N,N-dimethylamino)acrylate and cyclopropylamine were purchased from Acros. (±)-2-Mercaptopropionic acid, (±)-2-mercaptosuccinic acid and mercaptoacetic acid were purchased from Aldrich. Melting points were determined on a Gallenkamp capillary melting point apparatus and are uncorrected. 1H- and 13C-NMR spectra were measured on a Bruker DPX-300 instrument with Me4Si as internal reference. High resolution mass spectra (HRMS) were measured in positive ion mode by Electrospray (ESI) on APEX-Qe 94 instrument. The samples were dissolved in acetonitrile, diluted in spray solution (methanol/water 1:1 v/v + 0.1% formic acid) and infused using a syringe pump with a flow of 2 uL/min. External calibration was conducted using Arginine cluster in a mass range m/z 175-871. MS/MS spectra for 7a and 7b were performed in the external Qh of the APEX-Q. For all HRMS data, the mass error was 0.00-0.50 ppm. IR spectra were recorded as KBr discs on a Nicolet Impact-400 FT-IR spectrophotometer. Elemental analyses were preformed at the Microanalytical Laboratory of the Hashemite University, Zarqa, Jordan.
2-[(Carboxymethyl)thio]-7-cyclopropyl-3-nitro-4-oxo-4,7-dihydrothieno[2,3-b]pyridine-5-carboxylic acid (7a).
Mercaptoacetic acid (0.46 g, 5 mmol) was added to a stirred solution of 2-chloro-7-cyclopropyl-3-nitro-4-oxo-4,7-dihydrothieno[2,3-b]pyridine-5-carboxylic acid (6, 1.32 g, 4.2 mmol) in aqueous acetone (1:2 v/v, 54 mL) and triethylamine (6 mL) at rt, and then kept in the dark for 7 h. The reaction mixture was then washed with chloroform (2 x 10 mL), the aqueous layer was acidified with 3N HCl and the precipitated product was collected and dried. The title compound was purified by stirring in boiling chloroform (10 mL) in which the soluble impurities are removed. Yield 1.37 g (88 %); mp 236-237 oC (decomp); IR (cm-1) 3601, 3529, 3408, 3016, 2932, 1744, 1728, 1695, 1615, 1525, 1502, 1479, 1426, 1337, 1236, 1183; 1H-NMR (300 MHz, DMSO-d6) δ 1.16/1.29 (2 m, 4H, 2H-2' / 2H-3'), 3.85 (m, 1H, H-1'), 4.06 (s, 2H, 2H-2˝), 8.61 (s, 1H, H-6), 13.28 [br s, 1H, C(2˝)-CO2H], 14.56 [br s, 1H, C(5)-CO2H]; 13C-NMR (75 MHz, DMSO-d6) δ 7.8 (C-2'/C-3'), 38.2 (C-2''), 38.9 (C-1'), 113.0 (C-5), 120.3 (C-3), 133.7 (C-2), 142.3 (C-3a), 147.0 (C-6), 152.8 (C-7a), 165.4 [C(5)-CO2H], 169.9 [C(1˝)-CO2H], 171.9 (C-4); HRMS: calcd. for C13H11N2O7S2+ [M+H]+: 371.00077, found: 371.00040; calcd. for C13H10N2O7S2Na+ [M+Na]+: 392. 98271, found: 392.98237; MS/MS (of m/z 371): C8H6NS2+ (179.99367), C11H6NO2S2+ (247.98351), C8H3N2O4S2+ (255.95301); Anal. calcd. for C13H10N2O7S2 (370.36): C, 42.16; H, 2.72; N, 7.56; S, 17.32. Found: C, 41.78; H, 2.93; N, 7.60; S, 17.50.
2-[(Carboxyethyl)thio]-7-cyclopropyl-3-nitro-4-oxo-4,7-dihydrothieno[2,3-b]pyridine-5-carboxylic acid [(±)-7b]
Prepared from (±)-2-mercaptopropionic acid (0.53 g, 5 mmol) and 6 (1.32 g, 4.2 mmol) using the procedure and experimental conditions described above for the preparation of 7a. The title compound was isolated as a yellow solid which was recrystallized from chloroform. Yield 1.26 g (78 %); mp 221-222oC (decomp); IR (cm-1) 3427, 3205, 3106, 3078, 3003, 2925, 1736, 1691, 1602, 1546, 1470, 1335, 1294, 1215, 1182; 1H-NMR (300 MHz, DMSO-d6) δ 1.17 / 1.31 (m, 4H, 2H-2'/2H-3'), 1.40 (d, J = 7.1 Hz, 3H, CH3), 3.87 (m, 1H, H-1'), 4.10 (q, J = 7.1 Hz, 1H, H-2˝), 8.64 (s, 1H, H-6), 13.34 [br s, 1H, C(2˝)-CO2H], 14.40 [br s, 1H, C(5)-CO2H]; 13C-NMR (75 MHz, DMSO-d6) δ 7.7 (C-2'/C3'), 18.0 (CH3), 38.3 (C-1'), 47.9 (C-2˝), 112.9 (C-5), 120.1 (C-3), 126.6 (C-2), 145.6 (C-3a), 147.6 (C-6), 154.2 (C-7a), 165.2 [C(5)-CO2H], 172.1 (C-4), 172.5 [C(1˝)-CO2H]; HRMS: calcd. for C14H13N2O7S2+ [M+H]+: 385.01642, found: 385.01592; calcd. for C14H12N2O7S2Na+ [M+Na]+: 406.99836, found: 406.99781. MS/MS (of m/z 385): C8H6NS2+ (179.99363), C11H6NO2S2+ (247.98347), C8H3N2O4S2+ (255.95303), C11H9NO3S2+ (267.00185), C11H8N2O5S2+ (311.98696); Anal. calcd. for C14H12N2O7S2 (384.39): C, 43.74; H, 3.15; N, 7.29; S, 16.68. Found: C, 43.63; H, 3.14; N, 7.04; S, 16.66.
