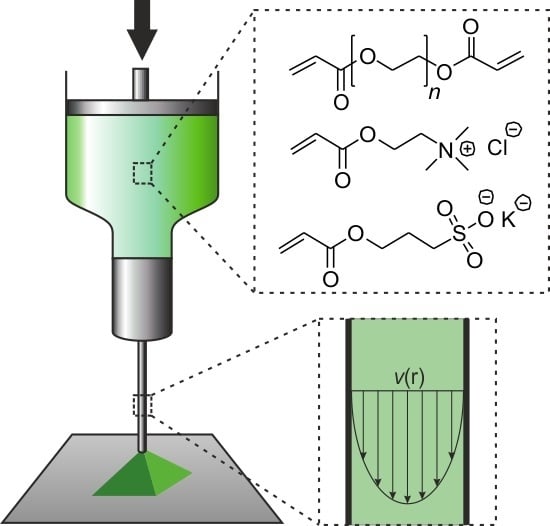
Extrusion-Based 3D Printing of Poly(ethylene glycol) Diacrylate Hydrogels Containing Positively and Negatively Charged Groups
Abstract
:1. Introduction
2. Results
2.1. Physical Properties of Cross-Linked Hydrogels
2.2. Physical Gelation of Hydrogel Formulations with Charged Monomers
2.3. Flow Properties of Hydrogel Formulations with Charged Monomers
2.4. Influence of Yield Stress and Shear Thinning on Flow Profiles
2.5. Viscosity Recovery after Shearing
2.6. Extrusion-Based 3D Printing of Hydrogel Formulations
3. Discussion
3.1. Physical Properties of Cross-Linked Hydrogels
3.2. Physical Gelation of Hydrogel Formulations with Charged Monomers
3.3. Yield Stress, Shear Thinning, Viscosity Recovery after Shearing, and 3D Printing of Hydrogel Formulations with Charged Monomers
4. Materials and Methods
4.1. Chemicals
4.2. Analytical Methods
4.3. Preparation of Hydrogel Formulations
4.4. Preparation and Characterization of Chemical Hydrogels
4.5. File Preparation and Device Setup for Extrusion-Based 3D Printing
4.6. Calculation of Flow Profiles during Extrusion-Based 3D Printing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bakarich, S.E.; Gorkin, R., III; Gately, R.; Naficy, S.; in het Panhuis, M.; Spinks, G.M. 3D printing of tough hydrogel composites with spatially varying materials properties. Addit. Manuf. 2017, 14, 24–30. [Google Scholar] [CrossRef]
- Panwar, A.; Tan, L. Current Status of Bioinks for Micro-Extrusion-Based 3D Bioprinting. Molecules 2016, 21, 685. [Google Scholar] [CrossRef] [PubMed]
- Jungst, T.; Smolan, W.; Schacht, K.; Scheibel, T.; Groll, J. Strategies and Molecular Design Criteria for 3D Printable Hydrogels. Chem. Rev. 2016, 116, 1496–1539. [Google Scholar] [CrossRef] [PubMed]
- Oyen, M.L. Mechanical characterisation of hydrogel materials. Int. Mater. Rev. 2014, 59, 44–59. [Google Scholar] [CrossRef]
- Anseth, K.S.; Bowman, C.N.; Brannon-Peppas, L. Mechanical properties of hydrogels and their experimental determination. Biomaterials 1996, 17, 1647–1657. [Google Scholar] [CrossRef]
- Chawla, P.; Srivastava, A.R.; Pandey, P.; Chawla, V. Hydrogels: A journey from diapers to gene delivery. Mini-Rev. Med. Chem. 2014, 14, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Southan, A.; Hagel, V.; Mateescu, M.; Bach, M.; Schuh, C.; Kleinhans, C.; Kluger, P.J.; Tussetschläger, S.; Nuss, I.; Haraszti, T.; et al. Towards Controlling the Formation, Degradation Behavior and Properties of Hydrogels Synthesized by Aza-Michael Reactions. Macromol. Chem. Phys. 2013, 214, 1865–1873. [Google Scholar] [CrossRef]
- Williams, C.G.; Malik, A.N.; Kim, T.K.; Manson, P.N.; Elisseeff, J.H. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials 2005, 26, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.S.; Schon, B.S.; Mekhileri, N.V.; Brown, G.C.J.; Chia, C.M.; Prabakar, S.; Hooper, G.J.; Woodfield, T.B.F. New Visible-Light Photoinitiating System for Improved Print Fidelity in Gelatin-Based Bioinks. ACS Biomater. Sci. Eng. 2016, 2, 1752–1762. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose–Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.W.; Dhert, W.J.A.; Hutmacher, D.W.; Malda, J. Development and characterisation of a new bioink for additive tissue manufacturing. J. Mater. Chem. B 2014, 2, 2282–2289. [Google Scholar] [CrossRef]
