Enantioselective Hydrogenations with Chiral Titanocenes
Abstract
:1. Introduction
2. Chirality and Synthesis of Titanocene Catalysts
2.1. Unbridged Titanocenes

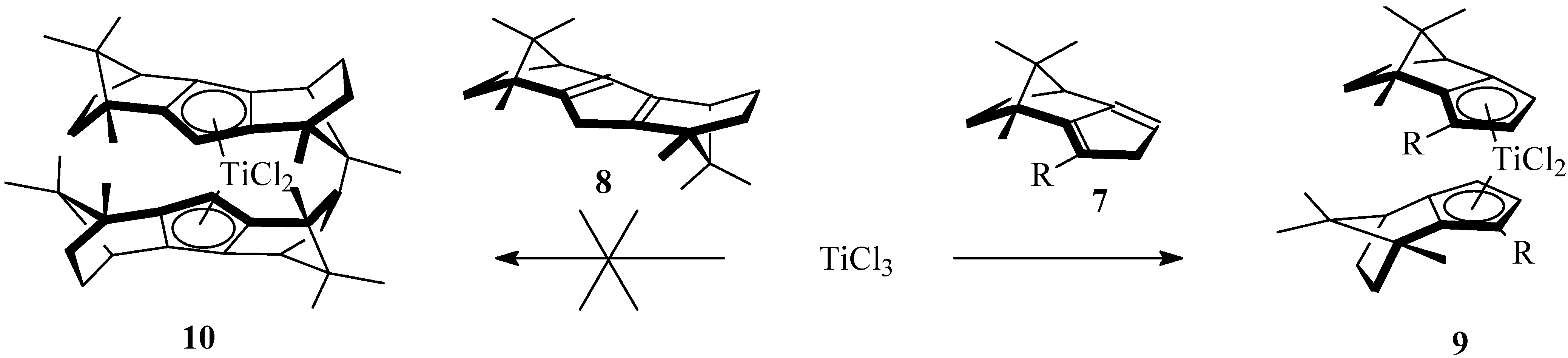

2.2. Ansa-Titanocenes
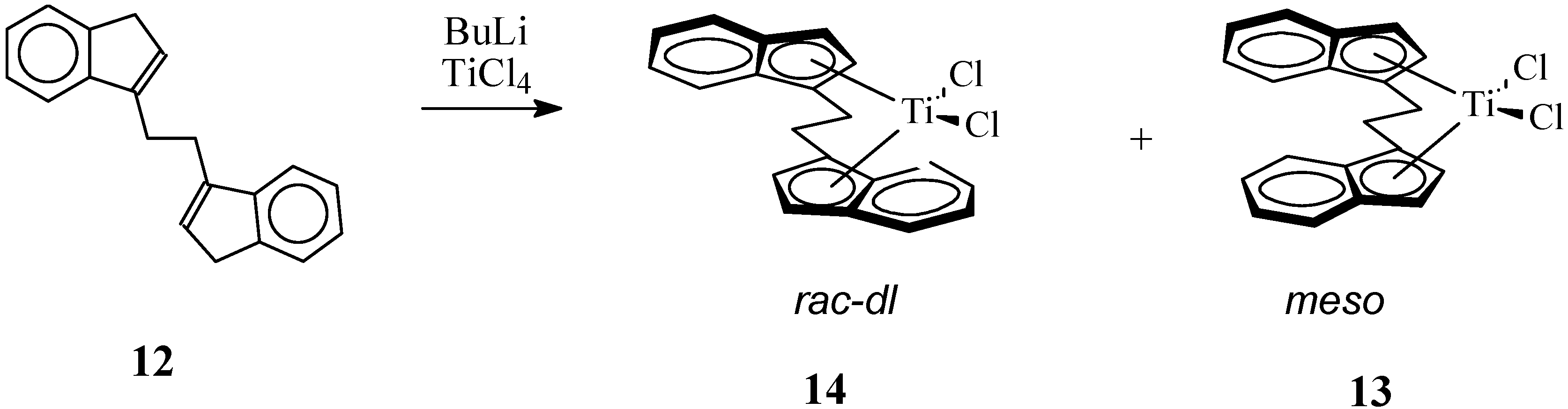

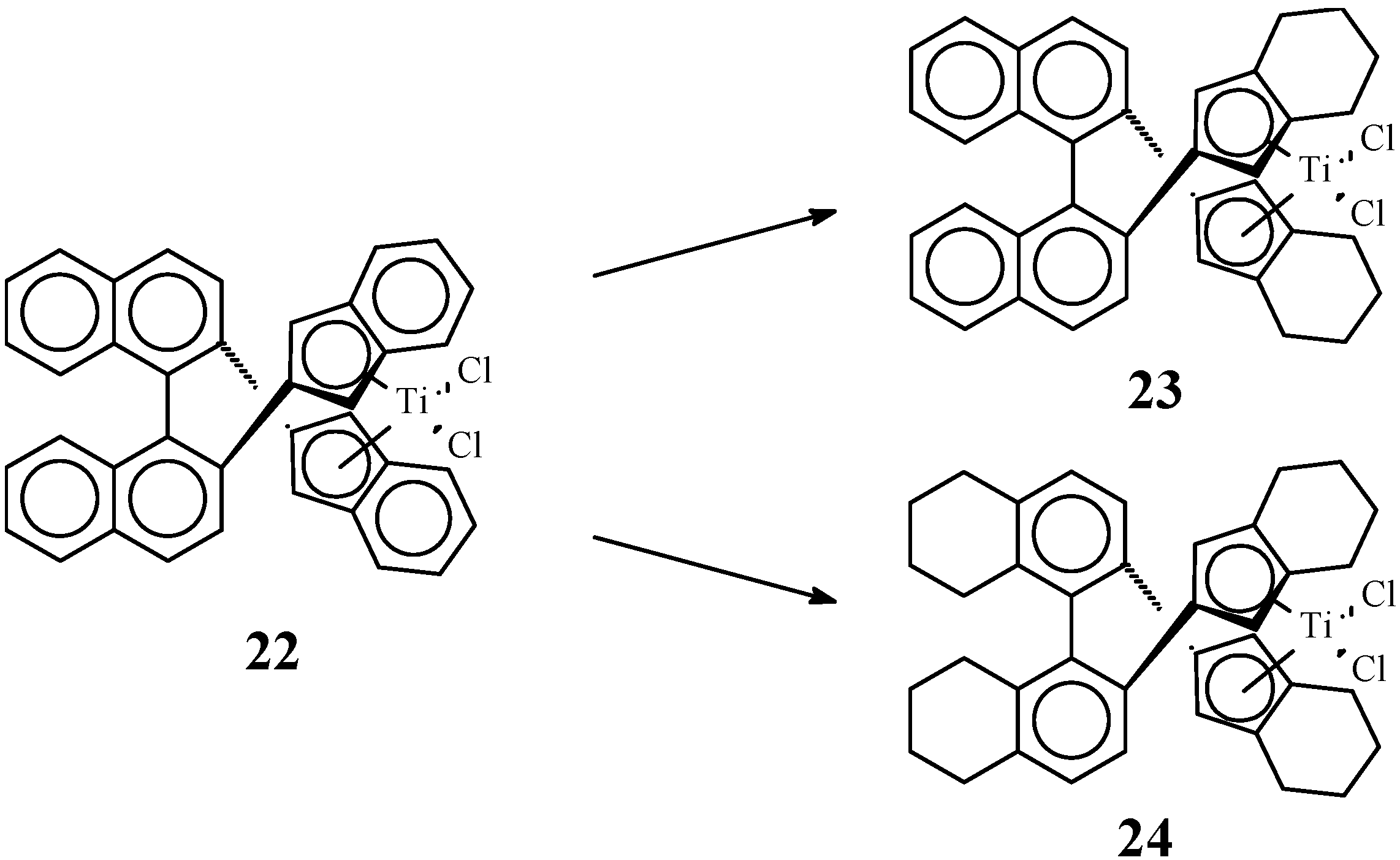

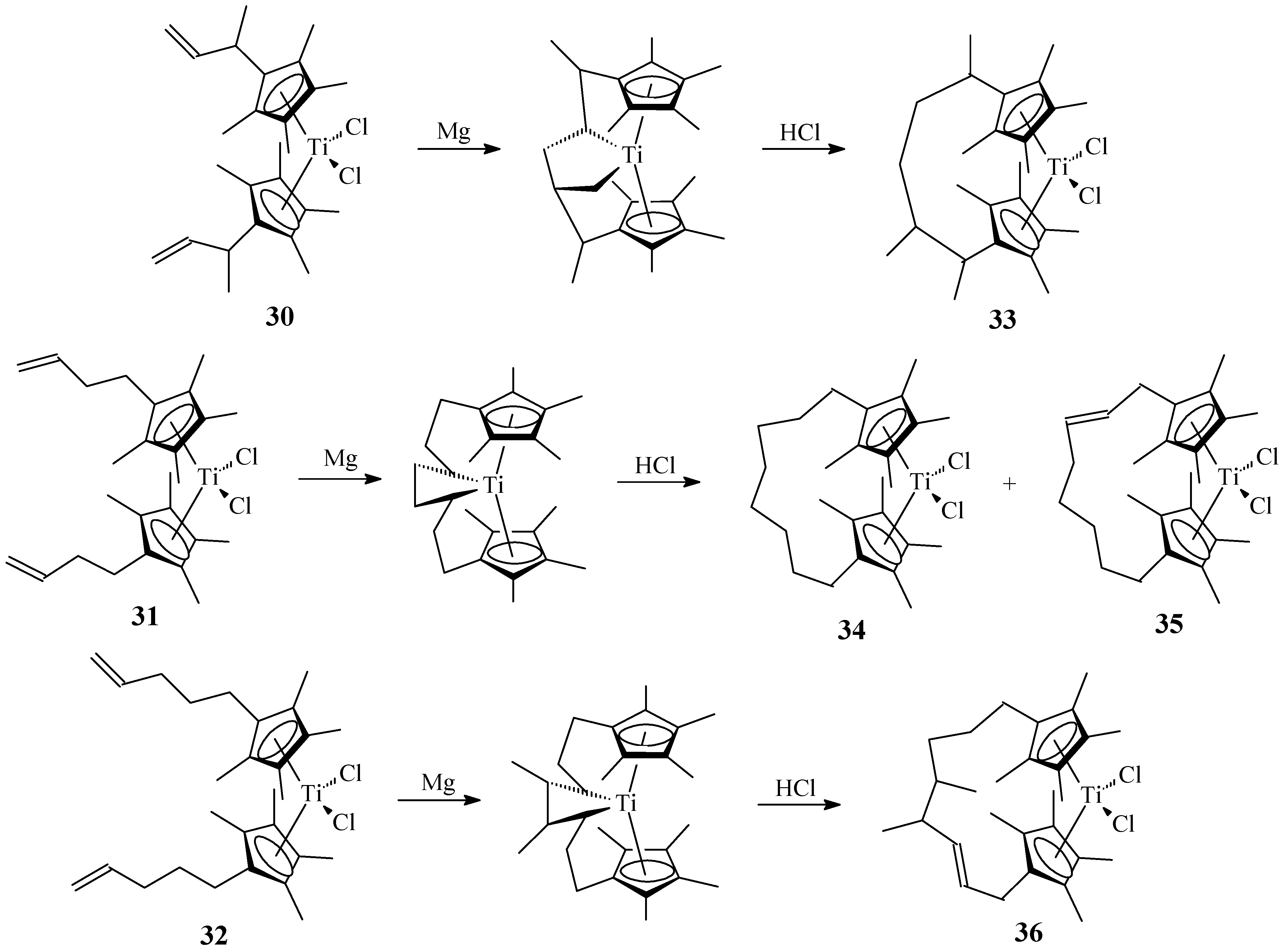
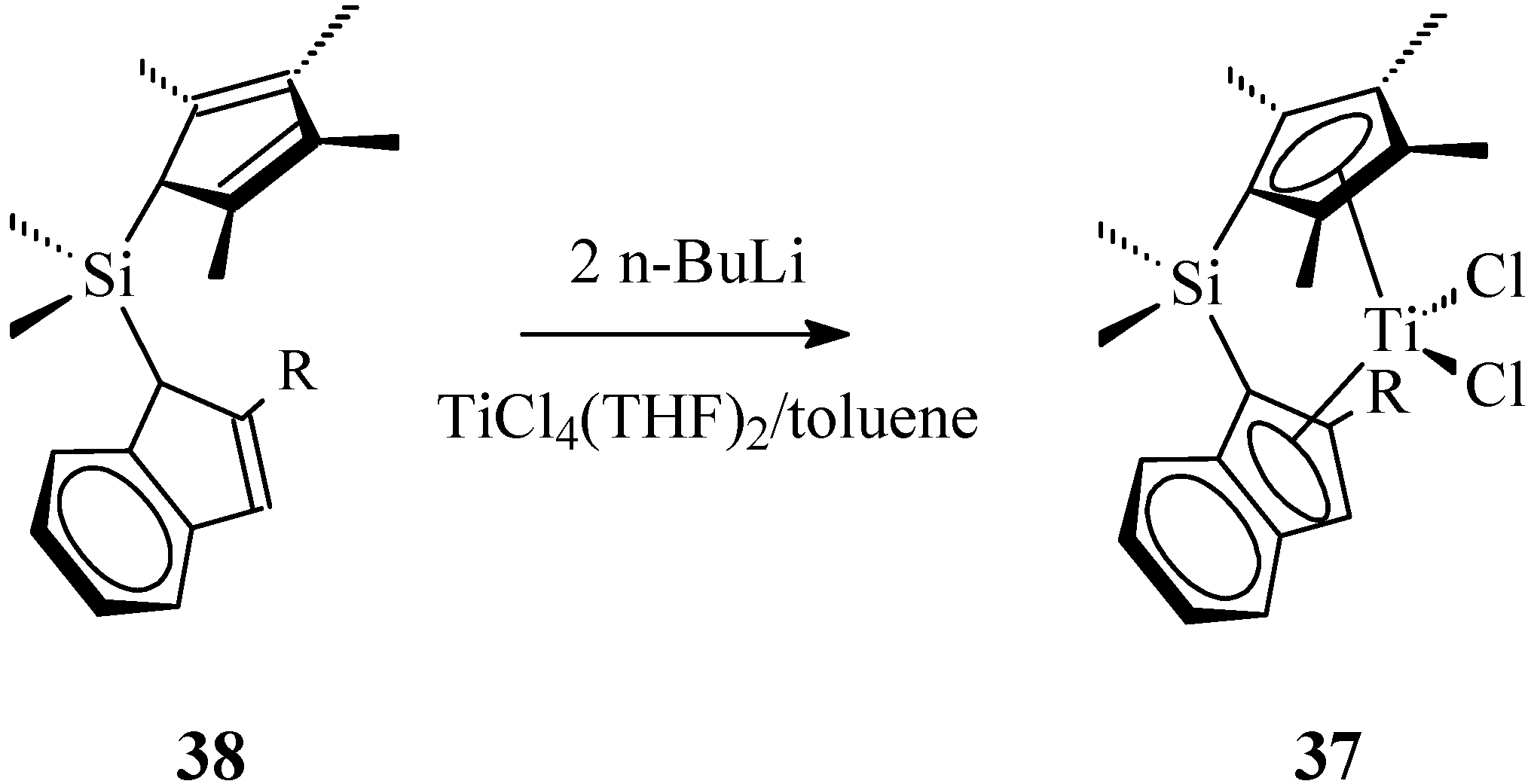

2.3. Active and Enantioselective Form of Titanocene Catalysts

3. Enantioselective Hydrogenation of Double Bonds
3.1 Activity of Titanocenes in Enantioselective Reductions
Alkenes
Imines

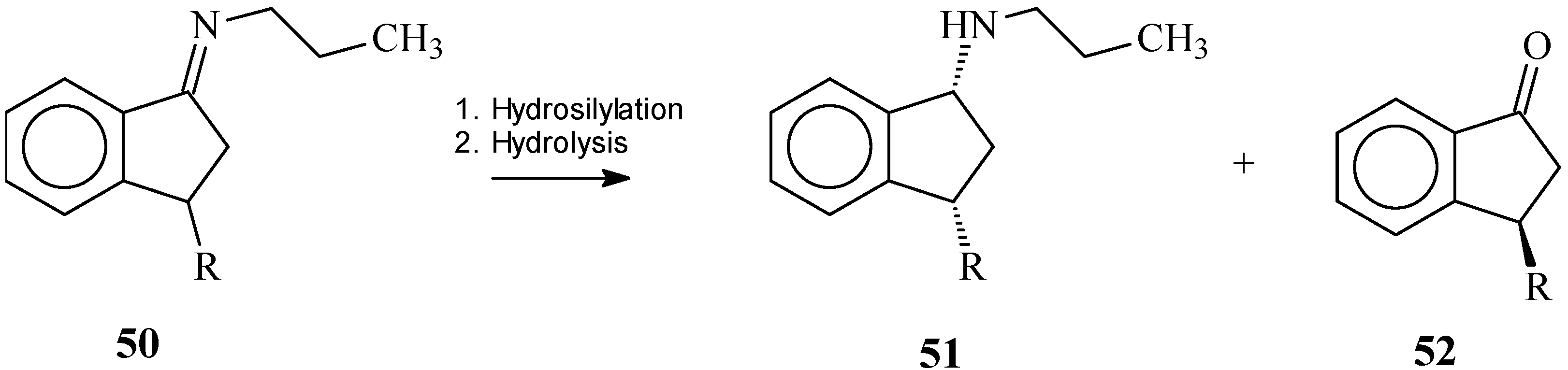
Ketones
| Substrate | Conv. % | TOF, 1/h | ee, % | Ref. | Substrate | Conv. % | TOF, 1/h | ee, % | Ref. |