2-[(Carboxyl-7-cyclopropyl-3-nitro-4-oxo-4,7-dihydrothieno[2,3-b]pyridin-2-yl)thio]succinic acid [(±)-7c]
Prepared from (±)-2-mercaptosuccinic acid (0.75 g, 5 mmol) and 6 (1.32 g, 4.2 mmol) using the procedure and experimental conditions described above for the preparation of 7a. The title compound was obtained as a yellow precipitate which was collected, washed successively with chloroform and methanol and dried. Yield 1.62 g (90 %); mp 211-212oC (decomp); IR (cm-1) 3440 (br), 3092, 2928, 1724 (br), 1708, 1596, 1529, 1501, 1454, 1416, 1376, 1325, 1260, 1178; 1H-NMR (300MHz, DMSO-d6) δ 1.17/1.31 (2 m, 4H, 2H-2' / 2H-3'), 2.74 (dd, J = 16.8, 6.2 Hz, 1H, HA-3˝), 2.81 (dd, J = 16.8, 7.7 Hz, 1H, HB-3˝), 3.87 (m, 1H, H-1'), 4.15 (dd, J = 6.2, 7.7 Hz, 1H, H-2˝), 8.68 (s, 1H, H-6), 13.05 [br s, 2H, 2CO2H], 14.38 [br s, 1H, C(5)-CO2H]; 13C-NMR (75 MHz, DMSO-d6) δ 7.8 (C-2'/C-3'), 36.5 (C-3˝), 38.4 ( C-1'), 48.4 (C-2˝), 112.9 (C-5), 120.0 (C-3), 126.5 (C-2), 145.6 (C-3a), 147.7 (C-6), 154.3 (C-7a), 165.2 [C(5)-CO2H], 171.3 [C(1˝)-CO2H], 171.8 [C(4˝)-CO2H], 172.1 (C-4); HRMS: calcd. for C15H11N2O9S2+ [M-H]+: 426.99060, found: 426.99128; Anal. calcd. for C15H12N2O9S2 (428.40): C, 42.05; H, 2.82; N, 6.54; S, 14.97. Found: C, 42.17; H, 3.01; N, 6.60; S, 15.06.
6-Cyclopropyl-2,9-dioxo-2,3,6,9-tetrahydro-1H-pyrido[3',2':4,5]thieno[2,3-b][1,4]thiazine-8-carboxylic acid (9a)
To a vigorously stirred suspension of 2-[(carboxymethyl)thio]-7-cyclopropyl-3-nitro-4-oxo-4,7-dihydrothieno[2,3-b]pyridine-5-carboxylic acid (7a, 0.37 g, 1 mmol) in concentrated hydrochloric acid (12 mL) was added, portionwise, stannous chloride dihydrate ( 1.13 g, 5 mmol). The mixture was stirred for 1h, then treated with water (12 mL) and was kept under stirring at rt until a clear solution was obtained. This solution was finally neutralized with aqueous sodium carbonate whereby a deep brown precipitate was formed, which was collected, washed with cold water, cold ethanol and dried. Yield 0.27 g (84 %); mp 274-275oC (decomp); IR (cm-1) 3440 (br), 3343, 3080, 3009, 2913, 1719, 1691, 1614, 1543, 1460, 1440, 1337, 1228, 1177; 1H-NMR (300 MHz, DMSO-d6) δ 1.15/1.26 (2 m, 4H, 2H-2' / 2H-3'), 3.64 (s, 2H, 2H-3), 3.82 (m, 1H, H-1'), 8.54 (s, 1H, H-7), 9.52 (s, 1H, H-1), 14.61 (br s, 1H, CO2H); 13C-NMR (75 MHz, DMSO-d6 ) δ 7.5 (C-2'/C-3'), 30.7 (C-3), 38.2 (C-1'), 106.6 (C-4a), 111.9 (C-9b), 119.8 (C-8), 132.6 (C-9a), 145.9 (C-7),151.9 (C-5a), 163.4 (C-2), 165.7 (CO2H), 173.7 (C-9); HRMS: calcd. for C13H11N2O4S2+ [M+H]+: 323.01603, found: 323.01544; calcd. for C13H10N2O4S2Na+ [M+Na]+: 344.99797, found: 344.99737; Anal. calcd. for C13H10N2O4S2 (322.36): C, 48.44; H, 3.13; N, 8.69; S, 19.89. Found: C, 48.39; H, 3.18; N, 8.54; S, 19.97.
