Protective Effects of Dendropanax morbifera against Cisplatin-Induced Nephrotoxicity without Altering Chemotherapeutic Efficacy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
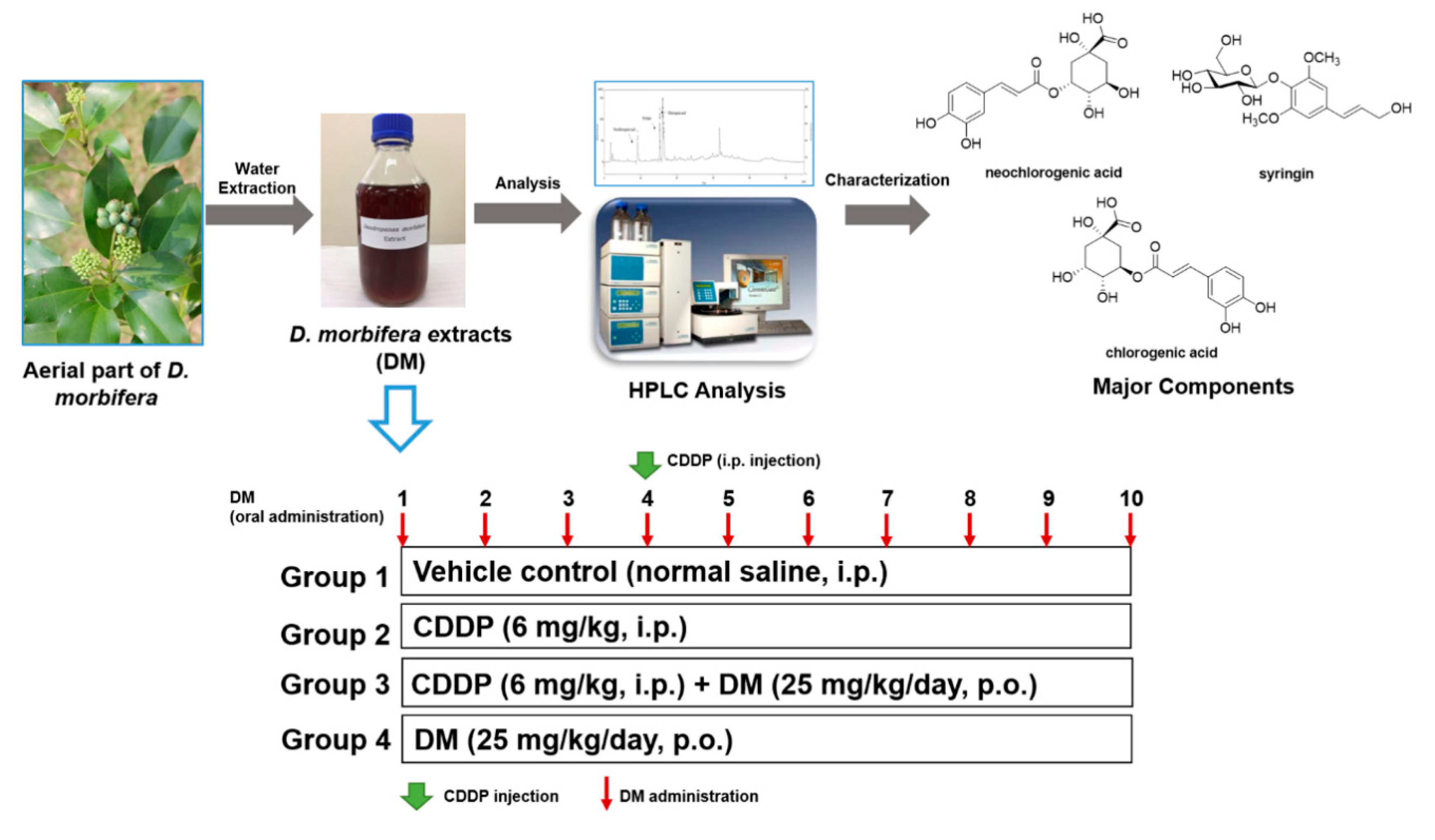
2.2. Plant Material and Preparation of Water Extracts from D. morbifera
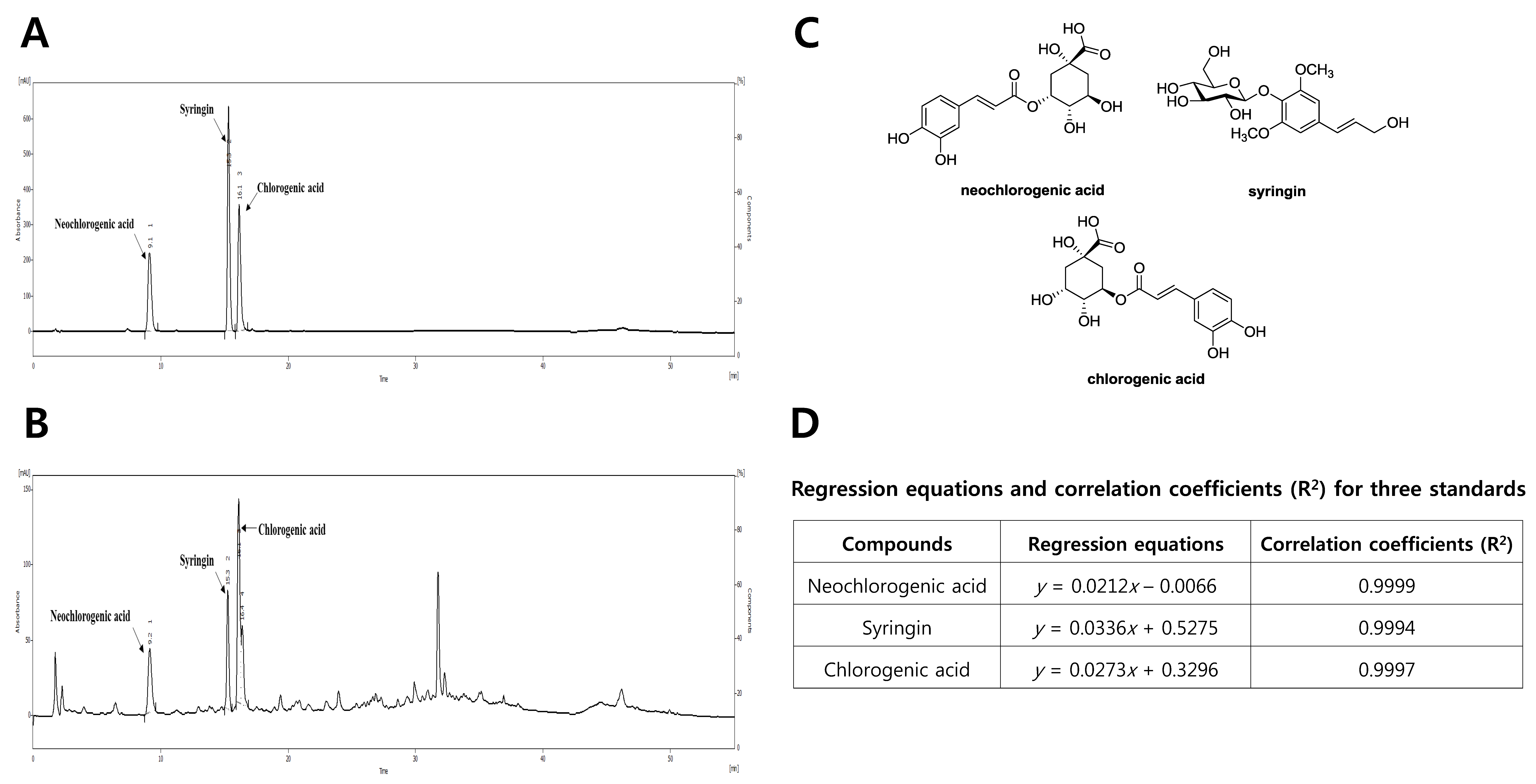
2.3. HPLC Analysis of the Water Extract of D. morbifera
2.4. Experimental Design
2.5. Urinalysis and Serum Biochemical Analysis
2.6. Histopathological Examination
2.7. Determination of Antioxidant Enzyme Activity
2.8. Analysis of Inflammatory Cytokines
2.9. Western Blot Analysis
2.10. Immunohistochemical Examination
2.11. TUNEL Assay
2.12. In Vivo Tumor Xenograft Model
2.13. Statistical Analysis
3. Results
3.1. Phytochemical Characterization of DM
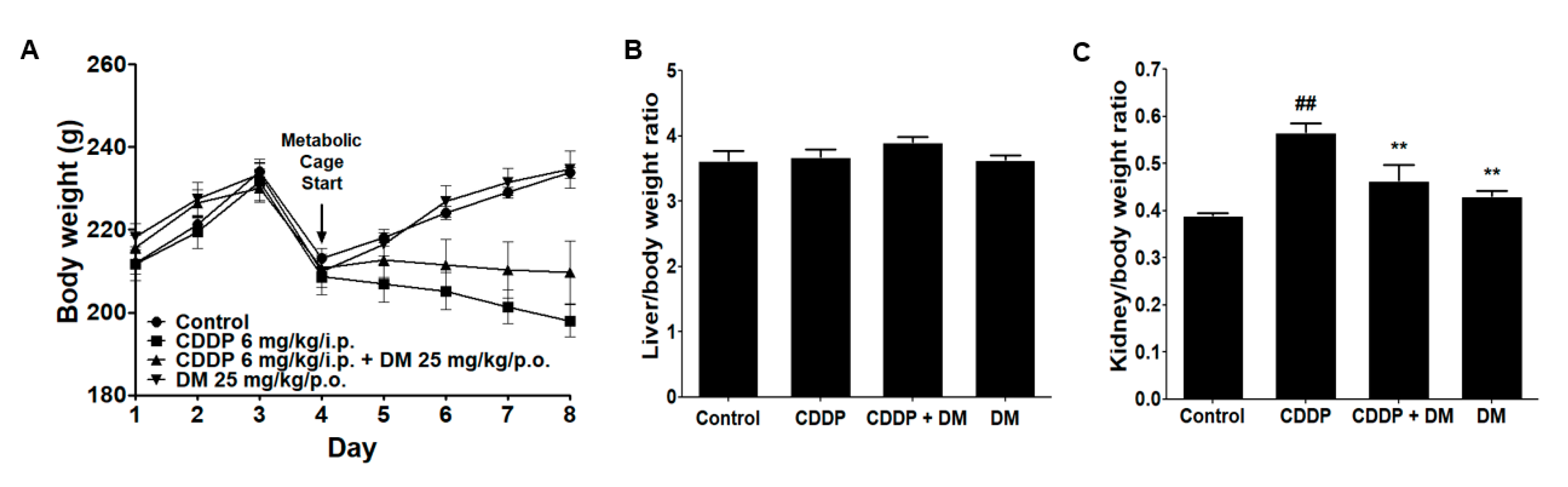
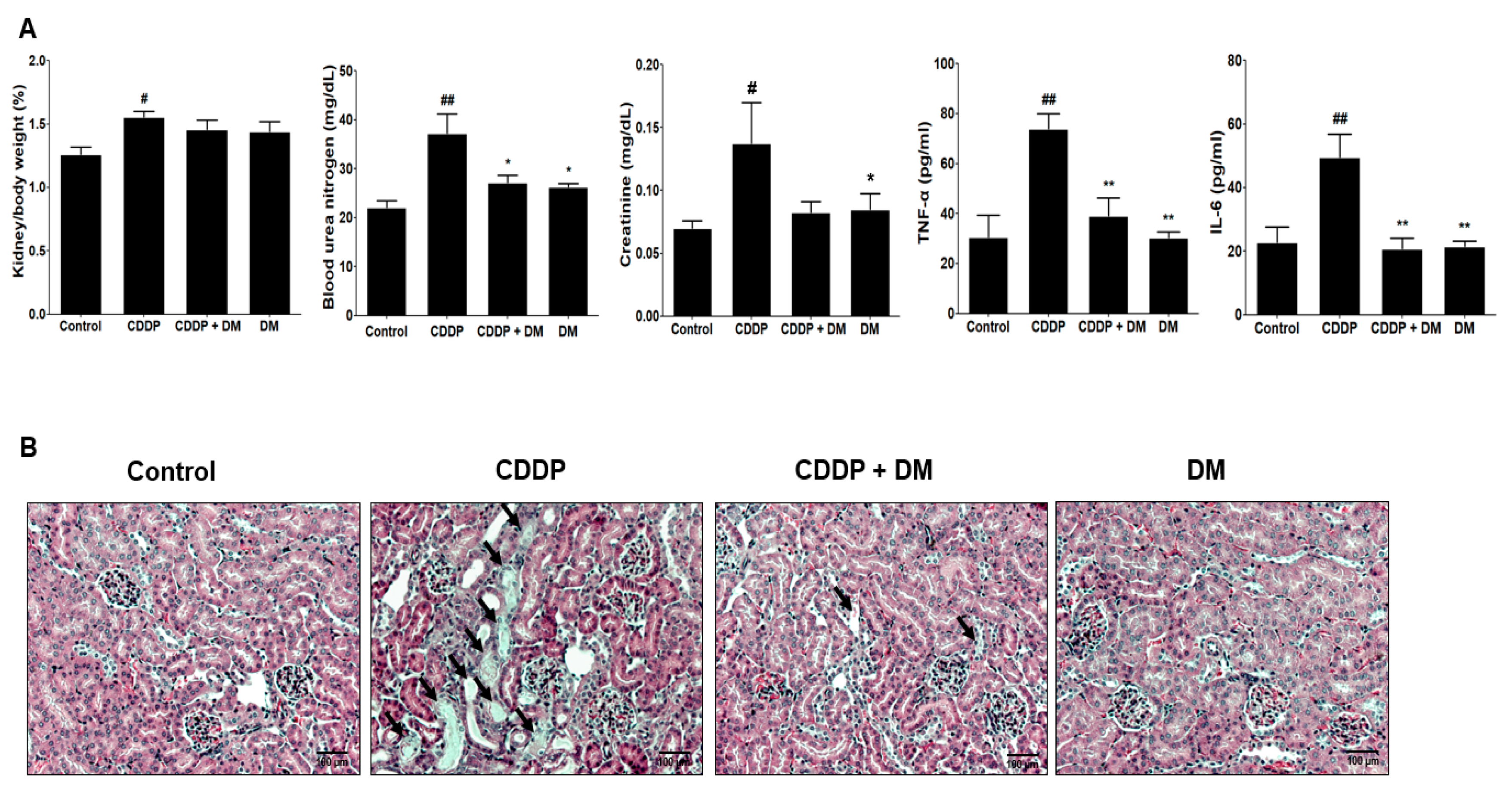
3.2. Effects of DM on Body and Kidney Weight Changes in CDDP-Treated Rats
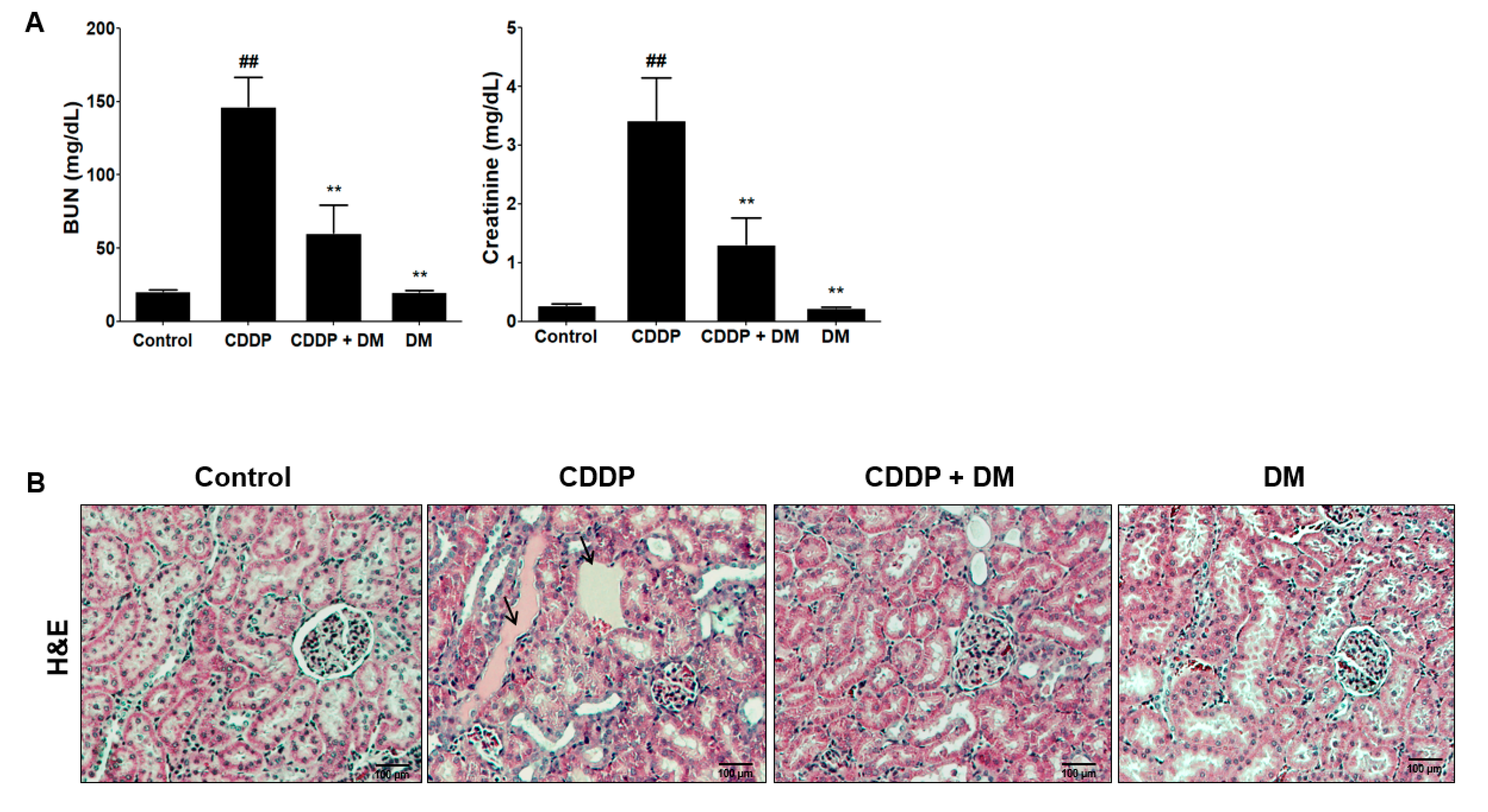
3.3. Protective Effect of DM on CDDP-Induced AKI
3.4. Effect of DM on the Urinary Excretion of AKI Biomarkers in CDDP-Treated Rats
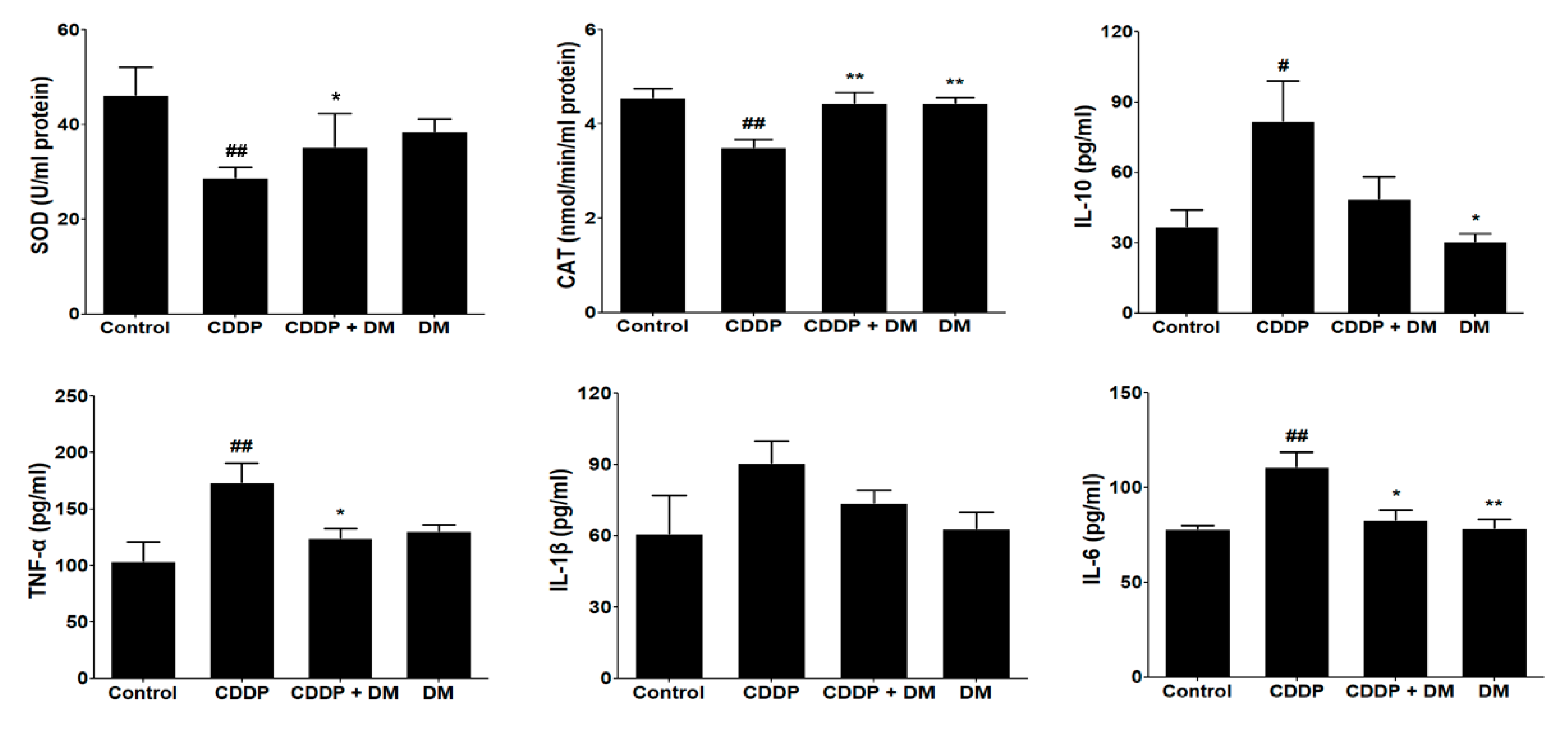
3.5. Effect of DM on Antioxidant Enzyme Activity and Pro-Inflammatory Cytokines in CDDP-Treated Rats
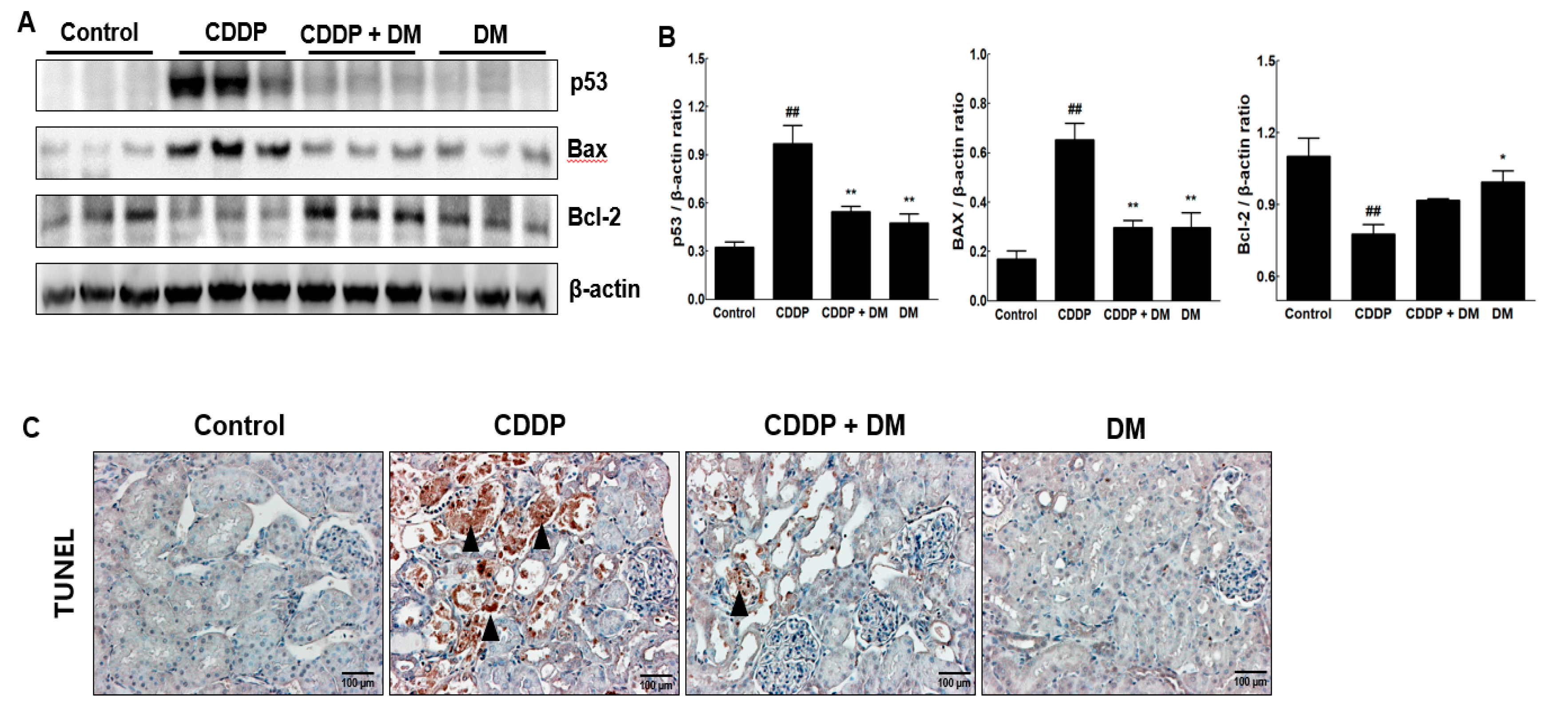