|---|---|---|---|---|---|---|---|---|---|
 | 80 96a 100b | 2.0 80 2.08 | 99 98 60 | 65 54 62 |  | 72d | 0.63 | 99 | 65 |
 | 78 | 0.65 | 98 | 65 |  | 84 | 0.26 | 95 | 73 |
 | 73 | 0.75 | 97 | 73 | 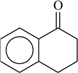 | 92 | 0.22 | 91 | 73 |
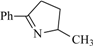 | 34 | 0.23 | 99 | 69 | 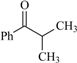 | 86a | 10.75 | 99 | 82 |
 | 78 | 0.65 | 94 | 66 | 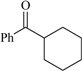 | 80a | 8.0 | 98 | 82 |
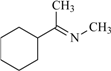 | 85 | 0.38 | 92 | 64 | 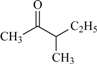 | 100 | 1.85 | 50 | 74 |
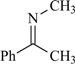 | 95a | 395.8 | 99 | 54 | 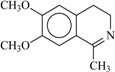 | 79 | 0.32 | 95 | 65 |
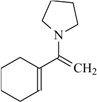 | 72f | 0.6 | 95 | 66 | 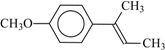 | 79 | 0.33 | 95 | 77 |
 | 94 | 2.09 | 99 | 77 | 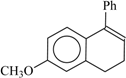 | 87 | 0.05 | 83 | 77 |
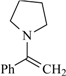 | 75 | 0.62 | 92 | 66 | 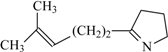 | 79e | 0.69 | 99 | 65 |
 | 100c | 1.04 | 61 | 12 |  | 96a | 38.4 | 92 | 81 |
3.2 Reaction Mechanisms
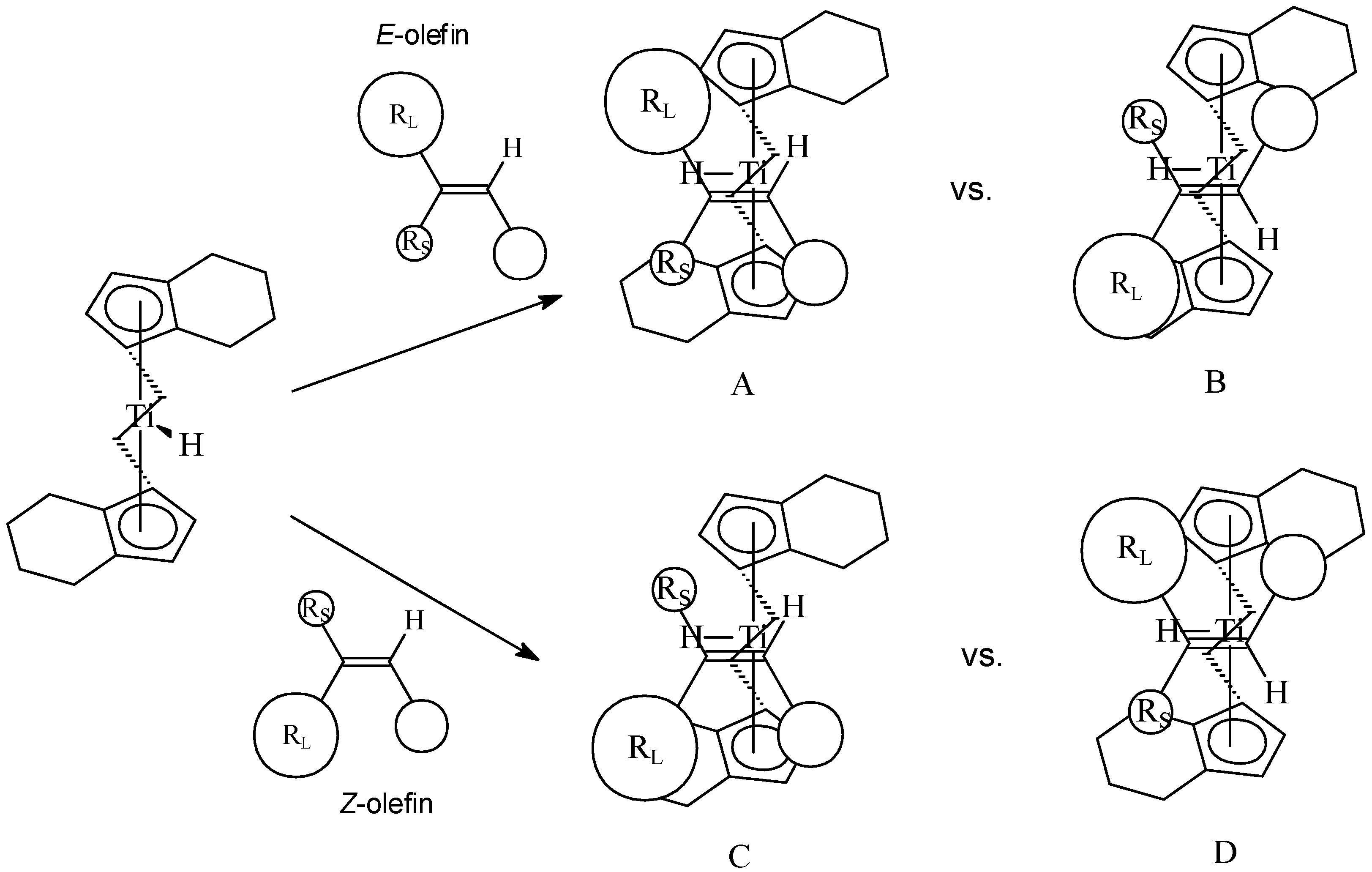
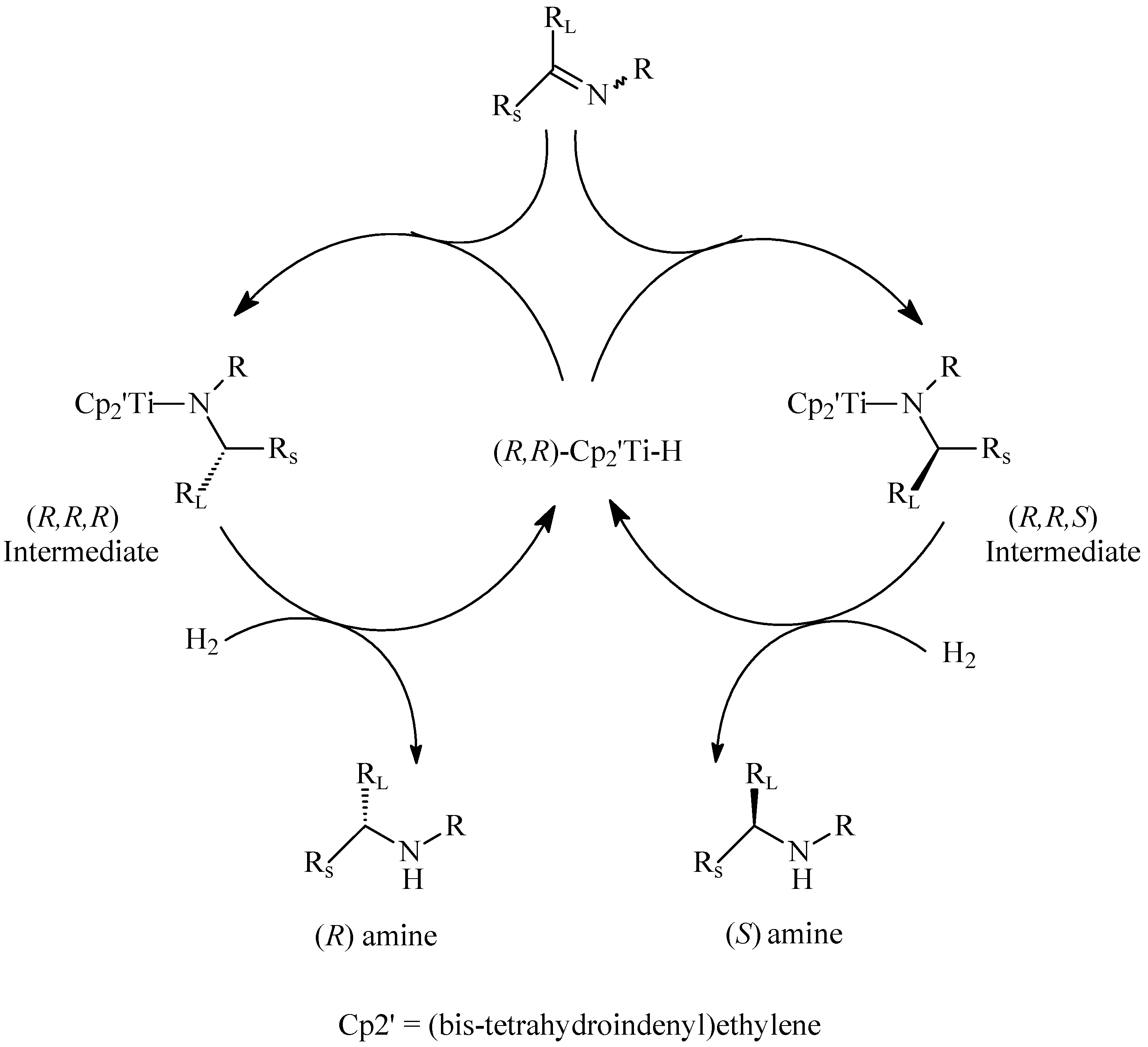
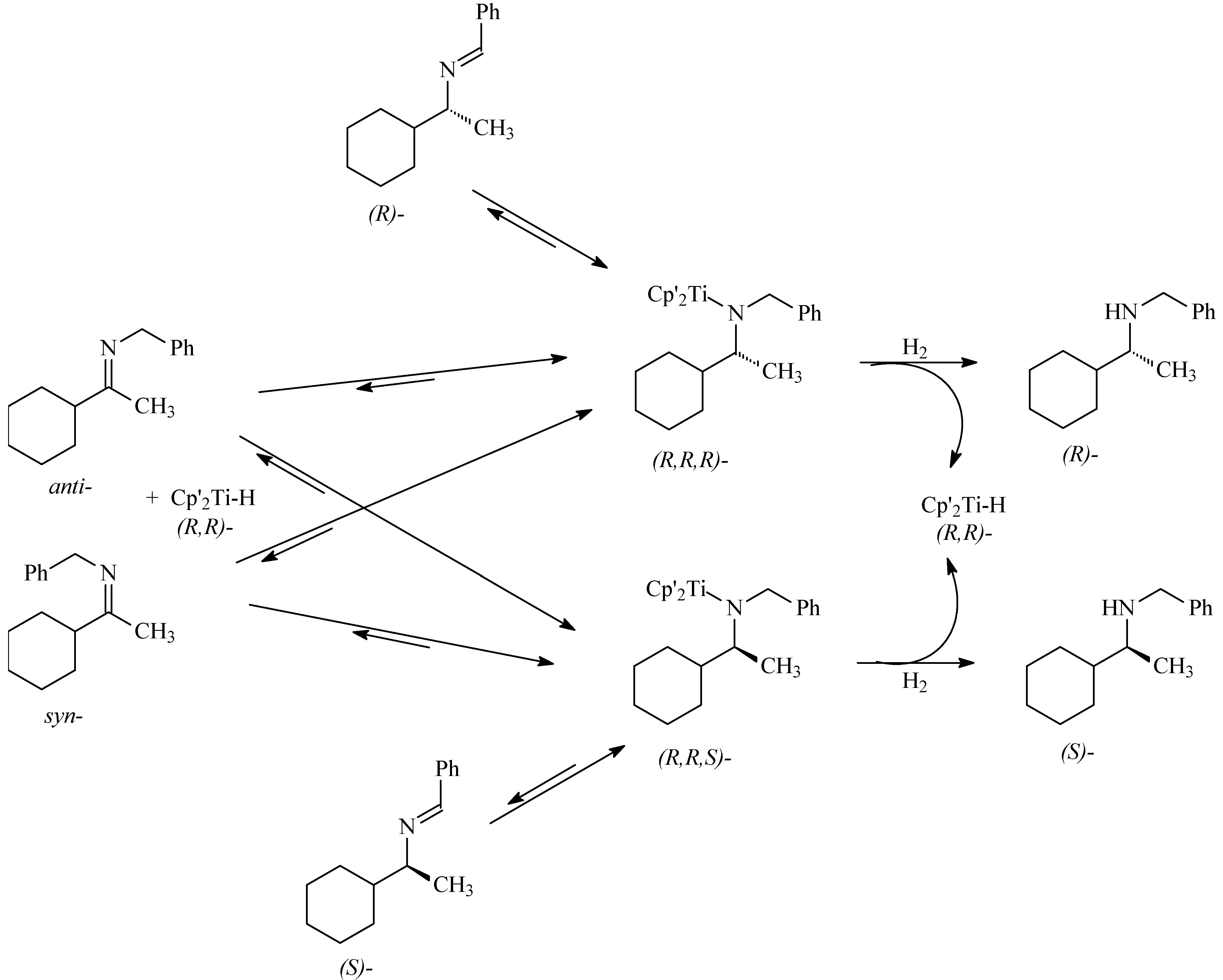
Hydrogenation
Hydrosilylation

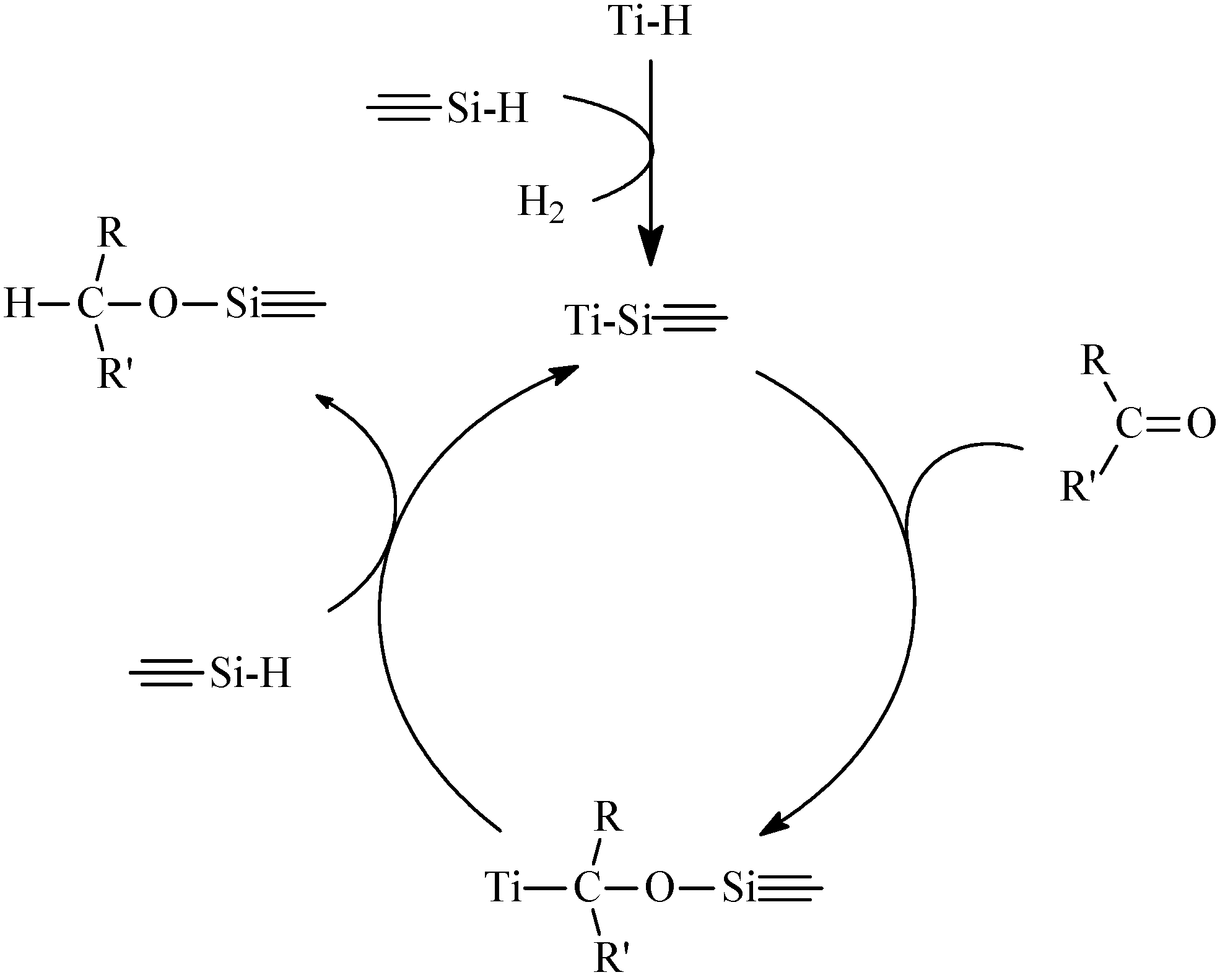
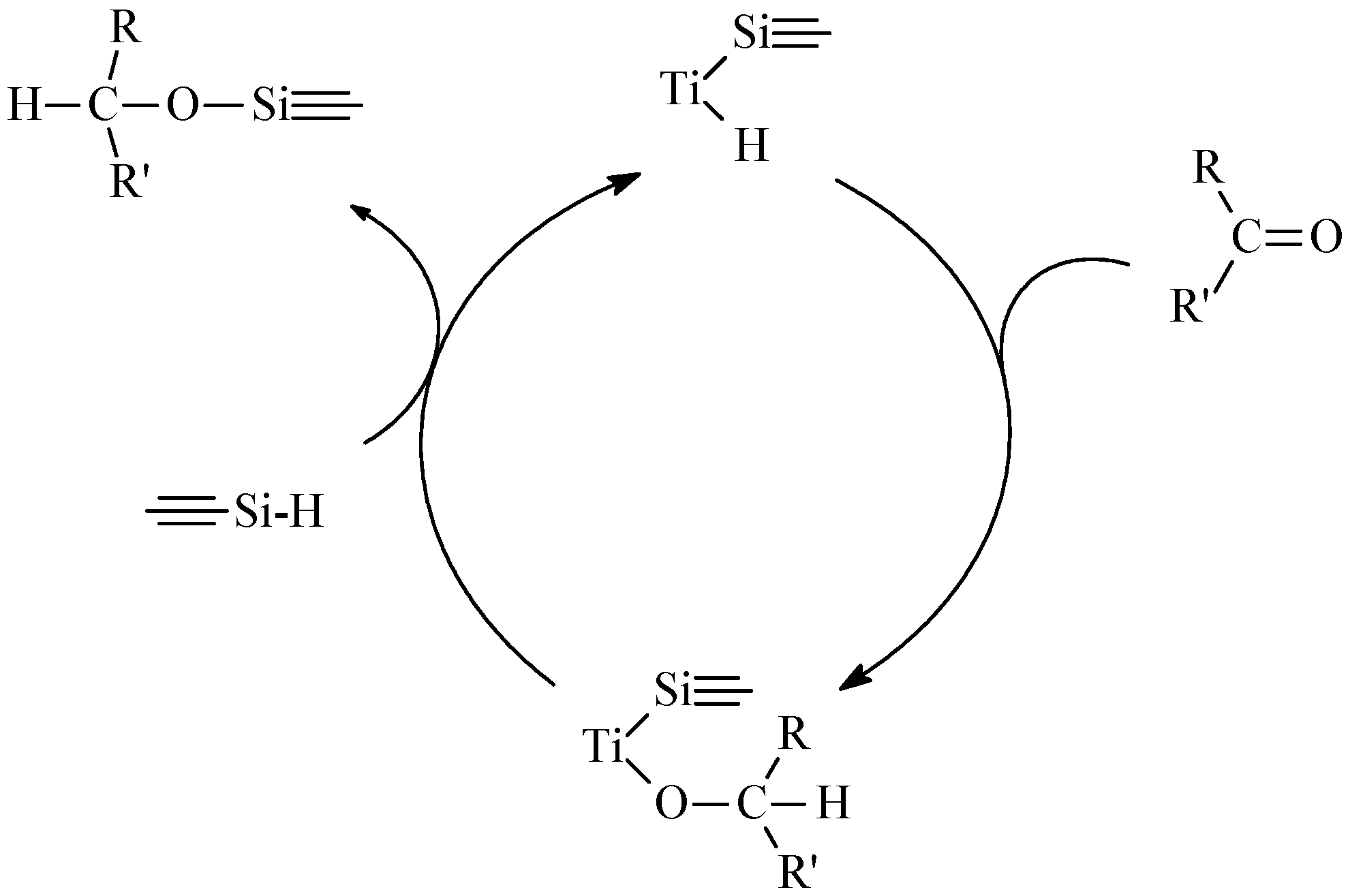
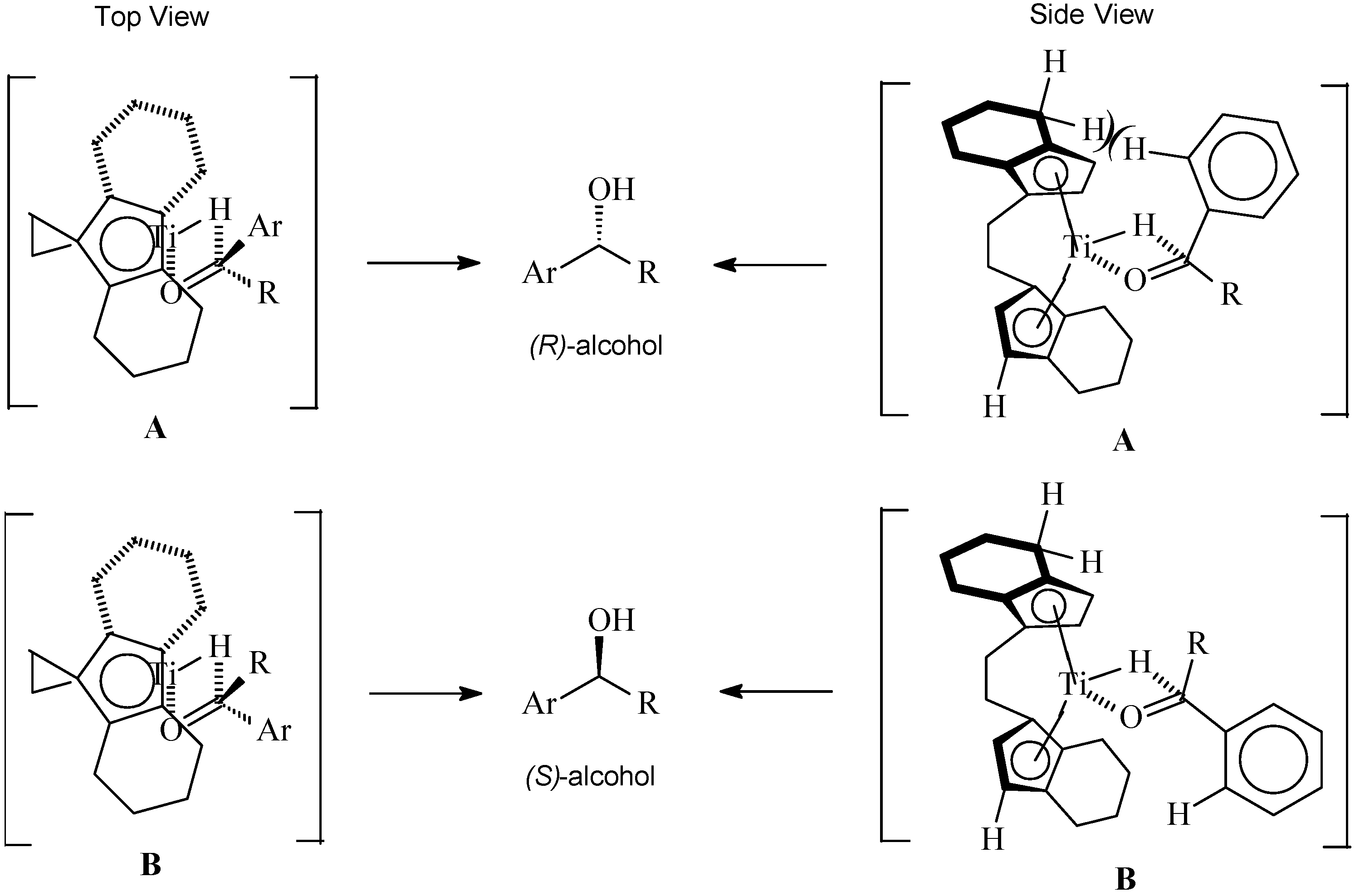
