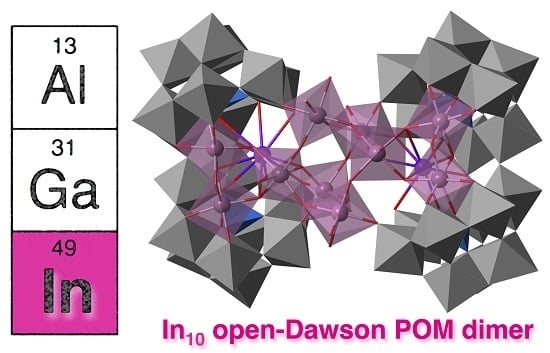
Synthesis, Structure, and Characterization of In10-Containing Open-Wells–Dawson Polyoxometalate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
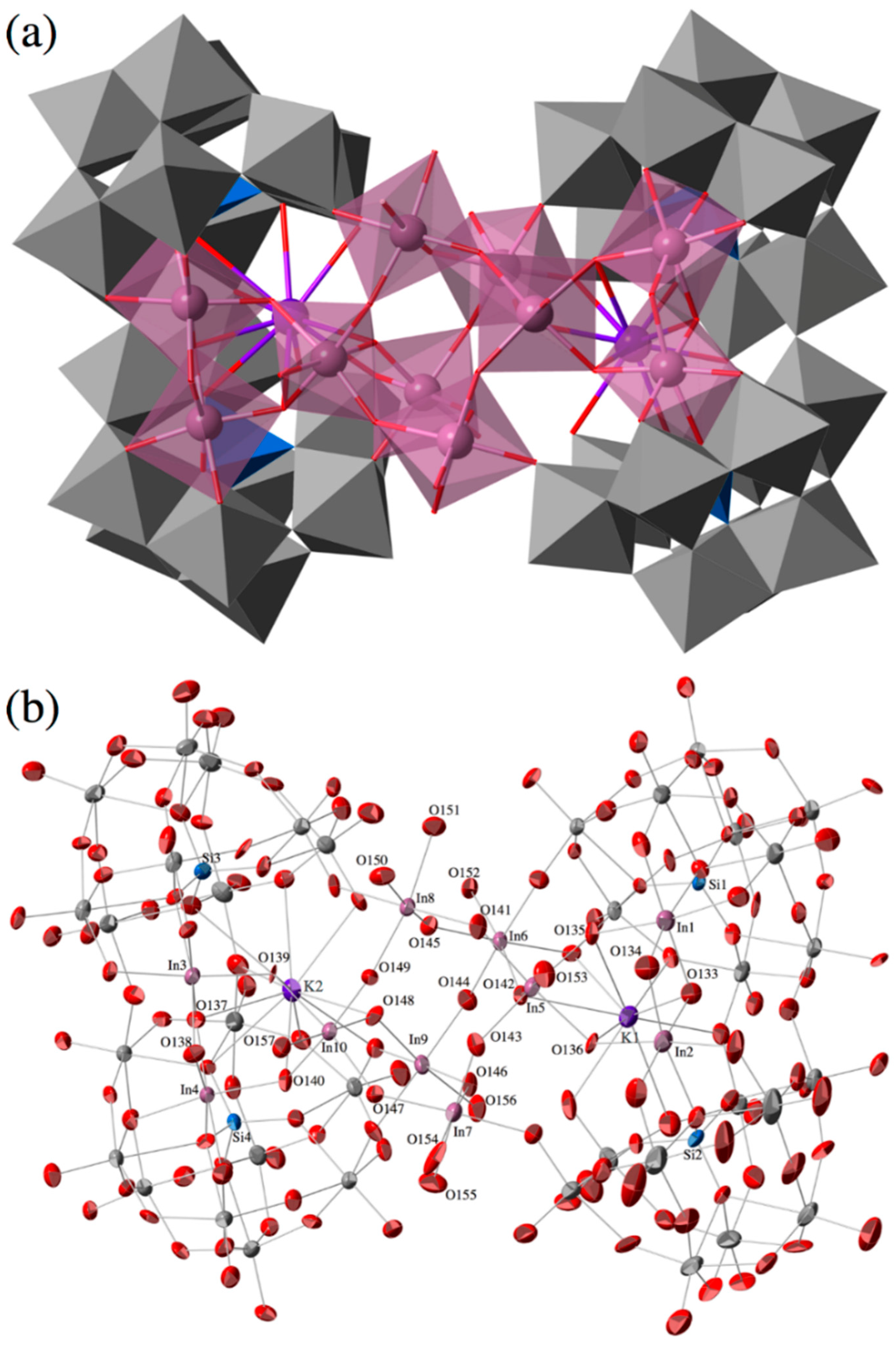
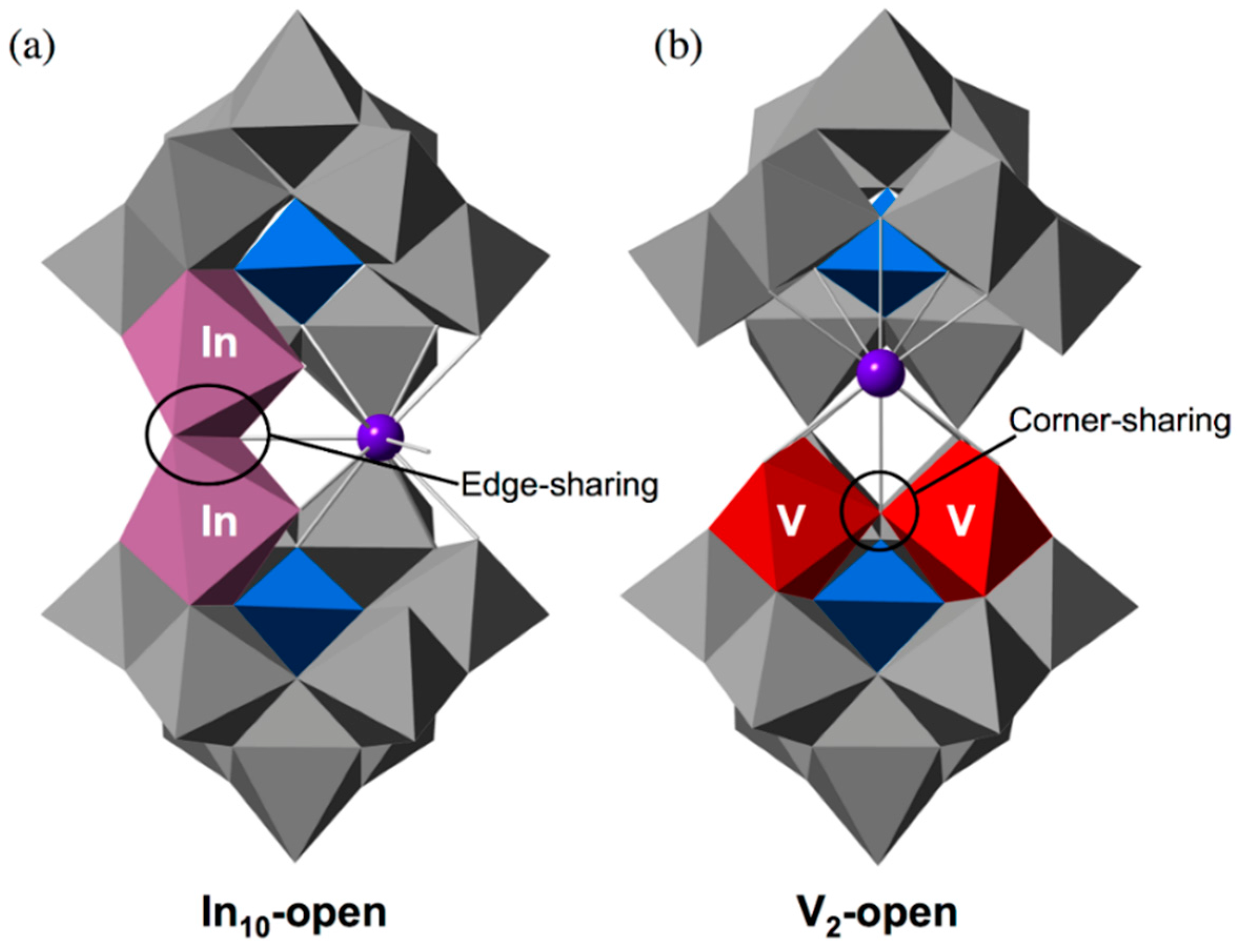
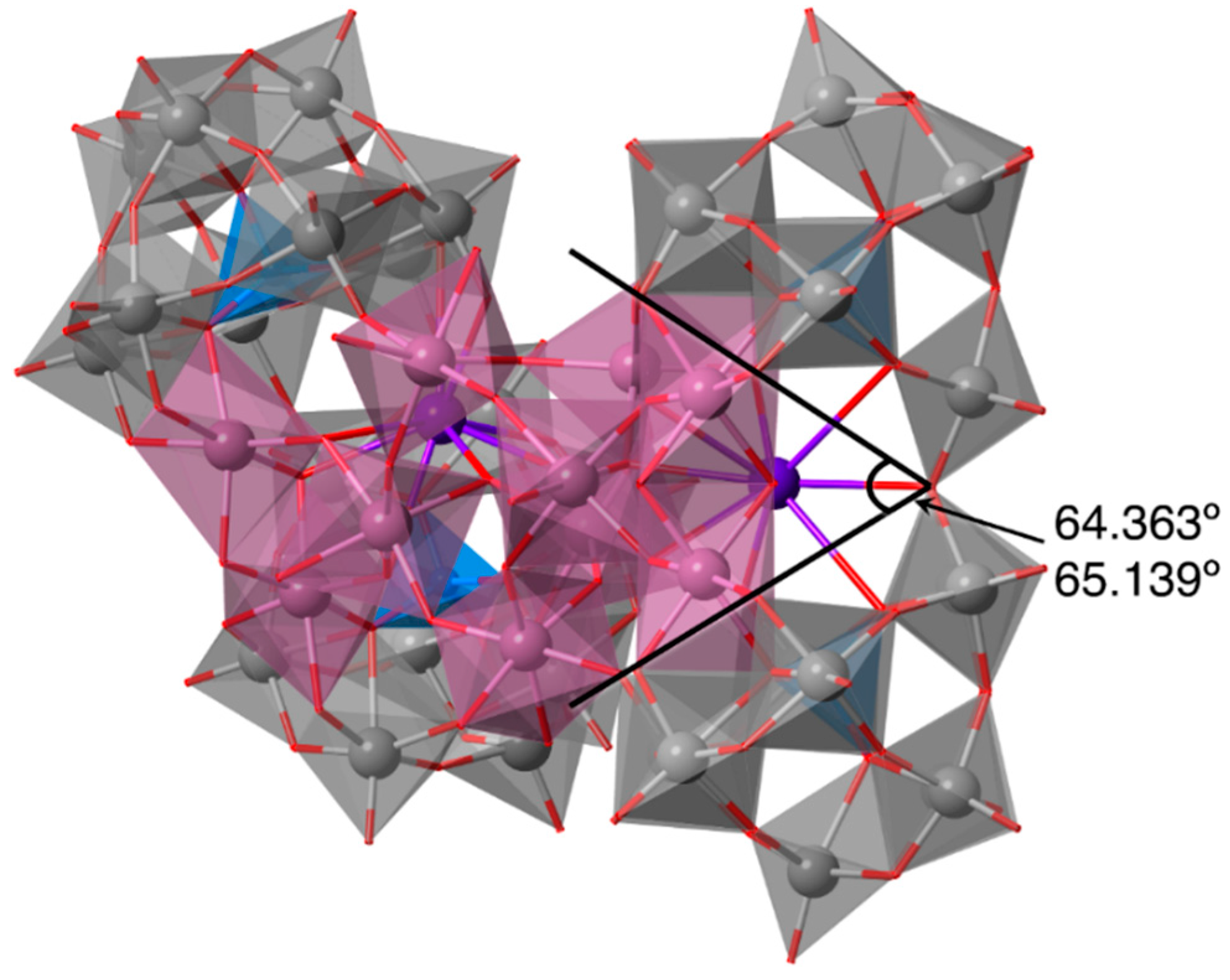
2.2. Molecular Structure
2.3. Solution 29Si NMR
3. Experimental Section
3.1. Materials
3.2. Instrumentation and Analytical Procedures
3.3. Synthesis of K17{[{KIn2(μ-OH)2}(α,α-Si2W18O66)]2[In6(μ-OH)13(H2O)8]}·35H2O (Potassium Salt of In10-open)
3.4. X-Ray Crystallography
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| POM | Polyoxometalate |
| TG/DTA | Thermogravimetric/differential thermal analyses |
| BVS | Bond valence sum |
References
- Pope, M.T.; Müller, A. Polyoxometalate chemistry: An old field with new dimensions in several disciplines. Angew. Chem. Int. Ed. 1991, 30, 34–48. [Google Scholar] [CrossRef]
- Pope, M.T. Heteropoly- and Isopolyoxometalates; Springer-Verlag: New York, NY, USA, 1983. [Google Scholar]
- Hill, C.L.; Prosser-McCartha, C.M. Homogeneous catalysis by transition metal oxygen anion clusters. Coord. Chem. Rev. 1995, 143, 407–455. [Google Scholar] [CrossRef]
- Neumann, R. Polyoxometalate complexes in organic oxidation chemistry. Prog. Inorg. Chem. 1998, 47, 317–370. [Google Scholar]
- Proust, A.; Thouvenot, R.; Gouzerh, P. Functionalization of polyoxometalates: Towards advanced applications in catalysis and materials science. Chem. Commun. 2008, 1837–1852. [Google Scholar] [CrossRef] [PubMed]
- Hasenknopf, B.; Micoine, K.; Lacôte, E.; Thorimbert, S.; Malacria, M.; Thouvenot, R. Chirality in polyoxometalate chemistry. Eur. J. Inorg. Chem. 2008, 2008, 5001–5013. [Google Scholar] [CrossRef]