- Bonten, C. Kunststofftechnik: Einführung und Grundlagen, 2nd ed.; Carl Hanser Verlag: München, Germany, 2016. [Google Scholar]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagel, V.; Mateescu, M.; Southan, A.; Wegner, S.V.; Nuss, I.; Haraszti, T.; Kleinhans, C.; Schuh, C.; Spatz, J.P.; Kluger, P.J.; et al. Desmosine-Inspired Cross-Linkers for Hyaluronan Hydrogels. Sci. Rep. 2013, 3, 2043. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lang, Q.; Yildirimer, L.; Lin, Z.Y.; Cui, W.; Annabi, N.; Ng, K.W.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Khademhosseini, A. Photocrosslinkable Gelatin Hydrogel for Epidermal Tissue Engineering. Adv. Healthc. Mater. 2016, 5, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Hoch, E.; Hirth, T.; Tovar, G.E.M.; Borchers, K. Chemical tailoring of gelatin to adjust its chemical and physical properties for functional bioprinting. J. Mater. Chem. B 2013, 1, 5675–5685. [Google Scholar] [CrossRef]
- Luckanagul, J.; Lee, L.A.; Nguyen, Q.L.; Sitasuwan, P.; Yang, X.; Shazly, T.; Wang, Q. Porous Alginate Hydrogel Functionalized with Virus as Three-Dimensional Scaffolds for Bone Differentiation. Biomacromolecules 2012, 13, 3949–3958. [Google Scholar] [CrossRef] [PubMed]
- Davidovich-Pinhas, M.; Harari, O.; Bianco-Peled, H. Evaluating the mucoadhesive properties of drug delivery systems based on hydrated thiolated alginate. J. Controll. Release 2009, 136, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.H.; Kurokawa, T.; Katsuyama, Y.; Tsukeshiba, H.; Gong, J.P.; Osada, Y.; Okabe, S.; Karino, T.; Shibayama, M. Structural Characteristics of Double Network Gels with Extremely High Mechanical Strength. Macromolecules 2004, 37, 5370–5374. [Google Scholar] [CrossRef]
- Leone, G.; Consumi, M.; Greco, G.; Bonechi, C.; Lamponi, S.; Rossi, C.; Magnani, A. A PVA/PVP hydrogel for human lens substitution: Synthesis, rheological characterization, and in vitro biocompatibility. J. Biomed. Mater. Res. Part B 2011, 97B, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Sokolovskaya, E.; Barner, L.; Bräse, S.; Lahann, J. Synthesis and On-Demand Gelation of Multifunctional Poly(ethylene glycol)-Based Polymers. Macromol. Rapid Commun. 2014, 35, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Tong, X.; Yang, F. The effects of varying poly(ethylene glycol) hydrogel crosslinking density and the crosslinking mechanism on protein accumulation in three-dimensional hydrogels. Acta Biomater. 2014, 10, 4167–4174. [Google Scholar] [CrossRef] [PubMed]
- Mouser, V.H.M.; Melchels, F.P.W.; Visser, J.; Dhert, W.J.A.; Gawlitta, D.; Malda, J. Yield stress determines bioprintability of hydrogels based on gelatin-methacryloyl and gellan gum for cartilage bioprinting. Biofabrication 2016, 8, 035003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraut, G.; Yenchesky, L.; Prieto, F.; Tovar, G.E.M.; Southan, A. Influence of shear thinning and material flow on robotic dispensing of poly(ethylene glycol) diacrylate/poloxamer 407 hydrogels. J. Appl. Polym. Sci. 2017. [Google Scholar] [CrossRef]