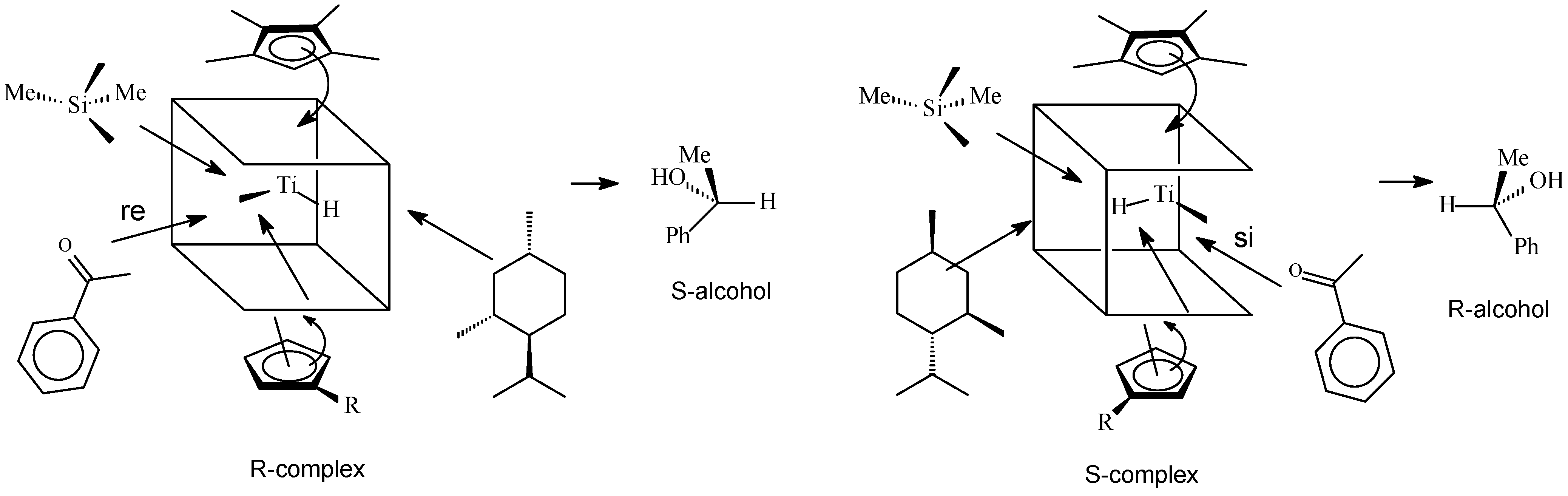
3.3. Effect of Additives and Solvents

3.4. Kinetic Studies
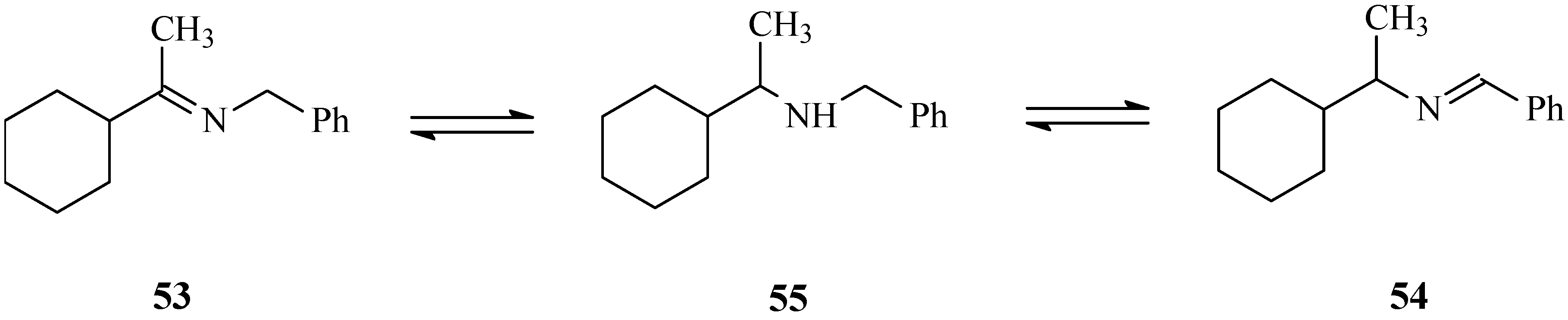
3.5. Catalyst Stability
| [(C5H5)2TiH]2 + CH2=CH2 → (C5H5)2TiCH2CH3 + (C5H5)2TiH | (1) |
| (C5H5)2TiCH2CH3 + (C5H5)2TiH → [(C5H5)2Ti]2 + CH3CH3 | (2) |
| [(C5H5)2Ti]2 + H2 → [(C5H5)2TiH]2 | (3) |
| [(C5H5)2Ti]2 → [(C5H5)(C5H4)TiH]2 (inactive) | (4) |
4. Heterogenization of Titanocene Catalysts
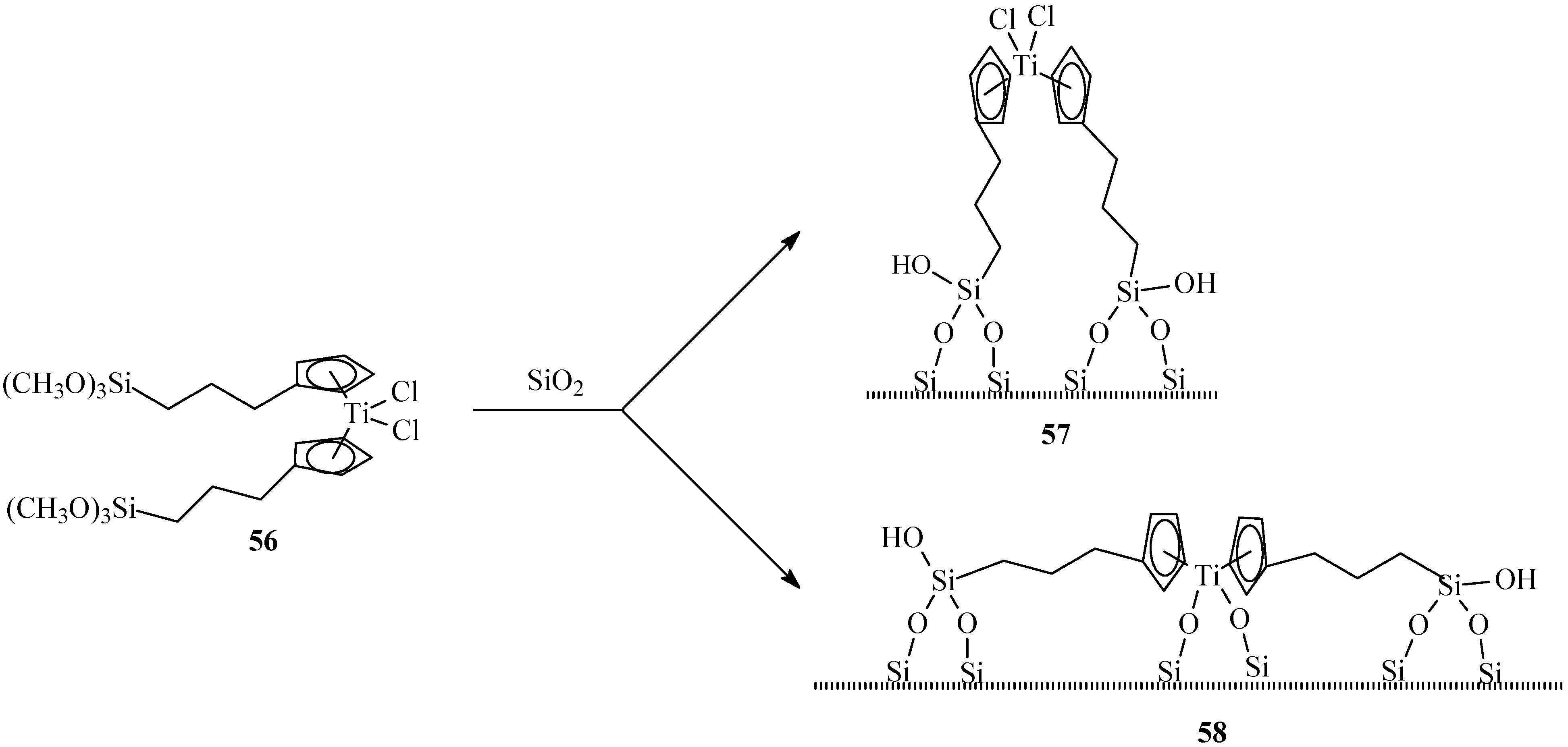
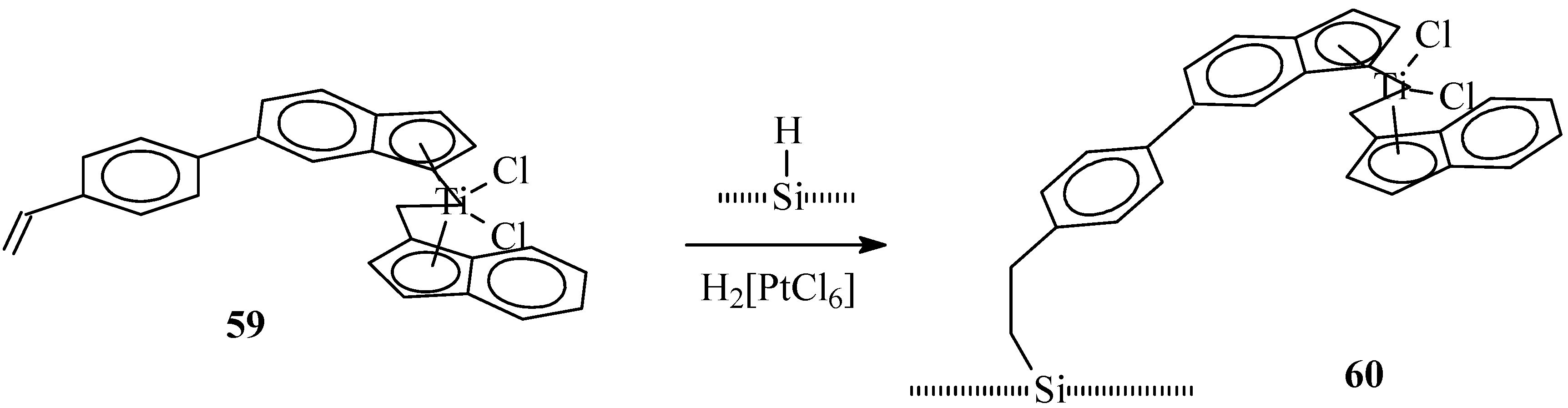
5. Summary and Outlook
- Increased academic research in close collaboration with industry should lead to a wider variety of ligands, complexes and reaction systems.
- Detailed and precise kinetic studies and the optimization of actual reaction systems need to be performed in order to fully exploit the potential of titanocenes in stereoselective hydrogenations. Furthermore, reactor and process design have to be integrated in the development of an actual catalytic reaction system [109]. Here, chemical engineers and chemists should interact closely.
- Computational chemistry should become a design tool for new catalysts. Computational chemistry has reached a level, where it is possible to make predictions to help guide experimental directions, resulting in more efficient use of experimental resources. Experiments will provide testing and calibration of the computational models, which in turn increases their value as experimental tools.
