The Role of Neodymium in the Optimization of a Ni/CeO2 and Ni/CeZrO2 Methane Dry Reforming Catalyst
Abstract
:1. Introduction
2. Results and Discussion
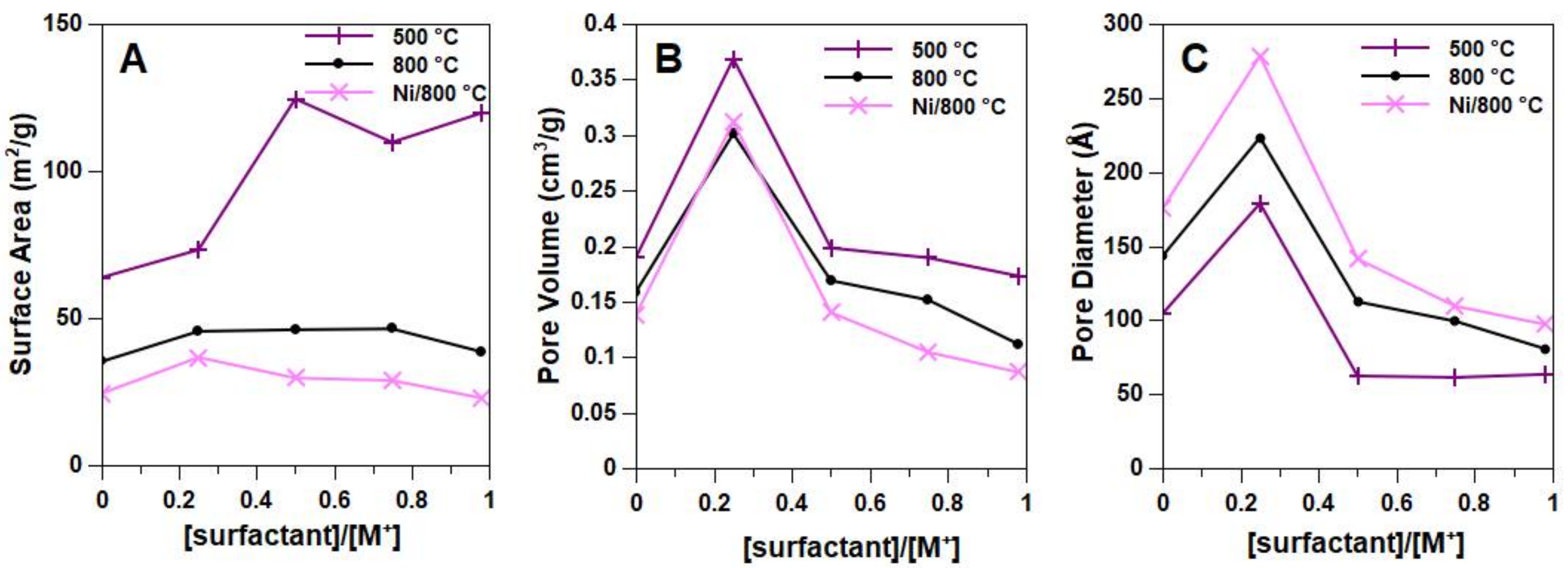
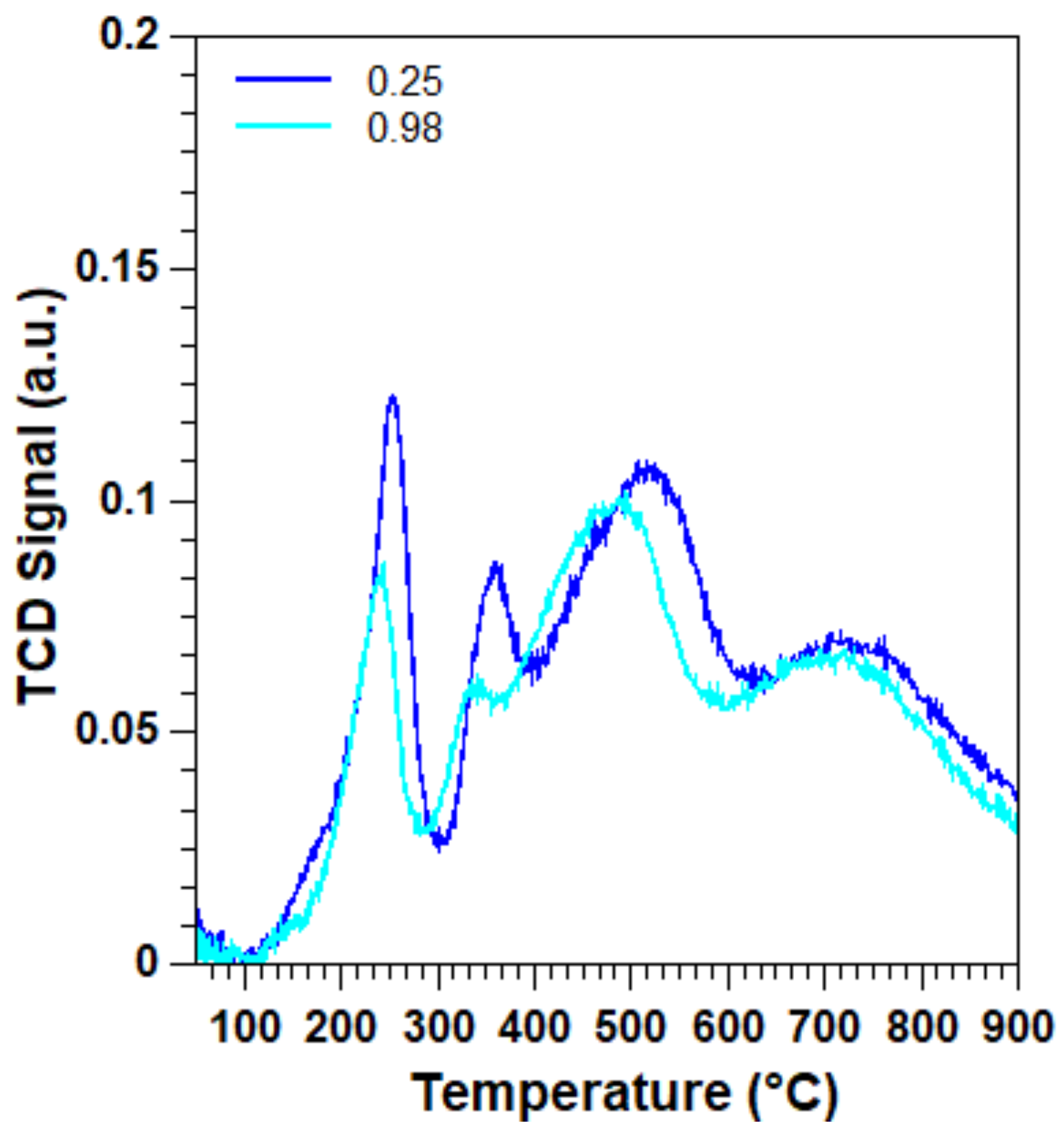
2.1. Effect of the Surfactant
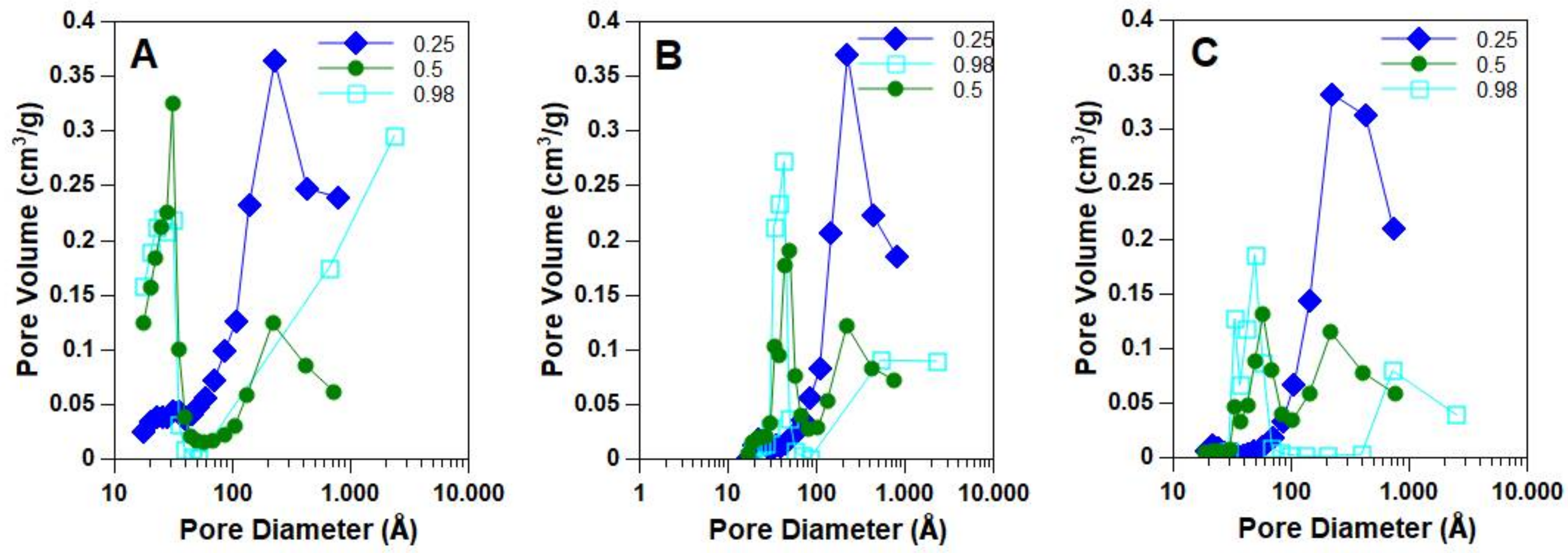
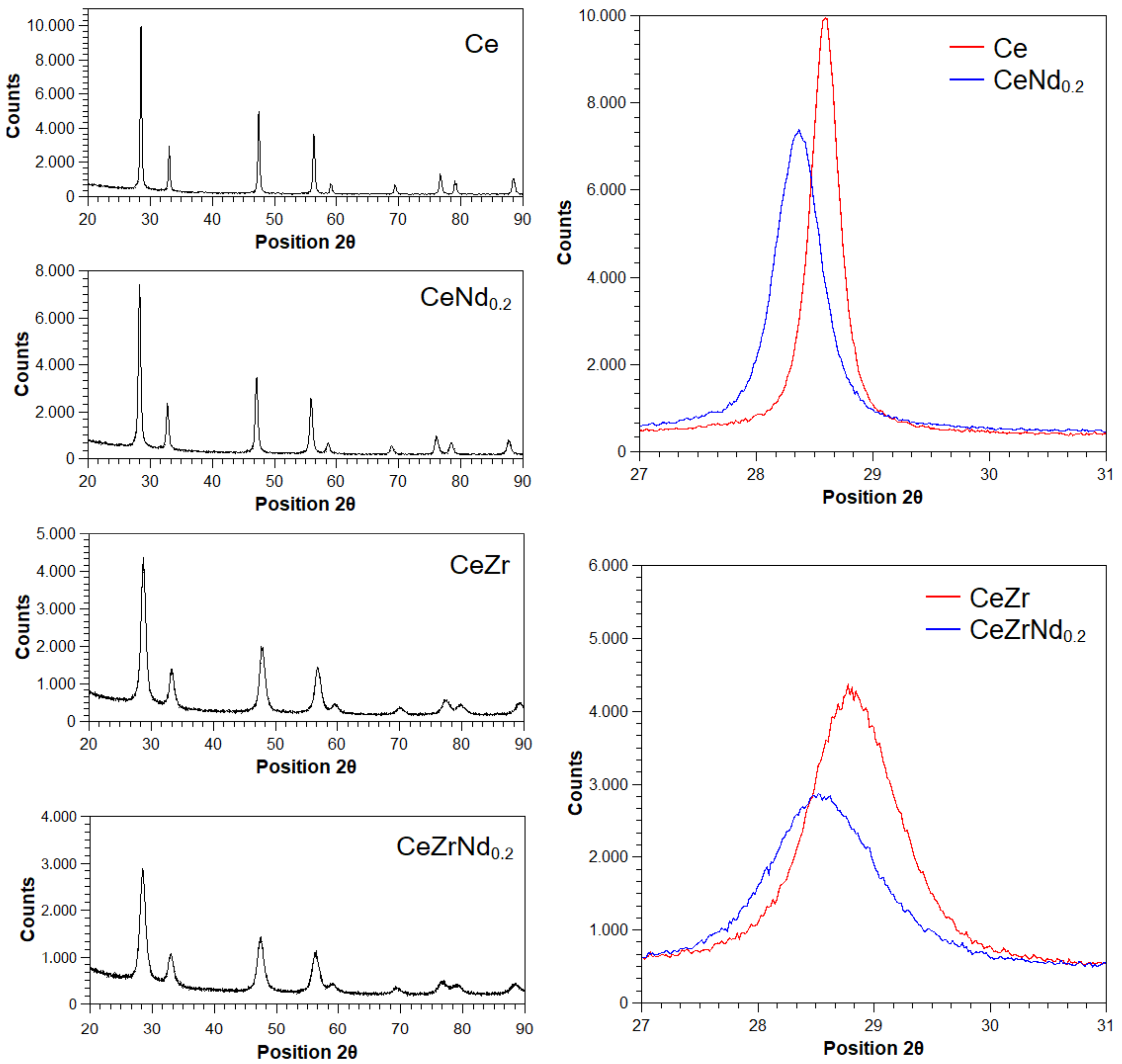
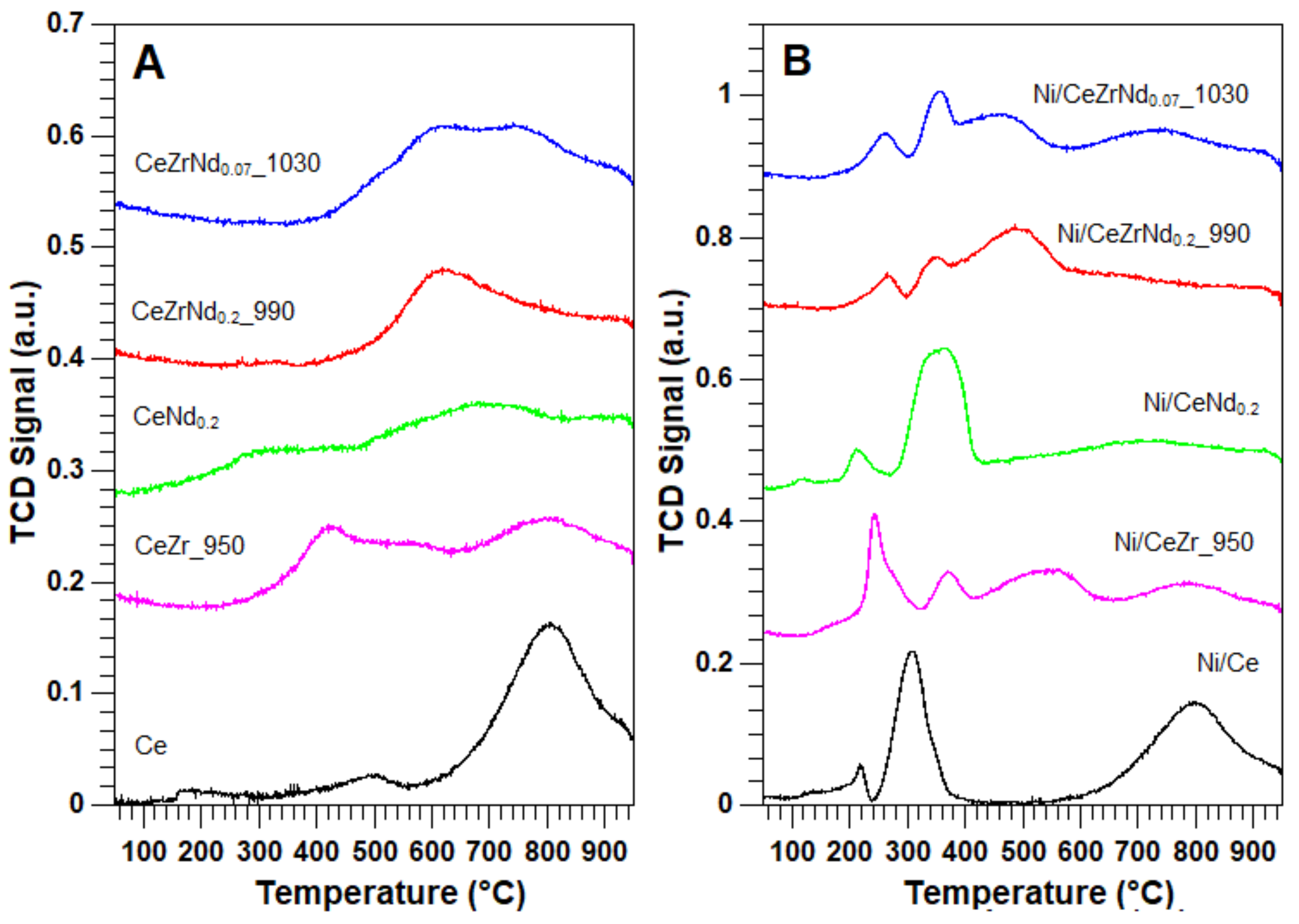
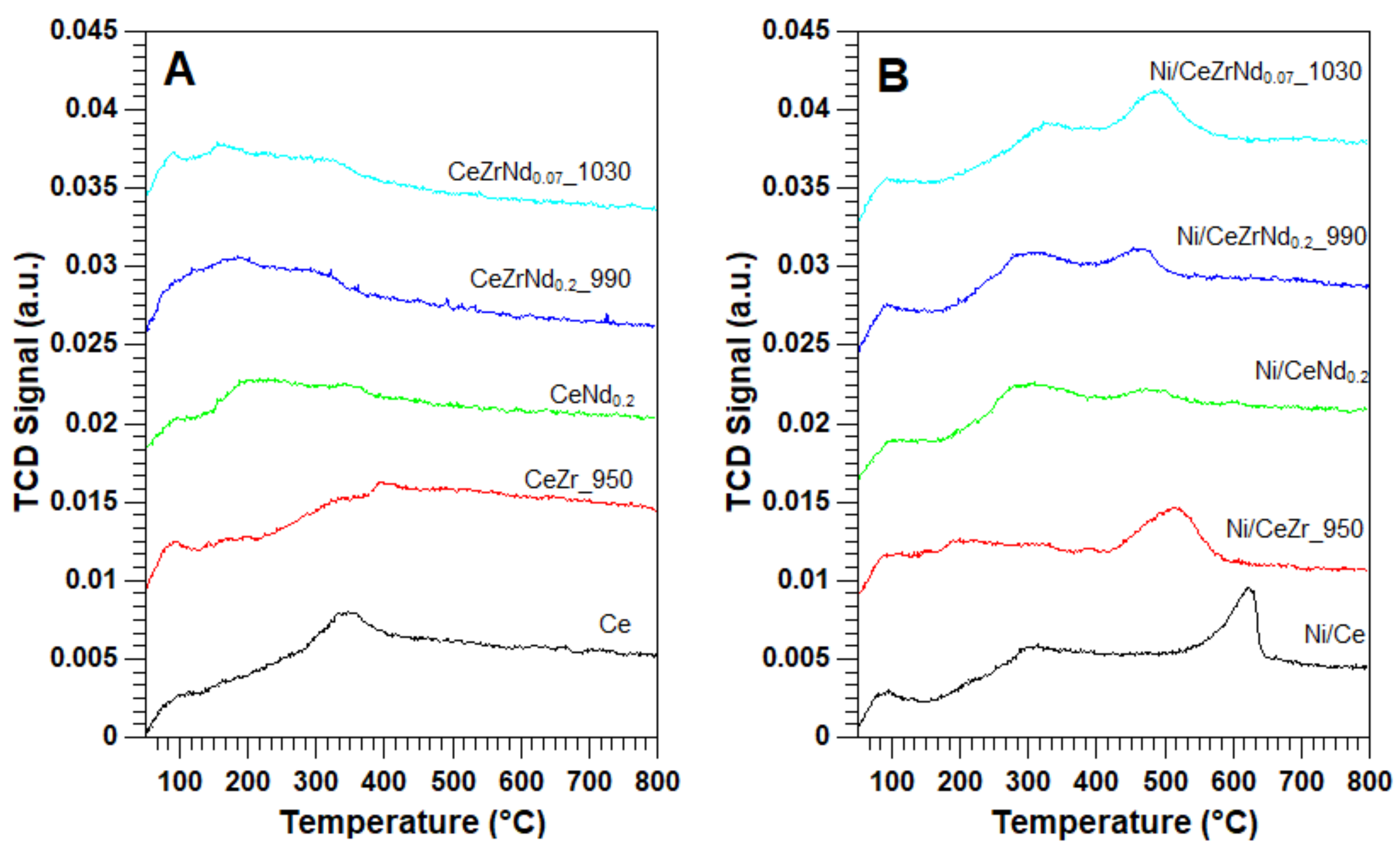
2.2. Morphological, Structural and Redox Characterization of Catalysts
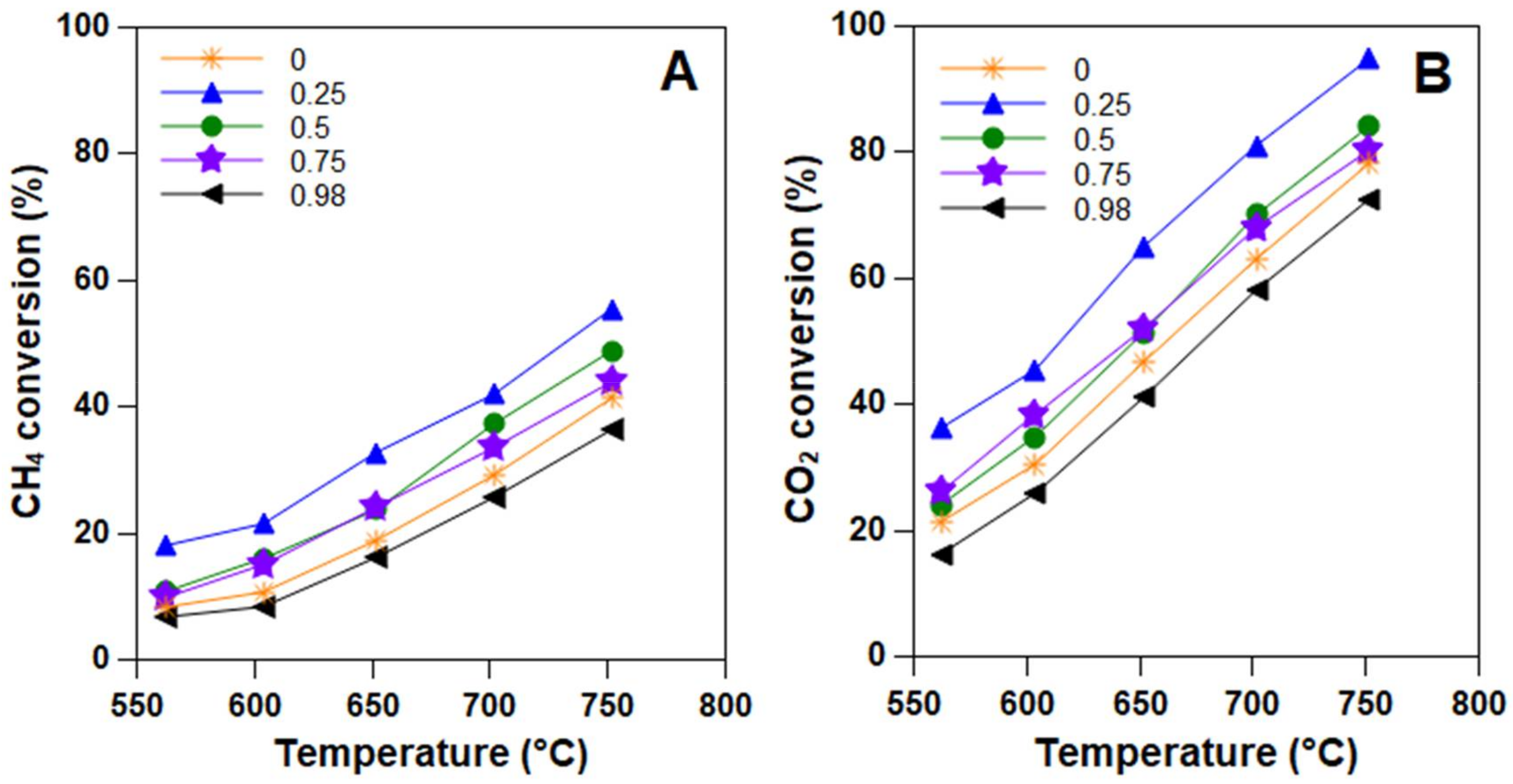
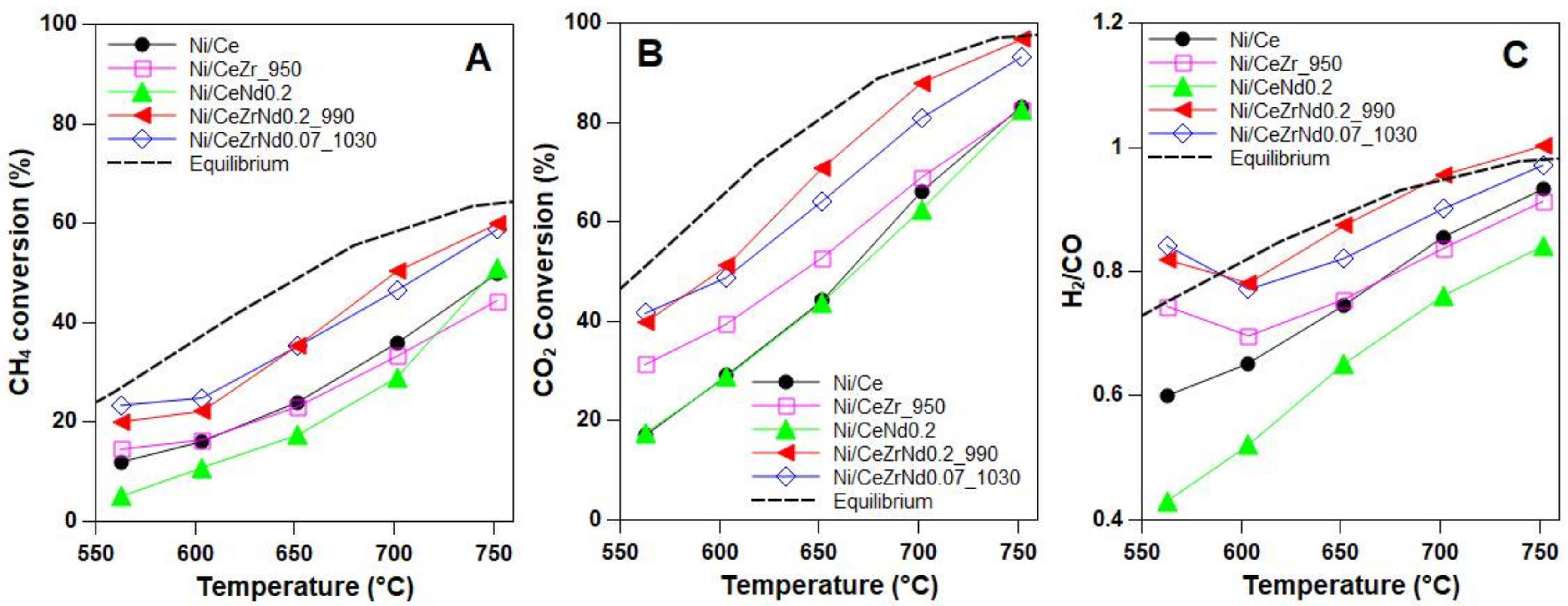
2.3. Catalytic Tests
3. Materials and Methods
3.1. Preparation of Catalysts
3.2. Characterization of Catalysts
3.3. Catalytic Tests
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ghoneim, S.A.; El Salamony, R.A.; El Temtamy, S.A. Review on innovative catalytic reforming of natural gas to syngas. World J. Eng. Technol. 2016, 4, 116–139. [Google Scholar] [CrossRef]
- Monaco, F.; Lanzini, A.; Santarelli, M. Making synthetic fuels for the road transportation sector via solid oxide electrolysis and catalytic upgrade using recovered carbon dioxide and residual. J. Clean. Prod. 2018, 170, 160–173. [Google Scholar] [CrossRef]
- Cheekatamarla, P.K.; Finnerty, C.M. Reforming catalysts for hydrogen generation in fuel cell applications. J. Power Sources 2006, 160, 490–499. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Candamano, S.; Barberio, M.; Crea, F.; Antonucci, P. CO2 conversion over supported Ni. Chem. Eng. Trans. 2017, 60, 229–234. [Google Scholar] [CrossRef]