- Long, D.-L.; Tsunashima, R.; Cronin, L. Polyoxometalates: Building blocks for functional nanoscale systems. Angew. Chem. Int. Ed. 2010, 49, 1736–1758. [Google Scholar] [CrossRef] [PubMed]
- Nomiya, K.; Sakai, Y.; Matsunaga, S. Chemistry of group IV metal ion-containing polyoxometalates. Eur. J. Inorg. Chem. 2011, 2011, 179–196. [Google Scholar] [CrossRef]
- Izarova, N.V.; Pope, M.T.; Kortz, U. Noble metals in polyoxometalates. Angew. Chem. Int. Ed. 2012, 51, 9492–9510. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-F.; Tsunashima, R. Recent advances on polyoxometalate-based molecular and composite materials. Chem. Soc. Rev. 2012, 41, 7384–7402. [Google Scholar] [CrossRef] [PubMed]
- Bijelic, A.; Rompel, A. The use of polyoxometalates in protein crystallography—An attempt to widen a well-known bottleneck. Coord. Chem. Rev. 2015, 299, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-S.; Yang, G.-Y. Recent advances in polyoxometalate-catalyzed reactions. Chem. Rev. 2015, 115, 4893–4962. [Google Scholar] [CrossRef] [PubMed]
- Blazevic, A.; Rompel, A. The Anderson–Evans polyoxometalate: From inorganic building blocks via hybrid organic–inorganic structures to tomorrows “Bio-POM”. Coord. Chem. Rev. 2016, 307, 42–64. [Google Scholar] [CrossRef]
- Laronze, N.; Marrot, J.; Hervé, G. Synthesis, molecular structure and chemical properties of a new tungstosilicate with an open Wells–Dawson structure, α-[Si2W18O66]16−. Chem. Commun. 2003, 21, 2360–2361. [Google Scholar] [CrossRef]
- Bi, L.-H.; Kortz, U. Synthesis and structure of the pentacopper(II) substituted tungstosilicate [Cu5(OH)4(H2O)2(A-α-SiW9O33)2]10−. Inorg. Chem. 2004, 43, 7961–7962. [Google Scholar] [CrossRef] [PubMed]