- Heterogenization of catalysts and the development of new and more effective heterogenization methods may further increase the applicability and value of these systems.
References
- Cole-Hamilton, D. Why asymmetric catalysis won the 2001 Nobel prize. IAS News 2002, 9, 4–5. [Google Scholar]
- Stinson, S. C. Chiral chemistry. Chem. Eng. News 2001, 79, 45–57. [Google Scholar] [CrossRef]
- Reetz, M. T. Strategies for the development of enantioselective catalysts. Pure Appl. Chem. 1999, 71, 1503–1509. [Google Scholar] [CrossRef]
- James, B. R. Synthesis of chiral amines catalyzed homogeneously by metal complexes. Catal Today 1997, 37, 209–221. [Google Scholar] [CrossRef]
- Blaser, H. U.; Malan, C.; Pugin, B.; Spindler, F.; Steiner, H.; Studer, M. Selective hydrogenation for fine chemicals: recent trends and new developments. Adv. Synth. Catal. 2003, 345, 103–151. [Google Scholar] [CrossRef]
- Blaser, H. U.; Spindler, F.; Studer, M. Enantioselective catalysis in fine chemicals production. Appl. Catal. A: (General) 2001, 221, 119–143. [Google Scholar]
- Cuenca, T.; Flores, J. C.; Royo, P. Dicyclopentadienyltitanium and -zirconium complexes as catalysts for hydrogenation of olefins. J. Organomet. Chem. 1993, 462, 191–201. [Google Scholar] [CrossRef]
- Halterman, R. L.; Togni, A. (Eds.) Metallocenes; Wiley: New York, 1998.
- Halterman, R. L. Synthesis and applications of chiral cyclopentadienylmetal complexes. Chem. Rev. 1992, 92, 965–94. [Google Scholar] [CrossRef]
- Besancon, J.; Tirouflet, J.; Top, S.; Ea, B. H. Titanocene derivatives having two pentahapto ligands with planar chirality. Separation and identification of different stereochemical forms. J. Organomet. Chem. 1977, 133, 37–51. [Google Scholar]
- Cesarotti, E.; Kagan, H. B.; Goddard, R.; Kruger, C. Synthesis of new ligands for transition metal complexes: menthyl- and neomenthylcyclopentadienes. J. Organomet. Chem. 1978, 162, 297–309. [Google Scholar]
- Paquette, L. A.; Sivik, M. R.; Bzowej, E. I.; Stanton, K. J. Catalytic enantioselective hydrogenation of 1,1-disubstituted olefins with optically active titanocene and zirconocene complexes containing either identical or different ligands. Organometallics 1995, 14, 4865–4878. [Google Scholar] [CrossRef]
- Halterman, R. L.; Vollhardt, K. P. C.; Welker, M. E.; Blaser, D.; Boese, R. A designed, enantiomerically pure, fused cyclopentadienyl ligand with C2 symmetry. Synthesis and use in enantioselective titanocene-catalyzed hydrogenations of alkenes. J. Am. Chem. Soc. 1987, 109, 8105–8107. [Google Scholar]
- Chen, Z.; Eriks, K.; Halterman, R. L. Asymmetric synthesis and metalation of C2-symmetric annulated bicyclooctylcyclopentadienes. Organometallics 1991, 10, 3449–3458. [Google Scholar] [CrossRef]
- Paquette, L. A.; Moriarty, K. J.; Menunier, P.; Gautheron, B.; Crocq, V. Isodicyclopentadienes and related molecules. Part 40. Stereocontrolled bifacial complexation of the isodicyclopenta-dienyl ligand to cyclopentadienyltitanium dichloride fragments. Organometallics 1988, 7, 1873–1875. [Google Scholar]
- Halterman, R. L.; Tretyakov, A. Synthesis of camphor-derived chiral cyclopentadienes via the Nazarov cyclization: preparation of chiral cis(cyclopentadienyl)zirconium and -titanium dichlorides. Tetrahedron 1995, 51, 4371–4382. [Google Scholar] [CrossRef]
- Yang, Q.; Jensen, M. D. Novel and efficient syntheses of bis(η5-tetrahydroindenyl) dichlorides of titanium, zirconium and hafnium. Synlett 1996, 6, 563–564. [Google Scholar] [CrossRef]
- Bajgur, C. S.; Tikkanen, W. R.; Petersen, J. L. Synthesis, structural characterization, and electrochemistry of [l]metallocenophane complexes, [Si(alkyl)2(C5H4)2]MCl2, M = Ti, Zr. Inorg. Chem. 1985, 24, 2539–2546. [Google Scholar] [CrossRef]
- Smith, J. A.; Brintzinger, H. H. Ansa-metallocene derivatives. III. Influence of an interannular ethylene bridge on the reactivity of titanocene derivatives. J. Organomet. Chem. 1981, 218, 159–67. [Google Scholar]
- Collins, S.; Kuntz, B. A.; Taylor, N. J.; Ward, D. G. X-ray structures of ethylenebis(tetrahydroindenyl)titanium and -zirconium dichlorides: a revision. J. Organomet. Chem. 1988, 342, 21–9. [Google Scholar] [CrossRef]
- Huttenloch, M. E.; Dorer, B.; Rief, U.; Prosenc, M.-H.; Schmidt, K.; Brintzinger, H. H. ansa-Metallocene derivatives. XXXIX. Biphenyl-bridged metallocene complexes of titanium, zirconium, and vanadium: syntheses, crystal structures and enantioseparation. J. Organomet. Chem. 1997, 541, 219–232. [Google Scholar]
- Shapiro, P. J. The evolution of the ansa-bridge and its effect on the scope of metallocene chemistry. Coord. Chem. Rev. 2002, 231, 67–81. [Google Scholar] [CrossRef]
- Schnutenhaus, H.; Britzinger, H.H. ansa-Metallocenes. 2. 1,1'-Trimethylene-bis(η5-3-tert-butylcyclopentadienyl)titanium(IV) dichloride, a chiral ansa-titanocene derivative. Angew. Chem. 1979, 91, 837–838. [Google Scholar]
- Wild, F. R. W. P.; Zsolnai, L.; Huttner, G.; Brintzinger, H. H. ansa-Metallocene derivatives. IV. Synthesis and molecular structures of chiral ansa-titanocene derivatives with bridged tetrahydroindenyl ligands. J. Organomet. Chem. 1982, 232, 233–47. [Google Scholar]
- Nantz, M. H.; Hitchcock, S. R.; Sutton, S. C.; Smith, M. D. A disulfone-based approach to ansa-titanocenes: synthesis of ethylene bis(2-indenyl)titanium dichloride. Organometallics 1993, 12, 5012–5015. [Google Scholar] [CrossRef]
- Hitchcock, S. R.; Situ, J. J.; Covel, J. A.; Olmstead, M. M.; Nantz, M. H. Synthesis of ansa-titanocenes from 1,2-bis(2-indenyl)ethane and structural comparisons in the catalytic epoxidation of unfunctionalized alkenes. Organometallics 1995, 14, 3732–3740. [Google Scholar] [CrossRef]
- Kelly, P. A.; Berger, G. O.; Wyatt, J. K.; Nantz, M. H. Synthesis of [ethylene-1-(η5-4,5,6,7-tetrahydro-1-indenyl)-2-(η5-4',5',6',7'-tetrahydro-2'-indenyl)]titanium dichloride, the elusive isomer of the Brintzinger-type ansa-titanocenes. J. Org. Chem. 2003, 68, 8447–8452. [Google Scholar] [CrossRef]
- Palandoken, H.; Wyatt, J. K.; Hitchcock, S. R.; Olmstead, M. M.; Nantz, M. H. Reductive dehydroxy coupling of 2-(hydroxymethyl)indenes to prepare ethano-bridged bis(2-indenyl) ansa-titanocenes. J. Organomet. Chem. 1999, 579, 338–347. [Google Scholar] [CrossRef]
- Halterman, R. L.; Ramsey, T. M.; Pailes, N. A.; Khan, M. A. Application of the double Pauson-Khand cyclization to the synthesis of bis(cyclopentadienes): preparation of phenyl-bridged bis(tetrahydroindenyl)titanium and zirconium dichlorides. J. Organomet. Chem. 1995, 497, 43–53. [Google Scholar] [CrossRef]
- Huttenloch, M. E.; Diebold, J.; Rief, U.; Brintzinger, H. H.; Gilbert, A. M.; Katz, T. J. ansa-Metallocene derivatives. 26. Biphenyl-bridged metallocenes that are chiral, configurationally stable, and free of diastereomers. Organometallics 1992, 11, 3600–3607. [Google Scholar]
- Halterman, R. L.; Ramsey, T. M. Asymmetric synthesis of a sterically rigid binaphthyl-bridged chiral metallocene: asymmetric catalytic epoxidation of unfunctionalized alkenes. Organometallics 1993, 12, 2879–80. [Google Scholar] [CrossRef]
- Burk, M. J.; Colletti, S. L.; Halterman, R. L. C2-symmetric 2,2'-dimethyl-1,1'-binaphthyl-bridged ansa-bis(1-indenyl)metal complexes. Organometallics 1991, 10, 2998–3000. [Google Scholar] [CrossRef]
- Halterman, R. L.; Zhu, C.; Chen, Z.; Dunlap, M. S.; Khan, M. A.; Nicholas; Kenneth, M. Preparation of [2,5-diisopropylcyclohexane-1,4-bis(indenyl)]titanium dichloride and [2,5-diisopropylcyclohexane-1,4-bis(tetrahydroindenyl)]- titanium dichloride and their comparison as catalysts for the enantioselective pinacol coupling of benzaldehyde. Organometallics 2000, 19, 3824–3829. [Google Scholar]
- Halterman, R. L.; Combs, D.; Kihega, J.; Khan, M. A. Synthesis of ansa-2,2'-bis[(4,7-dimethylinden-1-yl)methyl]-1,1'-binaphthyl and ansa-2,2'-bis[(4,5,6,7-tetrahydroinden-1-yl)methyl]-1,1'-binaphthyltitanium and -zirconium dichlorides. J. Organomet. Chem. 1996, 520, 163–170. [Google Scholar] [CrossRef]
- Halterman, R. L.; Schumann, H.; Dubner, F. Synthesis and structure of ansa-metallocene complexes (M = ZrCl2, TiCl2, YCl, and LuCl) containing the bis(2-methyl-4,5,6,7-tetrahydroindenyl)dimethylsilane ligand. J. Organomet. Chem. 2000, 604, 12–19. [Google Scholar] [CrossRef]
- Chen, Z.; Halterman, R. L. Enantioselective catalytic isomerization of an unfunctionalized achiral alkene. J. Amer. Chem. Soc. 1992, 114, 2276–2277. [Google Scholar] [CrossRef]