- Lavoie, J.M. Review on dry reforming of methane, a potentially more environmentally-friendly approach to the increasing natural gas exploitation. Front. Chem. 2014, 2, 81. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Wan Daud, W.M.A.; Abbas, H.F. Dry reforming of methane: Influence of process parameters-A review. Renew. Sustain. Energy Rev. 2015, 45, 710–744. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, L.; Wang, S.; Mao, D.; Hu, C. Low-temperature catalytic CO2 dry reforming of methane on Ni-based catalysis: A review. Fuel Process. Technol. 2018, 169, 199–206. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst design for dry reforming of methane: Analysis review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Nikoo, M.K.; Amin, N.A.S. Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process. Technol. 2011, 92, 678–691. [Google Scholar] [CrossRef] [Green Version]
- Shah, Y.T.; Gardner, T.H. Dry Reforming of Hydrocarbon Feedstocks. Catal. Rev. 2014, 5, 476–536. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lu, G.Q.; Millar, G.J. Carbon dioxide reforming of methane to produce synthesis over metal-supported catalysts: State of the art. Energy Fuels 1996, 10, 896–904. [Google Scholar] [CrossRef]
- Wang, S.; Lu, G.Q. A comprehensive study on carbon dioxide reforming of methane over Ni/γ-Al2O3 catalysts. Ind. Eng. Chem. Res. 1999, 38, 2615–26253. [Google Scholar] [CrossRef]