3.6. Effect of DM on Apoptosis in the Kidney of CDDP-Treated Rats
3.7. Renoprotective Effects of DM on CDDP-Induced Tumor Xenograft Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siddik, Z.H. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 2003, 22, 7265–7279. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lippard, S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef]
- Cepeda, V.; Fuertes, M.A.; Castilla, J.; Alonso, C.; Quevedo, C.; Pe’rez, J.M. Biochemical mechanisms of cisplatin cytotoxicity. Anticancer Agents Med. Chem. 2007, 7, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.B.; Madias, N.E. Cisplatin nephrotoxicity. Miner. Electrolyte Metab. 1994, 20, 201–213. [Google Scholar] [PubMed]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of Cisplatin Nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awdishu, L.; Mehta, R.L. The 6R’s of drug induced nephrotoxicity. BMC Nephrol. 2017, 18, 124. [Google Scholar] [CrossRef] [PubMed]
- Patzer, L. Nephrotoxicity as a cause of acute kidney injury in children. Pediatr. Nephrol. 2008, 23, 2159–2173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, G.S.; Kim, H.J.; Shen, A.; Lee, S.B.; Khadka, D.; Pandit, A.; So, H.S. Cisplatin-induced kidney dysfunction and perspectives on improving treatment strategies. Electrolyte Blood Press. 2014, 12, 55–65. [Google Scholar] [CrossRef]