- Chen, Z.; Halterman, R. L. Chiral cyclopentane-1,3-diyl-bridged ansa-titanocene dichlorides. Organometallics 1994, 13, 3932–3942. [Google Scholar] [CrossRef]
- Roell, W.; Zsolnai, L.; Huttner, G.; Brintzinger, H. H. ansa-Metallocene derivatives. XI. Synthesis and crystal structure of a chiral ansa-titanocene derivative with trimethylene bridged tetrahydroindenyl ligands. J. Organomet. Chem. 1987, 322, 65–70. [Google Scholar]
- Horacek, M.; Stepnicka, P.; Gyepes, R.; Cisarova, I.; Tislerova, I.; Zemanek, J.; Kubista, J.; Mach, K. Reduction of bis[η5-(ω-alkenyl)tetramethylcyclopentadienyl]titanium dichlorides: an efficient synthesis of long-chain ansa-bridged titanocene dichlorides by acidolysis of cyclopentadienyl-ring-tethered titanacyclopentanes. Chem. A Eur. J. 2000, 6, 2397–2408. [Google Scholar] [CrossRef]
- Gibis, K.-L.; Helmchen, G; Huttner, G.; Zsolnai, L. Enantiomerically pure C2-symmetric bridged ferrocene and titanocene derivatives. J. Organomet. Chem. 1993, 445, 181–186. [Google Scholar]
- Halterman, R. L.; Chen, Z.; Khan, M. A. Synthesis, structure determination, and reactivity of C2-symmetrical ethylene-bridged ansa-Bis(DiMeBCOCp)titanium dichlorides. Organometallics 1996, 15, 3957–3967. [Google Scholar]
- Willoughby, C. A.; Davis, W. M.; Buchwald, S. L. Preparation and X-ray structure of a novel chiral methylene bridged titanocene complex. J. Organomet. Chem. 1995, 497, 11–15. [Google Scholar] [CrossRef]
- Wiesenfeldt, H.; Reinmuth, A.; Barsties, E.; Evertz, K.; Brintzinger, H. H. ansa-Metallocene derivatives. XVII. Racemic and meso diastereomers of Group IV metallocene derivatives with symmetrically substituted, dimethylsilanediyl-bridged ligand frameworks. Crystal structure of R,S-Me2Si(3-tert-Bu-5-MeC5H2)2ZrCl2. J. Organomet. Chem. 1989, 369, 359–370. [Google Scholar]
- Mise, T.; Miya, S.; Yamazaki, H. Excellent stereoregular isotactic polymerizations of propylene with C2-symmetric silylene-bridged metallocene catalysts. Chem. Lett. 1989, 10, 1853–1856. [Google Scholar] [CrossRef]
- Lee, M. H.; Han, Y.; Kim, D.; Hwang, J.-W.; Do, Y. Isospecific propylene polymerization by C1-symmetric Me2Si(C5Me4)(2-R-Ind)MCl2 (M = Ti, Zr) complexes. Organometallics 2003, 22, 2790–2796. [Google Scholar] [CrossRef]
- Antinolo, A.; Fajardo, M.; Gomez-Ruiz, S.; Lopez-Solera, I.; Otero, A.; Prashar, S.; Rodriguez, A. M. Group 4 metallocene complexes incorporating vinyl or allyl substituted ansa ligands. X-Ray crystal structures of [Zr{Me(CH2:CH)Si(η5-C5Me4)2}Cl2], [Zr{Me(CH2:CHCH2)Si(η5-C5H4)2}Cl2] and [Zr{Me(CH2:CHCH2)Si(η5-C5Me4)( η5-C5H4)}Cl2]. J. Organomet. Chem. 2003, 683, 11–22. [Google Scholar]
- Grossman, R. B.; Tsai, J.-C.; Davis, W. M.; Gutierrez, A.; Buchwald, S. L. Synthesis and structure of a C2-symmetric, doubly bridged ansa-titanocene complex. Organometallics 1994, 13, 3892–3896. [Google Scholar] [CrossRef]
- Halterman, R. L.; Tretyakov, A.; Combs, D.; Chang, J.; Khan, M. A. Synthesis and structure of C2-symmetric, doubly bridged bis(indenyl)titanium and -zirconium dichlorides. Organometallics 1997, 16, 3333–3339. [Google Scholar] [CrossRef]
- Dorer, B.; Prosenc, M.-H.; Rief, U.; Brintzinger, H. H. ansa-Metallocene derivatives. 30. syntheses and structures of titanocene, zirconocene, and vanadocene dichloride complexes with two ethanediyl bridges. Organometallics 1994, 13, 3868–3872. [Google Scholar]
- Cano, A.; Cuenca, T.; Gomez-Sal, P.; Royo, B.; Royo, P. Double-dimethylsilyl-bridged dicyclopentadienyl group 4 metal complexes. X-ray molecular structures of M[(Me2Si)2(η5-C5H3)2]Cl2 (M = Ti, Zr) and (TiCl3)2{μ-[(Me2Si)2(η5-C5H3)2]}. Organometallics 1994, 13, 1688–1694. [Google Scholar]
- Cano, A.; Cuenca, T.; Gomez-Sai, P.; Manzanero, A; Royo, P. Dicyclopentadienyl titanium and zirconium complexes with the double bridged bis(dimethylsilanodiyl) dicyclopentadienyl [(Me2Si)2(η5-C5H3)2]2-ligand: X-ray molecular structure of [Ti{(SiMe2)2(η5-C5H3)2}Me2]. J. Organomet. Chem. 1996, 526, 227–235. [Google Scholar]
- Bercaw, J. E.; Brintzinger, H. H. A metastable form of titanocene. Formation from a hydride complex and reactions with hydrogen, nitrogen, and carbon monoxide. J. Am. Chem. Soc. 1971, 93, 2046–2048. [Google Scholar]
- Chin, B.; Buchwald, S. L. An Improved procedure for the resolution of (rac)-ethylenebis(tetrahydroindenyl)titanium derivatives. J. Org. Chem. 1996, 61, 5650–5651. [Google Scholar] [CrossRef]
- Verdaguer, X.; Lange, U. E. W.; Reding, M. T.; Buchwald, S. L. Highly enantioselective imine hydrosilylation using (S,S)-ethylenebis(-tetrahydroindenyl)titanium difluoride. J. Am. Chem. Soc. 1996, 118, 6784–6785. [Google Scholar] [CrossRef]
- Buchwald, S. L.; Broene, R. D. Catalytic asymmetric reduction of trisubstituted olefins. US Pat. 5442119, 1995. [Google Scholar]
- Buchwald, S. L.; Lee, N. E. Catalytic asymmetric reduction of enamines. US Pat. 5489682, 1996. [Google Scholar]
- Buchwald, S. L.; Broene, R. D.; Lee, N. E. Catalytic asymmetric reduction of trisubstituted olefins. US Pat. 5491233, 1996. [Google Scholar]
- Buchwald, S. L.; Lee, N. E.; Broene, R. D. Catalytic asymmetric reduction of trisubstituted olefins and enamines. WO Pat. 9502567 A1, 1995. [Google Scholar]
- Sloan, M. F.; Matlack, A. S.; Breslow, D. S. Soluble catalysts for the hydrogenation of olefins. J. Am. Chem. Soc. 1963, 85, 4014–4018. [Google Scholar] [CrossRef]
- Cesarotti, E.; Ugo, R.; Vitiello, R. Chiral cyclopentadienyl[s] as ligands in homogeneous asymmetric catalysis. Part 1. Asymmetric hydrogenation of simple olefins by titanium(IV) complexes. J. Mol. Catal. 1981, 12, 63–9. [Google Scholar]
- Halterman, R. L.; Vollhardt, K. P. C. Synthesis and asymmetric reactivity of enantiomerically pure cyclopentadienylmetal complexes derived from the chiral pool. Organometallics 1988, 7, 883–892. [Google Scholar] [CrossRef]
- Beagley, P.; Davies, P. J.; Blacker, A. J.; White, C. Chiral metallocenes. 3. The enantioselective reduction of C=X bonds (X=O or CH2) by the chiral ansa-metallocenes (R)- or (S)-[TiCl2(η5:η5-C5Me4SiMe2C5H3R*)] (R* = menthyl or neomenthyl). Organometallics 2002, 21, 5852–5858. [Google Scholar]
- Willoughby, C. A.; Buchwald, S. L. Asymmetric titanocene-catalyzed hydrogenation of imines. J. Am. Chem. Soc. 1992, 114, 7562–7564. [Google Scholar] [CrossRef]
- Willoughby, C. A.; Buchwald, S. L. Catalytic asymmetric hydrogenation of imines with a chiral titanocene catalyst: scope and limitations. J. Am. Chem. Soc. 1994, 116, 8952–8965. [Google Scholar] [CrossRef]
- Willoughby, C. A.; Buchwald, S. L. Synthesis of highly enantiomerically enriched cyclic amines by the catalytic asymmetric hydrogenation of cyclic imines. J. Org. Chem. 1993, 58, 7627–7629. [Google Scholar] [CrossRef]
- Lee, N. E.; Buchwald, S. L. Asymmetric Hydrogenation of enamines with a chiral titanocene catalyst. J. Am. Chem. Soc. 1994, 116, 5985–5986. [Google Scholar] [CrossRef]
- Verdaguer, X.; Lange, U. E. W.; Buchwald, S. L. Imine hydrosilylation, uses and reagents related thereto. US Pat. 6072085, 2000. [Google Scholar]
- Tillack, A.; Lefeber, C.; Peulecke, N.; Thomas, D.; Rosenthal, U. The hydrosilylation of ald- and ketimines catalyzed by titanocene complexes. Tetrahedron Lett. 1997, 38, 1533–1534. [Google Scholar]
- Viso, A.; Lee, N. E.; Buchwald, S. L. Kinetic resolution of racemic disubstituted 1-pyrrolines via asymmetric reduction with a chiral titanocene catalyst. J. Am. Chem. Soc. 1994, 116, 9373–9374. [Google Scholar]
- Yun, J.; Buchwald, S. L. Efficient kinetic resolution in the asymmetric hydrosilylation of imines of 3-substituted indanones and 4-substituted tetralones. J. Org. Chem. 2000, 65, 767–774. [Google Scholar] [CrossRef]
- Yamamoto, K.; Uramoto, Y.; Kumada, M. Asymmetric hydrosilylation with a chiral phosphine-nickel(II) complex. J. Organom. Chem. 1971, 31, C9–C10. [Google Scholar]
- Halterman, R. L.; Ramsey, T. M.; Chen, Z. Catalytic asymmetric hydrosilation of aryl alkyl ketones with C2-symmetric chiral metallocene complexes. J. Org. Chem. 1994, 59, 2642–2644. [Google Scholar] [CrossRef]
- Carter, M. B.; Schiott, B.; Gutierrez, A.; Buchwald, S. L. Enantioselective hydrosilylation of ketones with a chiral titanocene catalyst. J. Am. Chem. Soc. 1994, 116, 11667–11670. [Google Scholar] [CrossRef]