- Leclerc-Laronze, N.; Marrot, J.; Hervé, G. Cation-directed synthesis of tungstosilicates. 2. Synthesis, structure, and characterization of the open Wells–Dawson anion α-[{K(H2O)2}(Si2W18O66)]15− and its transiton-metal derivatives [{M(H2O)}(μ-H2O)2K(Si2W18O66)]13− and [{M(H2O)}(μ-H2O)2K{M(H2O)4}(Si2W18O66)]11−. Inorg. Chem. 2005, 44, 1275–1281. [Google Scholar] [PubMed]
- Nellutla, S.; Tol, J.V.; Dalal, N.S.; Bi, L.-H.; Kortz, U.; Keita, B.; Nadjo, L.; Khitrov, G.A.; Marshall, A.G. Magnetism, electron paramagnetic resonance, electrochemistry, and mass spectrometry of the pentacopper(II)-substituted tungstosilicate [Cu5(OH)4(H2O)2(A-α-SiW9O33)2]10−, A model five-spin frustrated cluster. Inorg. Chem. 2005, 44, 9795–9806. [Google Scholar] [CrossRef] [PubMed]
- Leclerc-Laronze, N.; Haouas, M.; Marrot, J.; Taulelle, F.; Hervé, G. Step-by-step assembly of trivacant tungstosilicates: Synthesis and characterization of tetrameric anions. Angew. Chem. Int. Ed. 2006, 45, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Leclerc-Laronze, N.; Marrot, J.; Hervé, G. Dinuclear vanadium and tetranuclear iron complexes obtained with the open Wells–Dawson [Si2W18O66]16− tungstosilicate. C. R. Chim. 2006, 9, 1467–1471. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Liu, S.-X.; Wang, C.-L.; Xie, L.-H.; Zhang, C.-D.; Gao, B.; Su, Z.-M.; Jia, H.-Q. Synthesis, structure and characterization of a new cobalt-containing germanotungstate with open Wells–Dawson structure: K13[{Co(H2O)}(μ-H2O)2K(Ge2W18O66)]. J. Mol. Struct. 2006, 785, 170–175. [Google Scholar] [CrossRef]