- Arora, S.; Prasad, R. An overview on dry reforming of methane: Strategies to reduce carbonaceous deactivation of catalysts. RSC Adv. 2016, 6, 108668–108688. [Google Scholar] [CrossRef]
- Goula, M.A.; Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Tsiaoussis, I.; Panagiotopoulou, P.; Goula, G.; Yentekakis, I.V. Syngs production via biogas dry reforming reaction over Ni Supported on zirconia modified with CeO2 or La2O3 catalysts. Int. J. Hydrogen Energy 2017, 42, 13724–13740. [Google Scholar] [CrossRef]
- Kumar, P.; Sun, Y.; Idem, O.R. Comparative study of Ni-based mxed oxide catalysts for carbon dioxide reforming of methane. Energy Fuels 2008, 22, 3575–3582. [Google Scholar] [CrossRef]
- Makri, M.M.; Vasiliades, M.A.; Petallidou, K.C.; Efstathiou, A.M. Effect of support composition on the origin and reactivity of carbon formed during dry reforming of methane over 5 wt % Ni/Ce1−xMxO2−δ (M = Zr4+, Pr3+) catalysts. Catal. Today 2016, 259, 150–164. [Google Scholar] [CrossRef]
- Trovarelli, A. Structural and oxygen storage/release properties of CeO2-based solid solutions. Comments Inorg. Chem. 1999, 20, 263–284. [Google Scholar] [CrossRef]
- Nahar, G.; Dupont, V. Hydrogen Production from simple alkane and oxygenated hydrocarbons over ceria–zirconia supported catalysts: Review. Renew. Sustain. Energy Rev. 2014, 32, 777–796. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, X.; Fu, X.; Zhang, L.; Su, H.; Hui, P. Co/CexZr1−xO2 solid-solution catalysts with cubic fluorite structure for carbon dioxide reforming methane. Appl. Catal. B Environ. 2013, 136–137, 308–316. [Google Scholar] [CrossRef]