- Rahimian, K.; Harrod, J. F. The Influence of the catalyst preparation protocol and silane structure on the rate and enantioselectivity of ansa-titanocene catalysed hydrosilylation of prochiral ketones. Inorg. Chim. Acta 1998, 270, 330–336. [Google Scholar] [CrossRef]
- Hironori, I.; Mori, M.; Nakai, T. Asymmetric catalytic hydrosilylation of ketones with triethoxysilane using a chiral binaphtol-titanium complex. Synlett 1996, 12, 1229–1230. [Google Scholar]
- Beagley, P.; Davies, P.; Adams, H.; White, C. Chiral metallocenes: the synthesis and X-ray crystal structures of TiCl2(η5:η5-C5Me4SiMe2C5H3R*) (R* = menthyl or neomenthyl) and related compounds. Can. J. Chem. 2001, 79, 731–741. [Google Scholar]
- Broene, R. D.; Buchwald, S. L. Asymmetric hydrogenation of unfunctionalized trisubstituted olefins with a chiral titanocene catalyst. J. Am. Chem. Soc. 1993, 115, 12569–12570. [Google Scholar] [CrossRef]
- Willoughby, C. A.; Buchwald, S. L. Catalytic asymmetric hydrogenation of imines with a chiral titanocene catalyst: kinetic and mechanistic investigations. J. Am. Chem. Soc. 1994, 116, 11703–11714. [Google Scholar] [CrossRef]
- Lee, S. J.; Han, B. H. Hydrosilylation of olefins catalyzed by activated titanocene prepared from the reduction of titanocene dichloride with excess lithium. Main Group Metal Chem. 1998, 21, 315–318. [Google Scholar] [CrossRef]
- Takahashi, T.; Bao, F.; Gao, G.; Ogasawara, M. Titanocene-catalyzed regioselective syn-hydrosilylation of alkynes. Org. Lett. 2003, 5, 3479–3481. [Google Scholar] [CrossRef]
- Verdaguer, X.; Lange, U. E. W.; Buchwald, S. L. Amine additives greatly expand the scope of asymmetric hydrosilylation of imines. Angew. Chem. Int. Ed. 1998, 37, 1103–1107. [Google Scholar] [CrossRef]
- Yun, J.; Buchwald, S. L. Titanocene-catalyzed asymmetric ketone hydrosilylation: the effect of catalyst activation protocol and additives on the reaction rate and enantioselectivity. J. Am. Chem. Soc. 1999, 121, 5640–5644. [Google Scholar] [CrossRef]
- Fan, Y.-H.; Liao, S.-J.; Xu, J.; Wang, F.-D.; Qian, Y.-L.; Huang, J.-L. Extremely active catalysts for the hydrogenation of terminal alkenes. J. Catal. 2002, 205, 294–298. [Google Scholar] [CrossRef]
- Woelfler, H.; Panarello, A.; Zaborenko, N.; Raupenstrauch, H.; Khinast, J. G. Homogeneous titanocene catalysts for stereoselective reductions: kinetic investigations and mechanistic studies. In Abstr. 14 Int. Symp. On Homogeneous Catalysis, Munich, Germany; 2004. [Google Scholar]
- Bercaw, J. E.; Marvich, R. H.; Bell, L. G.; Brintzinger, H. H. Titanocene as an intermediate in reactions involving molecular hydrogen and nitrogen. J. Am. Chem. Soc. 1972, 94, 1219–1232. [Google Scholar] [CrossRef]
- Sun, Q.; Liao, S.; Xu, Y.; Zhang, Y.; Yang, R.; Sun, R. Activity and stability of Cp2TiCl2/n-BuLi catalyst system for the hydrogenation of olefins. Cuihua Xuebao 1997, 18, 147–151, [Chem. Abstr. 126:239863]. [Google Scholar]
- Liao, S. J.; Xu, Y.; Zhang, Y. P.; Sun, Q.; Sun, R. A.; Yang, R. W. Factors affecting the catalytic activities of the Cp2TiCl2/n-BuLi system for the olefin hydrogenation. Chin. Chem. Lett. 1994, 5, 689–692. [Google Scholar]
- Fan, Y.-H.; Liao, S.-J.; Xu, J.; Qian, Y.-L.; Huang, J.-L. Highly active hydrogenation catalysts from titanocenes and sodium hydride of nanometric size. Gaodeng Xuexiao Huaxue Xuebao 1997, 18, 1683–1687, [Chem. Abstr. 127:307091]. [Google Scholar]
- Sun, Q.; Sun, R. Hydrogenation of olefins catalyzed by highly active titanocene/NaH or n-BuLi catalyst systems. Chem. Res. Chin. Univ. 2002, 18, 307–310, [Chem. Abstr. 138:122308]. [Google Scholar]
- De Vos, D. E.; Vankelecom, I. F. J.; Jacobs, P. A. (Eds.) Chiral catalyst immobilization and recycling; Wiley-VCH: Weinheim (Germany), 2000.
- Clark, J. H.; Macquarrie, D. J. Catalysis of liquid phase organic reactions using chemically modified mesoporous inorganic solids. Chem. Commun. 1998, 8, 853–860. [Google Scholar] [CrossRef]
- Clark, J. H. Catalysis for green chemistry. Pure Appl. Chem. 2001, 73, 103–111. [Google Scholar] [CrossRef]
- Bonds, W. D., Jr.; Brubaker, C. H., Jr.; Chandrasekaran, E. S.; Gibbons, C.; Grubbs, R. H.; Kroll, L. C. Polystyrene attached titanocene species. Preparation and reactions. J. Amer. Chem. Soc. 1975, 97, 2128–2132. [Google Scholar] [CrossRef]
- Corma, A.; Diaz, U.; Fornes, V.; Jorda, M. D.; Rey, F. Ti/ITQ-2, a new material highly active and selective for the epoxidation of olefins with organic hydroperoxides. Chem. Commun. 1999, 9, 779–780. [Google Scholar] [CrossRef]
- Maschmeyer, T.; Rey, F.; Sankar, G.; Thomas, J. M. Heterogeneous catalysts obtained by grafting metallocene complexes onto mesoporous silica. Nature (London) 1995, 378, 159–62. [Google Scholar] [CrossRef]
- Capka, M.; Reissova, A. Hydrogenation activity of homogeneous and heterogenized cyclopentadienyl titanium complexes. Coll. Czech. Chem. Comm. 1989, 54, 1760–1769. [Google Scholar] [CrossRef]
- Ferreira, P.; Goncalves, I. S.; Kuhn, F. E.; Pillinger, M.; Rocha, J.; Santos, A. M.; Thursfield, A. Structural studies and catalytic activity of MCM-41 and MCM-48 modified with the titanocenophane [SiMe2(η5-C5H4)2]TiCl2. Eur. J. Inorg. Chem. 2000, 3, 551–557. [Google Scholar] [CrossRef]
- Guimaraes, R.; Stedile, F. C.; dos Santos, J. H. Z. Ethylene polymerization with catalyst systems based on supported metallocenes with varying steric hindrance. J. Mol. Catal. A: Chem. 2003, 206, 353–362. [Google Scholar]
- Yun, S.-H.; Bu, J.; Rhee, H.-K. Grafting titanocene on hydrophilic amorphous silica: synthesis of an effective catalyst for olefin epoxidation. React. Kinet. Catal. Lett. 2001, 72, 343–353. [Google Scholar] [CrossRef]
- Panarello, A. P.; Khinast, J. G. Synthesis of a novel ethylene-bis(tetrahydroindenyl) ligand containing a functionalized four-carbon tether. Tetr. Lett. 2003, 44, 4095–4098. [Google Scholar] [CrossRef]
- Panarello, A.; Vassylyev, O.; Khinast, J. G. Selective alkylation and Suzuki Coupling as a strategy for introducing functional anchors to the ethylene-bis(indenyl) ligand. Tetrahedron Lett. 2005, 46, 1353–1356. [Google Scholar] [CrossRef]
- Panarello, A.; Woelfler, H.; Vassylyev, O.; Raupenstrauch, H.; Khinast, J. G. Synthesis and kinetic investigation of a novel chiral heterogeneous titanocene catalyst. In Abstr. 2003 AIChE Ann. Meet., San Francisco, CA; 2003. abstr. 511e. [Google Scholar]
- Ofunne, G. C.; Booth, B. L.; Tait, P. J. T. Characterization and polymerization studies on silica-supported titanium (IV) complexes. Ind. J. Chem. 1988, 27A, 1040–1046. [Google Scholar]
- Antberg, M.; Boehm, L.; Rohrmann, J. Process for the preparation of a heterogeneous metallocene catalyst component. US Pat. 5071808, 1991. [Google Scholar]
- Booth, B. L.; Ofunne, G. C.; Stasey, C.; Tait, P. J. T. Silica-supported cyclopentadienyl-rhodium(I), -cobalt(I), and –titanium(IV) complexes. J. Organomet. Chem. 1986, 315, 143–156. [Google Scholar]
- Panarello, A.; Vassylyev, O.; Khinast, J. G. Preparation of a novel heterogeneous titanocene catalyst for chiral reduction and asymmetric polymerization. In Abstr. 228th ACS Nat. Meet., Philadelphia, PA; 2004. [Google Scholar]
- Wiles, J. A.; Bergens, S. H. Mechanistic investigations of an enantioselective hydrogenation catalyzed by a ruthenium-BINAP complex. 1. Stoichiometric and catalytic labelling studies. Organometallics 1998, 17, 2228–2240. [Google Scholar]
- Surpateanu, G.; Agbossou, F.; Carpentier, J.-F.; Mortreux, A. Rhodium dihydride complexes as models for the theoretical analysis of enantioselective hydrogenation reactions. Tetr. Asymmetr. 1998, 9, 2259–2270. [Google Scholar] [CrossRef]
- Chaudhari, R. V. Kinetics of catalytic reactions. In “Catalysis: Principles and Applications”; Viswanathan, B., Sivasanker, S., Ramaswamy, A. V., Eds.; Narosa Publishing House: Madras (India), 2002; pp. 184–205. [Google Scholar]
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Vassylyev, O.; Panarello, A.; Khinast, J. Enantioselective Hydrogenations with Chiral Titanocenes. Molecules 2005, 10, 587-619. https://doi.org/10.3390/10060587
Vassylyev O, Panarello A, Khinast J. Enantioselective Hydrogenations with Chiral Titanocenes. Molecules. 2005; 10(6):587-619. https://doi.org/10.3390/10060587
Chicago/Turabian StyleVassylyev, O., A. Panarello, and J. Khinast. 2005. "Enantioselective Hydrogenations with Chiral Titanocenes" Molecules 10, no. 6: 587-619. https://doi.org/10.3390/10060587