- Wang, C.-L.; Liu, S.-X.; Sun, C.-Y.; Xie, L.-H.; Ren, Y.-H.; Liang, D.-D.; Cheng, H.-Y. Bimetals substituted germanotungstate complexes with open Wells–Dawson structure: Synthesis, structure, and electrochemical behavior of [{M(H2O)}(μ-H2O)2K{M(H2O)4}(Ge2W18O66)]11− (M = Co, Ni, Mn). J. Mol. Struct. 2007, 841, 88–95. [Google Scholar] [CrossRef]
- Ni, L.; Hussain, F.; Spingler, B.; Weyeneth, S.; Patzke, G.R. Lanthanoid-containing open Wells–Dawson silicotungstates: Synthesis, crystal structures, and properties. Inorg. Chem. 2011, 50, 4944–4955. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Geletii, Y.V.; Zhao, C.; Musaev, D.G.; Song, J.; Hill, C.L. A dodecanuclear Zn cluster sandwiched by polyoxometalate ligands. Dalton Trans. 2012, 41, 9908–9913. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Glass, E.N.; Zhao, C.; Lv, H.; Vickers, J.W.; Geletii, Y.V.; Musaev, D.G.; Song, J.; Hill, C.L. A nickel containing polyoxometalate water oxidation catalyst. Dalton Trans. 2012, 41, 13043–13049. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Geletii, Y.V.; Song, J.; Zhao, C.; Glass, E.N.; Bacsa, J.; Hill, C.L. Di- and tri-cobalt silicotungstates: Synthesis, characterization, and stability studies. Inorg. Chem. 2013, 52, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Spingler, B.; Weyeneth, S.; Patzke, G.R. Trilacunary Keggin-type POMs as versatile building blocks for lanthanoid silicotungstates. Eur. J. Inorg. Chem. 2013, 2013, 1681–1692. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, D.; Chen, L.; Song, Y.; Zhu, D.; Xu, Y. Syntheses, structures and magnetic properties of two unprecedented hybrid compounds constructed from open Wells–Dawson anions and high-nuclear transition metal clusters. Dalton Trans. 2013, 42, 8454–8459. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.; Inoue, Y.; Otaki, T.; Osada, H.; Nomiya, K. Aluminum-and gallium-containing open-Dawson polyoxometalates. Z. Anorg. Allg. Chem. 2016, 642, 539–545. [Google Scholar] [CrossRef]