- He, Y.; Yang, F.; Zhao, H.; Gao, Q.; Xia, B.; Fu, J. Research on the printability of hydrogels in 3D bioprinting. Sci. Rep. 2016, 6, 29977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellott, M.B.; Searcy, K.; Pishko, M.V. Release of protein from highly cross-linked hydrogels of poly(ethylene glycol) diacrylate fabricated by UV polymerization. Biomaterials 2001, 22, 929–941. [Google Scholar] [CrossRef]
- Jo, Y.S.; Gantz, J.; Hubbell, J.A.; Lutolf, M.P. Tailoring hydrogel degradation and drug release via neighboring amino acid controlled ester hydrolysis. Soft Matter 2009, 5, 440–446. [Google Scholar] [CrossRef]
- Lin, C.C.; Raza, A.; Shih, H. PEG hydrogels formed by thiol-ene photo-click chemistry and their effect on the formation and recovery of insulin-secreting cell spheroids. Biomaterials 2011, 32, 9685–9695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperinde, J.J.; Griffith, L.G. Synthesis and Characterization of Enzymatically-Cross-Linked Poly(ethylene glycol) Hydrogels. Macromolecules 1997, 30, 5255–5264. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Hubbell, J.A. Synthesis and Physicochemical Characterization of End-Linked Poly(ethylene glycol)-co-peptide Hydrogels Formed by Michael-Type Addition. Biomacromolecules 2003, 4, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Bragg, J.C.; Lin, C.C. Designing Visible Light-Cured Thiol-Acrylate Hydrogels for Studying the HIPPO Pathway Activation in Hepatocellular Carcinoma Cells. Macromol. Biosci. 2016, 16, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G.B.; English, A.; Abraham, M.; Zaharias, R.; Stanford, C.; Keller, J. The effect of hydrogel charge density on cell attachment. Biomaterials 2004, 25, 3023–3028. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.J.; Quinn, T.; Bryant, S.J. Nondestructive evaluation of a new hydrolytically degradable and photo-clickable PEG hydrogel for cartilage tissue engineering. Acta Biomater. 2016, 39, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kermis, H.R.; Kostov, Y.; Rao, G. Rapid method for the preparation of a robust optical pH sensor. Analyst 2003, 128, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Xu, X.; Deng, T.; Yin, M.; Zhang, X.; Wang, J. Fabrication of positively charged poly(ethylene glycol)-diacrylate hydrogel as a bone tissue engineering scaffold. Biomed. Mater. 2012, 7, 055009. [Google Scholar] [CrossRef] [PubMed]
- Cha, C.; Kim, E.S.; Kim, I.W.; Kong, H. Integrative design of a poly(ethylene glycol)-poly(propylene glycol)-alginate hydrogel to control three dimensional biomineralization. Biomaterials 2011, 32, 2695–2703. [Google Scholar] [CrossRef] [PubMed]
- Southan, A.; Hoch, E.; Schönhaar, V.; Borchers, K.; Schuh, C.; Müller, M.; Bach, M.; Tovar, G.E.M. Side Chain Thiol-functionalized Poly(ethylene glycol) by Post-polymerization Modification of Hydroxyl Groups: Synthesis, Crosslinking and Inkjet Printing. Polym. Chem. 2014, 5, 5350–5359. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarker, M.; Chen, X.B. Modeling the Flow Behavior and Flow Rate of Medium Viscosity Alginate for Scaffold Fabrication With a Three-Dimensional Bioplotter. J. Manuf. Sci. Eng. 2017, 139, 081002. [Google Scholar] [CrossRef]