- Dobyan, D.C.; Levi, J.; Jacobs, C.; Kosek, J.; Weiner, M.W. Mechanism of cis-platinum nephrotoxicity: II. Morphologic observations. J. Pharmacol. Exp. Ther. 1980, 213, 551–556. [Google Scholar]
- Gowda, S.; Desai, P.B.; Kulkarni, S.S.; Hull, V.V.; Math, A.A.K.; Vernekar, S.N. Markers of renal function tests. N. Am. J. Med. Sci. 2010, 2, 170–173. [Google Scholar]
- Dos Santos, N.A.; Carvalho Rodrigues, M.A.; Martins, N.M.; Dos Santos, A.C. Cisplatin-induced nephrotoxicity and targets of nephroprotection: An update. Arch. Toxicol. 2012, 86, 1233–1250. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.D.; Bolt, H.M. Cisplatin-induced nephrotoxicity. Arch. Toxicol. 2012, 86, 1155–1156. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.C.; Lee, S.S. Chemical constituents from Dendropanax dentiger. Nat. Prod. Commun. 2013, 8, 363–365. [Google Scholar] [CrossRef]
- Moon, H.I. Antidiabetic effects of dendropanoxide from leaves of Dendropanax morbifera Leveille in normal and streptozotocin induced diabetic rats. Hum. Exp. Toxicol. 2011, 30, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Park, B.Y.; Min, B.S.; Oh, S.R.; Kim, J.H.; Kim, T.J.; Kim, D.H.; Bae, K.H.; Lee, H.K. Isolation and anticomplement activity of compounds from Dendropanax morbifera. J. Ethnopharmacol. 