- Kumar, P.; Sun, Y.; Idem, R.O. Nickel-based Ceria, Zirconia and Ceria–Zirconia catalytic systems for low-temperature carbon dioxide reforming of methane. Energy Fuels 2007, 22, 3113–3123. [Google Scholar] [CrossRef]
- Wolfbeisser, A.; Sophiphun, O.; Bernardi, J.; Wittayakun, J.; Föttinger, K.; Rupprechter, G. Methane dry reforming over ceria–zirconia supported Ni catalysts. Catal. Today 2016, 277, 234–245. [Google Scholar] [CrossRef]
- Kambolis, A.; Matralis, H.; Trovarelli, A.; Papadopoulou, C. Ni/CeO2–ZrO2 catalysts for the dry reforming of methane. Appl. Catal. A Gen. 2010, 377, 16–26. [Google Scholar] [CrossRef]
- Djinovic, P.; Crnivec, G.I.O.; Pintar, A. Biogas to syngas conversion without carbonaceous deposits via the dry reforming reaction using transition metal catalysts. Catal. Today 2015, 253, 155–162. [Google Scholar] [CrossRef]
- Andriopoulou, C.; Trimpalis, A.; Petallidou, K.C.; Sgoura, C.; Efstathiou, A.M.; Boghosian, S. Structural and redox properties of Ce1−xZrxO2−δ and Ce0.80Zr0.15RE0.05O2−δ (RE: La Nd Pr, Y) solid studies by high temperature in situ Raman spectroscopy. J. Phys. Chem. C 2017, 121, 7931–7943. [Google Scholar] [CrossRef]