- Zhang, F.-Q.; Guan, W.; Yan, L.-K.; Zhang, Y.-T.; Xu, M.-T.; Hayfron-Benjamin, E.; Su, Z.-M. On the origin of the relative stability of Wells–Dawson isomers: A DFT study of α-, β-, γ-, α*-, β*-, and γ*-[(PO4)2W18O54]6− anions. Inorg. Chem. 2011, 50, 4967–4977. [Google Scholar] [CrossRef] [PubMed]
- Jordan, P.A.; Clayden, N.J.; Heath, S.L.; Moore, G.R.; Powell, A.K.; Tapparo, A. Defining speciation profiles of Al3+ complexed with small organic ligands: The Al3+-heidi system. Coord. Chem. Rev. 1996, 149, 281–309. [Google Scholar] [CrossRef]
- Casey, W.H. Large aqueous aluminum hydroxide molecules. Chem. Rev. 2006, 106, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mensinger, Z.L.; Wang, W.; Keszler, D.A.; Johnson, D.W. Oligomeric group 13 hydroxide compounds—A rare but varied class of molecules. Chem. Soc. Rev. 2012, 41, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Kikukawa, Y.; Yamaguchi, S.; Nakagawa, Y.; Uehara, K.; Uchida, S.; Yamaguchi, K.; Mizuno, N. Synthesis of a dialuminum-substituted silicotungstate and the diastereoselective cyclization of citronellal derivatives. J. Am. Chem. Soc. 2008, 130, 15872–15878. [Google Scholar] [CrossRef] [PubMed]
- Kato, C.N.; Katayama, Y.; Nagami, M.; Kato, M.; Yamasaki, M. A sandwich-type aluminium complex composed of tri-lacunary Keggin-type polyoxotungstate: Synthesis and X-ray crystal structure of [(A-PW9O34)2{W(OH)(OH2)}{Al(OH)(OH2)}{Al(μ-OH)(OH2)2}2]7−. Dalton Trans. 2010, 39, 11469–11474. [Google Scholar] [CrossRef] [PubMed]