2004, 90, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Hyun, T.K.; Kim, M.O.; Lee, H.; Kim, Y.; Kim, E.; Kim, J.S. Evaluation of anti-oxidant and anti-cancer properties of Dendropanax morbifera Léveille. Food Chem. 2013, 141, 1947–1955. [Google Scholar] [CrossRef]
- Akram, M.; Kim, K.A.; Kim, E.S.; Syed, A.S.; Kim, C.Y.; Lee, J.S.; Bae, O.N. Potent anti-inflammatory and analgesic actions of the chloroform extract of Dendropanax morbifera mediated by the Nrf2/HO-1 pathway. Biol. Pharm. Bull. 2016, 39, 728–736. [Google Scholar] [CrossRef]
- Yang, H.Y.; Kim, K.S.; Lee, Y.H.; Park, J.H.; Kim, J.H.; Lee, S.Y.; Kim, Y.M.; Kim, I.S.; Kacew, S.; Lee, B.M.; et al. Dendropanax morbifera ameliorates thioacetamide-induced hepatic fibrosis via TGF-β1/Smads pathways. Int. J. Biol. Sci. 2019, 15, 800–811. [Google Scholar] [CrossRef]
- Choo, G.S.; Lim, D.P.; Kim, S.M.; Yoo, E.S.; Kim, S.H.; Kim, C.H.; Woo, J.S.; Kim, H.J.; Jung, J.Y. Anti-inflammatory effects of Dendropanax morbifera in lipopolysaccharide-stimulated RAW264.7 macrophages and in an animal model of atopic dermatitis. Mol. Med. Rep. 2019, 19, 2087–2096. [Google Scholar] [CrossRef]