- Mikulova, J.; Rossignol, S.; Gérard, F.; Mesnard, D.; Kappenstein, C.; Duprez, D. Properties of cerium–zirconium mixed oxides partially substituted by neodymium: Comparison with Zr–Ce–Pr–O ternary oxides. J. Solid State Chem. 2006, 179, 2511–2520. [Google Scholar] [CrossRef]
- Nandi, C.; Grover, V.; Sahu, M.; Krishnan, K.; Guleria, A.; Kaity, S.; Prakash, A.; Tyagi, A.K. Nd3+ substituted (Zr1−xCex)O2 (0.0 ≤ X ≤ 1.0) system: Synthesis, structural and thermophysical investigations. J. Nucl. Mater. 2016, 479, 152–161. [Google Scholar] [CrossRef]
- Wang, Q.; Li, G.; Zhao, B.; Zhou, R. The effect of Nd on the properties of ceria-zirconia solid solution and the catalytic performance of its supported Pd-only three-way catalysts for gasoline engine exhaust reduction. J. Hazard. Mater. 2011, 189, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Gimenez, A.M.; dos Santos Xavier, L.P.; Bueno-Lopez, A. Improving ceria–zirconia soot combustion catalysts by neodymium doping. Appl. Catal. A Gen. 2013, 462–463, 100–106. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Hossain, S.S.; Lam, S.S.; Osazuwa, O.U.; Khan, M.R.; Cheng, C.K. Syngas production from CO2 reforming of methane over neodymium sesquioxide supported cobalt catalysts. J. Nat. Gas Sci. Eng. 2016, 34, 873–885. [Google Scholar] [CrossRef]
- Boaro, M.; Vicario, M.; De Leitenburg, C.; Dolcetti, G.; Trovarelli, A. The use of temperature-programmed and dynamic/transient methods in catalysis: Characterization of ceria-based, model three-way catalysts. Catal. Today 2003, 77, 407–417. [Google Scholar] [CrossRef]