- Kikukawa, Y.; Yamaguchi, K.; Hibino, M.; Mizuno, N. Layered assemblies of a dialuminum-substituted silicotungstate trimer and the reversible interlayer cation-exchange properties. Inorg. Chem. 2011, 50, 12411–12413. [Google Scholar] [CrossRef] [PubMed]
- Carraro, M.; Bassil, B.S.; Sorarù, A.; Berardi, S.; Suchopar, A.; Kortz, U.; Bonchio, M. A Lewis acid catalytic core sandwiched by inorganic polyoxoanion caps: Selective H2O2-based oxidations with [AlIII4(H2O)10(β-XW9O33H)2]6− (X = AsIII, SbIII). Chem. Commun. 2013, 49, 7914–7916. [Google Scholar] [CrossRef] [PubMed]
- Kato, C.N.; Makino, Y.; Unno, W.; Uno, H. Synthesis, molecular structure, and stability of a zirconocene derivative with α-Keggin mono-aluminum substituted polyoxotungstate. Dalton Trans. 2013, 42, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Kato, C.N.; Kashiwagi, T.; Unno, W.; Nakagawa, M.; Uno, H. Syntheses and molecular structures of monomeric and hydrogen-bonded dimeric Dawson-type trialuminum-substituted polyoxotungstates derived under acidic and basic conditions. Inorg. Chem. 2014, 53, 4823–4832. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Matsunaga, S.; Nomiya, K. Al16-hydroxide cluster-containing tetrameric polyoxometalate, [{α-Al3SiW9O34(μ-OH)6}4{Al4(μ-OH)6}]22−. Chem. Lett. 2015, 44, 1649–1651. [Google Scholar] [CrossRef]
- Allmen, K.; Car, P.-E.; Blacque, O.; Fox, T.; Müller, R.; Patzke, G.R. Structure and properties of new gallium-containing polyoxotungstates with hexanuclear and tetranuclear cores. Z. Anorg. Allg. Chem. 2014, 640, 781–789. [Google Scholar] [CrossRef]