6-Cyclopropyl-3-methyl-2,9-dioxo-2,3,6,9-tetrahydro-1H-pyrido[3', 2' : 4,5]thieno[2,3-b][1,4]thiazine-8-carboxylic acid [(±)-9b]
This compound was prepared via reductive cyclization of (±)- 2-[(1-carboxyethyl)thio]-7-cyclopropyl-3-nitro-4-oxo-4,7-dihydrothieno[2,3-b]pyridine-5-carboxylic acid [(±)-7b, 0.38 g, 1 mmol] using stannous chloride dihydrate (1.13 g, 5 mmol), and following the same procedure and experimental conditions described above for obtaining 9a. The title product was formed as a yellow precipitate which was collected, washed with ethanol and dried. Yield 0.26 g (77 %); mp 246-247 oC (decomp); IR (cm-1) 3453 (br), 3318, 3096, 3048, 1738, 1682, 1649, 1612, 1520, 1467, 1431, 1332, 1297, 1249, 1186; 1H-NMR (300 MHz, DMSO-d6) δ 1.15/1.28 (2 m, 4H, 2H-2' / 2H-3'), 1.35 (d, J = 7 Hz, 3H, CH3), 3.83 (m, 1H, H-1'), 3.85 (q, J = 7 Hz, 1H, H-3), 8.54 (s, 1H, H-7), 9.54 (s, 1H, H-1), 14.83 (br s, CO2H); 13C-NMR (75 MHz, DMSO-d6) δ 7.5 (C-2'/C-3'), 15.5 (CH3), 37.9 (C-3), 38.2 (C-1'), 105.7 (C-4a), 112.0 (C-9b), 119.7 (C-8), 131.9 (C-9a), 145.9 (C-7), 152.0 (C-5a), 165.6 (CO2H), 165.7 (C-2), 173.7 (C-9); HRMS: calcd. for C14H13N2O4S2+ [M+H]+: 337.03168, found: 337.03114; calcd. for C14H12N2O4S2Na+ [M+Na]+: 359.01362, found: 359.01313; Anal. calcd. for C14H12N2O4S2 (336.39): C, 49.99; H, 3.60; N, 8.33; S, 19.06. Found: C, 49.97; H, 3.53; N, 8.42; S, 18.80.
3-(Carboxymethyl)-6-cyclopropyl-2,9-dioxo-2,3,6,9-tetrahydro-1H-pyrido-[3',2':4,5]thieno[2,3-b][1,4]-thiazine-8-carboxylic acid [ (±)-9c]
This compound was prepared via reductive cyclization of (±)-2-[(5-carboxy-7-cyclopropyl-3-nitro-4-oxo-4,7-dihydrothieno[2,3-b]pyridin-2-yl)thio]succinic acid [(±)-7c, 0.43 g, 1 mmol] using stannous chloride dihydrate (1.13 g, 5 mmol), and following the same procedure and experimental conditions described above for the preparation of 9a. The title compound was isolated as a yellow solid which was washed with ethanol and dried. Yield 0.28 g (74 %); mp 239-240oC (decomp); IR (cm-1) 3464 (br), 3342, 3095, 3016, 2940, 1717, 1697, 1601, 1549, 1471, 1439, 1363, 1325, 1244, 1202, 1171; 1H-NMR (300 MHz, DMSO-d6) δ 1.17/1.27 (2 m, 4H, 2H-2' / 2H-3'), 2.55 (dd, J = 16.6, 7.8 Hz, 1H, HA-3˝), 2.87 (dd, J = 16.6, 6.1 Hz, 1H, HB-3˝), 3.84 (m, 1H, H-1'), 3.98 (dd, J = 6.1, 7.8 Hz, 1H, H-3), 8.54 (s, 1H, H-7), 9.66 (s, 1H, H-1), 12.69 [br s, 1H, C(4˝)-O2H], 14.56 [br s, 1H, C(8)-CO2H]; 13C-NMR (75 MHz, DMSO-d6) δ 7.5 (C-2'/C-3'), 33.9 (C-3˝), 38.2 (C-1'), 39.5 (C-3), 106.1 (C-4a), 112.0 (C-9b), 119.8 (C-8), 132.0 (C-9a), 145.9 (C-7), 152.2 (C-5a), 164.1 (C-2), 165.7 [C(8)-CO2H], 171.3 [C(4˝)-O2H], 173.6 (C-9); HRMS: calcd. for C15H13N2O6S2+ [M+H]+: 381.02151, found: 381.02090; calcd. for C15H12N2O6S2Na+ [M+Na]+: 403.00345, found: 403.00280; Anal. calcd. for C15H12N2O6S2 (380.40): C, 47.36; H, 3.18; N, 7.36; S, 16.86. Found : C, 47.02; H, 3.17; N, 7.24; S, 16.65.