- Binet, C.; Daturi, M.; Lavalley, J.-C. IR study of polycrystalline ceria properties in oxidised and reduced states. Catal. Today 1999, 50, 207–225. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Crystal structures, acid-base properties, and reactivity of CexZr1−xO2. Catal. Today 2009, 148, 323–328. [Google Scholar] [CrossRef]
- Sato, S.; Takahashi, R.; Kobune, M.; Gotoh, H. Basic properties of rare earth oxides. Appl. Catal. A Gen. 2009, 356, 57–63. [Google Scholar] [CrossRef]
- Sokolov, S.; Kondratenko, E.V.; Pohl, M.-M. Effect of calcination conditios on time on-stream performance of Ni/La2O3–ZrO2 in low temperature dry reforming of methane. Int. J. Hydrogen Energy 2013, 38, 16121–16132. [Google Scholar] [CrossRef]
- Vasiliades, M.A.; Dijnovic, P.; Pintar, A.; Kovac, J.; Efstathious, A.M. The effect of CeO2–ZrO2 structural differences on origin and reactivity of carbon formed during methane dry reforming over NiCo/CeO2–ZrO2 catalysts studied by transient techniques. Catal. Sci. Technol. 2017, 7, 5422–5434. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous catalyst deactivation and regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Carbon deposition in steam reforming and methanation. Catal. Rev. Sci. Eng. 1982, 24, 67–112. [Google Scholar] [CrossRef]
- Pappacena, A.; Schermanz, K.; Sagar, A.; Aneggi, E.; Trovarelli, A. Development of a modified co-precipitation route for thermally resistant, high surface area ceria–zirconia based solid solutions. Stud. Surf. Sci. Catal. 2010, 175, 835–838. [Google Scholar] [CrossRef]