- Kang, M.J.; Kwon, E.B.; Ryu, H.W.; Lee, S.; Lee, J.W.; Kim, D.Y.; Lee, M.K.; Oh, S.R.; Lee, H.S.; Lee, S.U.; et al. Polyacetylene from Dendropanax morbifera alleviates diet-induced obesity and hepatic steatosis by activating AMPK signaling pathway. Front. Pharmacol. 2018, 9, 537. [Google Scholar] [CrossRef]
- Birhanu, B.T.; Kim, J.Y.; Hossain, M.A.; Choi, J.W.; Lee, S.P.; Park, S.C. An in vivo immunomodulatory and anti-inflammatory study of fermented Dendropanax morbifera Léveille leaf extract. BMC Complement. Altern. Med. 2018, 18, 222. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.; Leung, N. Cisplatin nephrotoxicity: A review of the literature. J. Nephrol. 2018, 31, 15–25. [Google Scholar]
- Kim, J.S.; Son, J.Y.; Kim, K.S.; Lim, H.J.; Ahn, M.Y.; Kwack, S.J.; Kim, Y.M.; Lee, K.Y.; Lee, J.; Lee, B.M.; et al. Hepatic damage exacerbates cisplatin-induced acute kidney injury in Sprague-Dawley rats. J. Toxicol. Environ. Health Part A 2018, 81, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Horie, S.; Oya, M.; Nangaku, M.; Yasuda, Y.; Komatsu, Y.; Yanagita, M.; Kitagawa, Y.; Kuwano, H.; Nishiyama, H.; Ishioka, C.; et al. Guidelines for treatment of renal injury during cancer chemotherapy 2016. Clin. Exp. Nephrol. 2018, 22, 210–244. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kong, L.; Zhang, J.; Yu, G.; Lv, G.; Zhang, F.; Chen, X.; Tian, J.; Fu, F. The pseudoginsenoside F11 ameliorates cisplatin-induced nephrotoxicity without compromising its anti-tumor activity in vivo. Sci. Rep. 2014, 4, 4986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; Zhang, Y.; Kang, N.; Wang, Y.; Zhang, Z.; Zha, Z.; Yang, S.; Xu, Q.; Liu, Y. Anemoside B4 attenuates nephrotoxicity of cisplatin without reducing anti-tumor activity of cisplatin. Phytomedicine 2019, 56, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Lee, J.S.; Akram, M.; Kim, K.A.; Shin, Y.J.; Yu, J.H.; Bae, O.N. Protective activity of Dendropanax morbifera against cisplatin-induced acute kidney injury. Kidney Blood Press. Res. 2015, 40, 1–12. [Google Scholar] [CrossRef] [PubMed]
- An, N.Y.; Kim, J.E.; Hwang, D.; Ryu, H.K. Anti-diabetic effects of aqueous and ethanol extract of Dendropanax morbifera Leveille in streptozotocin-induced diabetes model. J. Nutr. Health 2014, 47, 394–402. [Google Scholar] [CrossRef]
- Kim, K.S.; Yang, H.Y.; Song, H.; Kang, Y.R.; Kwon, J.; An, J.; Son, J.Y.; Kwack, S.J.; Kim, Y.M.; Bae, O.N.; et al. Identification of a sensitive urinary biomarker, selenium-binding protein 1, for early detection of acute kidney injury. J. Toxicol. Environ. Health A 2017, 80, 453–464. [Google Scholar] [CrossRef]