- Markus, F.; Dreher, F.; Laschat, S.; Baudis, S.; Tovar, G.E.M.; Southan, A. Physically and chemically gelling hydrogel formulations based on poly(ethylene glycol) diacrylate and Poloxamer 407. Polymer 2017, 108, 21–28. [Google Scholar] [CrossRef]
- Hiller, A.; Borchers, K.; Tovar, G.E.M.; Southan, A. Impact of intermediate UV curing and yield stress of 3D printed poly(ethylene glycol) diacrylate hydrogels on interlayer connectivity and maximum build height. Addit. Manuf. 2017, 18, 136–144. [Google Scholar] [CrossRef]
- Naomi, P.; Willi, S.; Thomas, B.; Ferry, M.; Jürgen, G.; Tomasz, J. Proposal to assess printability of bioinks for extrusion-based bioprinting and evaluation of rheological properties governing bioprintability. Biofabrication 2017, 9, 044107. [Google Scholar] [Green Version]
- Olsen, B.D.; Kornfield, J.A.; Tirrell, D.A. Yielding Behavior in Injectable Hydrogels from Telechelic Proteins. Macromolecules 2010, 43, 9094–9099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, C.; Mackay, M.E.; Czymmek, K.; Nagarkar, R.P.; Schneider, J.P.; Pochan, D.J. Injectable Solid Peptide Hydrogel as a Cell Carrier: Effects of Shear Flow on Hydrogels and Cell Payload. Langmuir 2012, 28, 6076–6087. [Google Scholar] [CrossRef] [PubMed]
- Aguado, B.A.; Mulyasasmita, W.; Su, J.; Lampe, K.J.; Heilshorn, S.C. Improving Viability of Stem Cells During Syringe Needle Flow Through the Design of Hydrogel Cell Carriers. Tissue Eng. Part A 2012, 18, 806–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalaal, M.; Cottrell, G.; Balmforth, N.; Stoeber, B. On the rheology of Pluronic F127 aqueous solutions. J. Rheol. 2017, 61, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Goel, N.K.; Kumar, V.; Bhardwaj, Y.K.; Chaudhari, C.V.; Dubey, K.A.; Sabharwal, S. Swelling Response of Radiation Synthesized 2-Hydroxyethylmethacrylate-co-[2-(methacryloyloxy)ethyl] Trimethylammonium Chloride Hydrogels Under Various In Vitro Conditions. J. Biomater. Sci. Polym. Ed. 2009, 20, 785–805. [Google Scholar] [CrossRef] [PubMed]
- Quesada-Perez, M.; Alberto Maroto-Centeno, J.; Forcada, J.; Hidalgo-Alvarez, R. Gel swelling theories: The classical formalism and recent approaches. Soft Matter 2011, 7, 10536–10547. [Google Scholar] [CrossRef]
- Wada, N.; Yagi, Y.; Inomata, H.; Saito, S. Swelling behavior of N-isopropylacrylamide-based amphoteric gels. J. Polym. Sci. Part A 1993, 31, 2647–2651. [Google Scholar] [CrossRef] [Green Version]
- Melekaslan, D.; Okay, O. Swelling of strong polyelectrolyte hydrogels in polymer solutions: Effect of ion pair formation on the polymer collapse. Polymer 2000, 41, 5737–5747. [Google Scholar] [CrossRef]