- Brooks, C.S.; Christopher, G.L.M. Measurement of the state of metal dispersion on supported Nickel catalysis by gas chemisorption. J. Catal. 1968, 10, 211–223. [Google Scholar] [CrossRef]
| Composition | Name | Temp/Time Calcination (°C/h) | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (Å) | Crystallite Size 1 (Å) |
|---|---|---|---|---|---|---|
| CeO2 | Ce | 800/3 | 22 | 0.195 | 295 | 298 |
| Ce0.80Zr0.20O2 | CeZr | 800/3 | 44 | 0.374 | 270 | 58 |
| Ce0.80Zr0.20O2 | CeZr_950 | 950/3 | 26 | 0.313 | 379 | 151 |
| Ce0.80Nd0.20O1.9 | CeNd0.2 | 800/3 | 25 | 0.221 | 280 | 178 |
| Ce0.64Zr0.16Nd0.2O1.9 | CeZrNd0.2 | 800/3 | 41 | 0.310 | 249 | 77 |
| Ce0.64Zr0.16Nd0.2O1.9 | CeZrNd0.2_990 | 990/3 | 22 | 0.282 | 384 | 134 |
| Ce0.80Zr0.13Nd0.07O1.96 | CeZrNd0.07 | 800/3 | 45 | 0.301 | 223 | 87 |
| Ce0.80Zr0.13Nd0.07O1.96 | CeZrNd0.07_1030 | 1030/3 | 20 | 0.259 | 395 | 170 |
| Catalysts | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (Å) | Metal Dispersion (%) | Support Crystallite Size 1 (Å) |
|---|---|---|---|---|---|
| Ni/Ce | 19 | 0.180 | 316 | 0.59 | 296 |
| Ni/CeZr | 34 | 0.261 | 255 | 1.08 | 100 |
| Ni/CeZr_950 | 22 | 0.240 | 349 | 1.58 | 154 |
| Ni/CeNd0.2 | 19 | 0.196 | 307 | 0.58 | 195 |
| Ni/CeZrNd0.07 | 37 | 0.311 | 278 | 1.56 | 95 |
| Ni/CeZrNd0.2 | 32 | 0.311 | 309 | 1.28 | 86 |
| Ni/CeZrNd0.07_1030 | 19 | 0.243 | 382 | 1.93 | 170 |
| Ni/CeZrNd0.2_990 | 21 | 0.193 | 310 | 1.44 | 134 |