- Cheon, J.H.; Kim, S.Y.; Son, J.Y.; Kang, Y.R.; An, J.H.; Kwon, J.H.; Song, H.S.; Moon, A.; Lee, B.M.; Kim, H.S. Pyruvate kinase M2: A novel biomarker for the early detection of acute kidney injury. Toxicol. Res. 2016, 32, 47–56. [Google Scholar] [CrossRef]
- Hoffmann, D.; Fuchs, T.C.; Henzler, T.; Matheis, K.A.; Herget, T.; Dekant, W.; Hewitt, P.; Mally, A. Evaluation of a urinary kidney biomarker panel in rat models of acute and subchronic nephrotoxicity. Toxicology 2010, 277, 49–58. [Google Scholar] [CrossRef] [PubMed]
- McDuffie, J.E.; Ma, J.Y.; Sablad, M.; Sonee, M.; Varacallo, L.; Louden, C.; Guy, A.; Vegas, J.; Liu, X.; La, D.; et al. Time course of renal proximal tubule injury, reversal, and related biomarker changes in rats following cisplatin administration. Int. J. Toxicol. 2013, 32, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, G.; Reeves, W.B. TNF-α mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J. Clin. Invest. 2002, 110, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Humanes, B.; Camaño, S.; Lara, J.M.; Sabbisetti, V.; González-Nicolás, M.Á.; Bonventre, J.V.; Tejedor, A.; Lázaro, A. Cisplatin-induced renal inflammation is ameliorated by cilastatin nephroprotection. Nephrol. Dial. Transplant. 