Department of Pharmaceutical and Toxicological Chemistry, Medical Institute, Peoples’ Friendship University of Russia (RUDN University), 6 Miklukho-Maklaya Street, 117198 Moscow, Russia
Pharmaceutics 2022, 14(4), 767; https://doi.org/10.3390/pharmaceutics14040767 - 31 Mar 2022
Cited by 12 | Viewed by 2947
Abstract
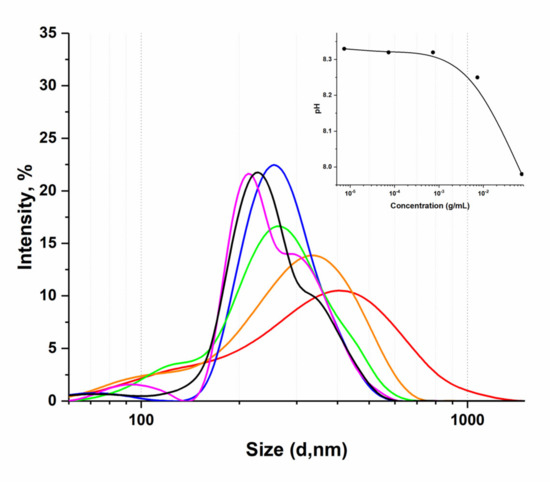
In the present work, the methods of dynamic light scattering and fluorescence spectroscopy were applied to study the optical properties of aqueous dilutions of the humic substances complex (HC) as a potential drug delivery system. The supramolecular structures in the humate solution were
[...] Read more.
In the present work, the methods of dynamic light scattering and fluorescence spectroscopy were applied to study the optical properties of aqueous dilutions of the humic substances complex (HC) as a potential drug delivery system. The supramolecular structures in the humate solution were characterized as monodisperse systems of the submicron range with a tendency to decrease in particle size with a decrease in the dry matter concentration. The slightly alkaline medium (8.3) of the studied aqueous dilutions of HC causes the absence of a pronounced fluorescence maximum in the region from 400 to 500 nm. However, the presence of an analytically significant, inversely proportional to the concentration second-order scattering (SOS) signal at 2λex = λem was shown. In the examples of the antiviral substances mangiferin and favipiravir, it was shown that the use of the humic complex as a drug carrier makes it possible to increase the solubility by several times and simultaneously obtain a system with a smaller particle size of the dispersed phase. It has been shown that HC can interact with mangiferin and favipiravir to form stable structures, which lead to a significant decrease in SOS intensities on HC SOS spectra. The scattering wavelengths, λex/λem, were registered at 350 nm/750 nm for mangiferin and 365 nm/730 nm for favipiravir, respectively. The increments of the scattering intensities (I0/I) turned out to be proportional to the concentration of antiviral components in a certain range of concentrations.
Full article
(This article belongs to the Special Issue New Properties of Supramolecular Complexes and Drug Nanoparticles)
▼
Show Figures