- Limanski, E.M.; Drewes, D.; Krebs, B. Sandwich-like polyoxotungstates with indium(III) as a heteroatom synthesis and characterization of the first examples of a new type of anions. Z. Anorg. Allg. Chem. 2004, 630, 523–528. [Google Scholar] [CrossRef]
- Hussain, F.; Reicke, M.; Janowski, V.; Silva, S.D.; Futuwi, J.; Kortz, U. Some indium(III)-substituted polyoxotungstates of the Keggin and Dawson types. C. R. Chim. 2005, 8, 1045–1056. [Google Scholar] [CrossRef]
- Zhao, D.; Ye, R.-H. Solvothermal synthesis and structure of a new indium-substituted polyoxotungstate: [(CH3)2NH2]4[In3(H2O)3(NO3)(A-α-H3PW9O34)2]·H2O. J. Clust. Sci. 2011, 22, 563–571. [Google Scholar] [CrossRef]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database. Acta Crystallogr. 1985, B41, 244–247. [Google Scholar] [CrossRef]
- Shannon, R.D.; Prewitt, C.T. Effective ionic radii in oxides and fluorides. Acta Cryst. 1969, B25, 925–946. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–761. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsunaga, S.; Otaki, T.; Inoue, Y.; Mihara, K.; Nomiya, K. Synthesis, Structure, and Characterization of In10-Containing Open-Wells–Dawson Polyoxometalate. Inorganics 2016, 4, 16. https://doi.org/10.3390/inorganics4020016
Matsunaga S, Otaki T, Inoue Y, Mihara K, Nomiya K. Synthesis, Structure, and Characterization of In10-Containing Open-Wells–Dawson Polyoxometalate. Inorganics. 2016; 4(2):16. https://doi.org/10.3390/inorganics4020016
Chicago/Turabian StyleMatsunaga, Satoshi, Takuya Otaki, Yusuke Inoue, Kohei Mihara, and Kenji Nomiya. 2016. "Synthesis, Structure, and Characterization of In10-Containing Open-Wells–Dawson Polyoxometalate" Inorganics 4, no. 2: 16. https://doi.org/10.3390/inorganics4020016