- Kloxin, A.M.; Kloxin, C.J.; Bowman, C.N.; Anseth, K.S. Mechanical Properties of Cellularly Responsive Hydrogels and Their Experimental Determination. Adv. Mater. 2010, 22, 3484–3494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belfer, S.; Fainchtain, R.; Purinson, Y.; Kedem, O. Surface characterization by FTIR-ATR spectroscopy of polyethersulfone membranes-unmodified, modified and protein fouled. J. Membr. Sci. 2000, 172, 113–124. [Google Scholar] [CrossRef]
- Khattak, S.F.; Bhatia, S.R.; Roberts, S.C. Pluronic F127 as a Cell Encapsulation Material: Utilization of Membrane-Stabilizing Agents. Tissue Eng. 2005, 11, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Schmolka, I.R. Artificial skin I. Preparation and properties of pluronic F-127 gels for treatment of burns. J. Biomed. Mater. Res. 1972, 6, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Li, C.; Lombardi, J.; Colby, R.H.; Rigas, B.; Rafailovich, M.H.; Sokolov, J.C. The effect of physiologically relevant additives on the rheological properties of concentrated Pluronic copolymer gels. Polymer 2008, 49, 3561–3567. [Google Scholar] [CrossRef]
- Gilbert, J.C.; Richardson, J.L.; Davies, M.C.; Palin, K.J.; Hadgraft, J. The effect of solutes and polymers on the gelation properties of pluronic F-127 solutions for controlled drug delivery. J. Controll. Release 1987, 5, 113–118. [Google Scholar] [CrossRef]
- Pandit, N.K.; Kisaka, J. Loss of gelation ability of Pluronic® F127 in the presence of some salts. Int. J. Pharm. 1996, 145, 129–136. [Google Scholar] [CrossRef]
- Perrot, A.; Rangeard, D.; Pierre, A. Structural built-up of cement-based materials used for 3D-printing extrusion techniques. Mater. Struct. 2016, 49, 1213–1220. [Google Scholar] [CrossRef]
- Ribeiro, A.; Blokzijl, M.M.; Levato, R.; Visser, C.W.; Castilho, M.; Hennink, W.E.; Vermonden, T.; Malda, J. Assessing bioink shape fidelity to aid material development in 3D bioprinting. Biofabrication 2018, 10, 014102. [Google Scholar] [CrossRef] [PubMed]









| [%, w/w] | [%, w/w] | [%, w/w] | n | log |
|---|---|---|---|---|
| 17.5 | 1.0 | - | 0.09 | 2.09 |
| 17.5 | 5.0 | - | 0.08 | 1.99 |
| 17.5 | - | 1.0 | 0.10 | 2.05 |
| 17.5 | - | 5.0 | 0.08 | 1.92 |
| [%, w/w] | [%, w/w] | [%, w/w] | m | log | [Pa] |
|---|---|---|---|---|---|
| 20.0 | 3.0 | - | 0.25 | 1.73 | 110 |
| 22.5 | 1.0 | - | 0.31 | 1.72 | 160 |
| 22.5 | 5.0 | - | 0.28 | 1.76 | 152 |
| 20.0 | - | 3.0 | 0.24 | 1.76 | 99 |
| 22.5 | - | 1.0 | 0.28 | 1.78 | 156 |
| 22.5 | - | 5.0 | 0.27 | 1.80 | 130 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joas, S.; Tovar, G.E.M.; Celik, O.; Bonten, C.; Southan, A. Extrusion-Based 3D Printing of Poly(ethylene glycol) Diacrylate Hydrogels Containing Positively and Negatively Charged Groups. Gels 2018, 4, 69. https://doi.org/10.3390/gels4030069
Joas S, Tovar GEM, Celik O, Bonten C, Southan A. Extrusion-Based 3D Printing of Poly(ethylene glycol) Diacrylate Hydrogels Containing Positively and Negatively Charged Groups. Gels. 2018; 4(3):69. https://doi.org/10.3390/gels4030069
Chicago/Turabian StyleJoas, Sebastian, Günter E. M. Tovar, Oguz Celik, Christian Bonten, and Alexander Southan. 2018. "Extrusion-Based 3D Printing of Poly(ethylene glycol) Diacrylate Hydrogels Containing Positively and Negatively Charged Groups" Gels 4, no. 3: 69. https://doi.org/10.3390/gels4030069