| Samples | Supports H2 Uptake (mL/g) a | Degree of Reduction of Supports b (%) | Ni Catalysts H2 Uptake (mL/g) c | Degree of Reduction of Ni Catalysts b (%) |
|---|---|---|---|---|
| Ni/Ce | 24.5 | 38 | 29.3 (38.0) d | 45 |
| Ni/CeZr_950 | 26.5 | 48 | 35.7 (40.0) | 51 |
| Ni/CeNd0.2 | 30.7 | 59 | 38.5 (44.2) | 44 |
| Ni/CeZrNd0.2_990 | 18.4 | 42 | 28.3 (31.9) | 40 |
| Ni/CeZrNd0.07_1030 | 23.5 | 43 | 32.2 (37.0) | 56 |
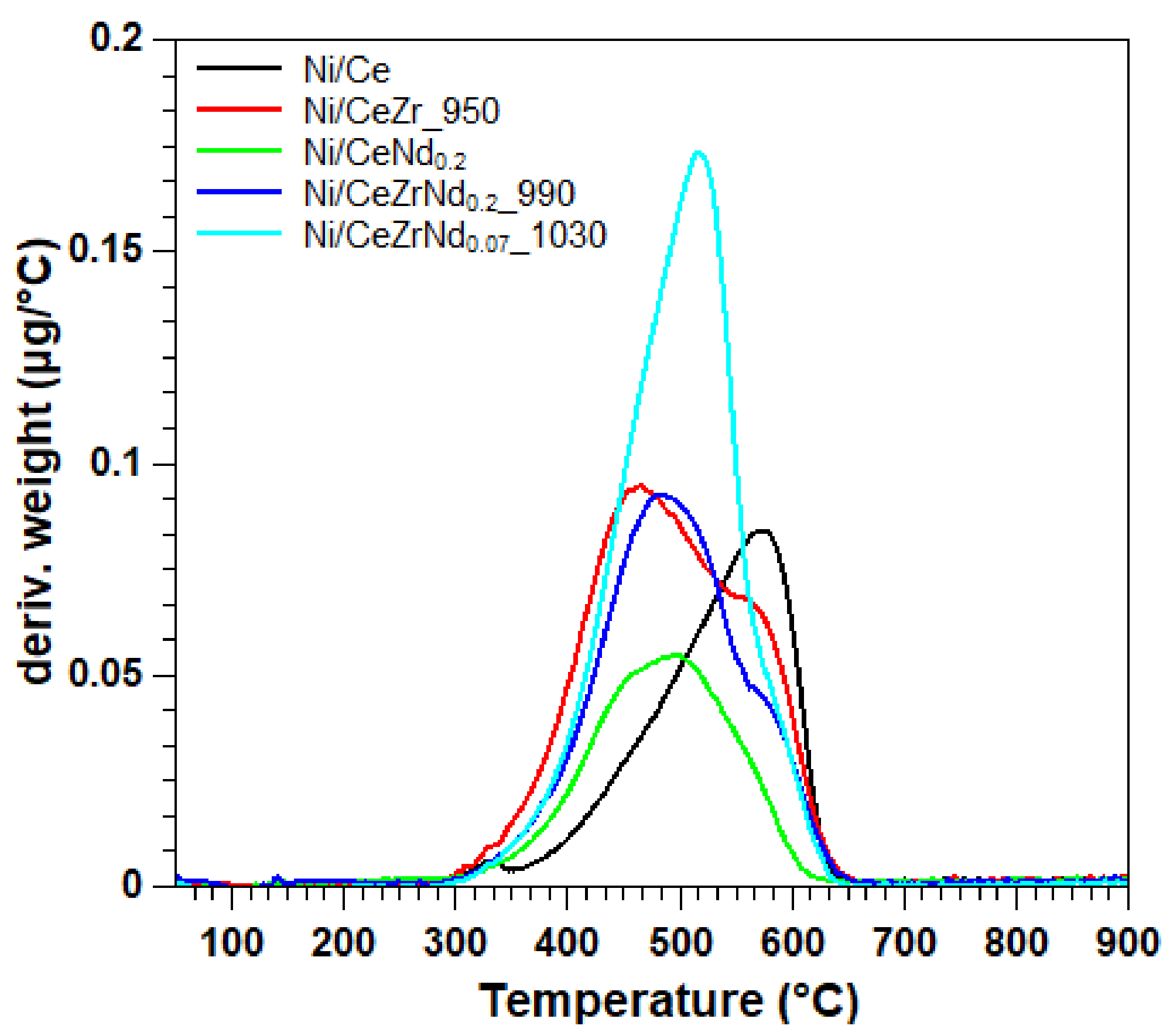
| Catalysts | Time on Stream (h) | Carbon % |
|---|---|---|
| Ni/Ce | 8 | 22 |
| Ni/CeNd0.2 | 8 | 17 |
| Ni/CeZr_950 | 8 | 31 |
| Ni/CeZrNd0.2_990 | 8 | 27 |
| Ni/CeZrNd0.07_1030 | 8 | 36 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pappacena, A.; Razzaq, R.; De Leitenburg, C.; Boaro, M.; Trovarelli, A. The Role of Neodymium in the Optimization of a Ni/CeO2 and Ni/CeZrO2 Methane Dry Reforming Catalyst. Inorganics 2018, 6, 39. https://doi.org/10.3390/inorganics6020039
Pappacena A, Razzaq R, De Leitenburg C, Boaro M, Trovarelli A. The Role of Neodymium in the Optimization of a Ni/CeO2 and Ni/CeZrO2 Methane Dry Reforming Catalyst. Inorganics. 2018; 6(2):39. https://doi.org/10.3390/inorganics6020039
Chicago/Turabian StylePappacena, Alfonsina, Rabil Razzaq, Carla De Leitenburg, Marta Boaro, and Alessandro Trovarelli. 2018. "The Role of Neodymium in the Optimization of a Ni/CeO2 and Ni/CeZrO2 Methane Dry Reforming Catalyst" Inorganics 6, no. 2: 39. https://doi.org/10.3390/inorganics6020039