2017, 32, 1645–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozkok, A.; Ravichandran, K.; Wang, Q.; Ljubanovic, D.; Edelstein, C.L. NF-κB transcriptional inhibition ameliorates cisplatin-induced acute kidney injury (AKI). Toxicol. Lett. 2016, 240, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, N.M.; Eladl, M.A.; Al-Gayyar, M.M. Renal protective effects of arjunolic acid in a cisplatin-induced nephrotoxicity model. Cytokine 2016, 77, 26–34. [Google Scholar] [CrossRef]
- Ahmed, L.A.; Shehata, N.I.; Abdelkader, N.F.; Khattab, M.M. Tempol, a superoxide dismutase mimetic agent, ameliorates cisplatin-induced nephrotoxicity through alleviation of mitochondrial dysfunction in mice. PLoS ONE 2014, 9, e108889. [Google Scholar] [CrossRef]
- Ning, Y.; Shi, Y.; Chen, J.; Song, N.; Cai, J.; Fang, Y.; Yu, X.; Ji, J.; Ding, X. Necrostatin-1 attenuates cisplatin-induced nephrotoxicity through suppression of apoptosis and oxidative stress and retains klotho expression. Front. Pharmacol. 2018, 9, 384. [Google Scholar] [CrossRef]
- Tristão, V.R.; Pessoa, E.A.; Nakamichi, R.; Reis, L.A.; Batista, M.C.; Durão Junior Mde, S.; Monte, J.C. Synergistic effect of apoptosis and necroptosis inhibitors in cisplatin-induced nephrotoxicity. Apoptosis 2016, 21, 51–59. [Google Scholar] [CrossRef]
- Bernal-Barquero, C.E.; Vázquez-Zapién, G.J.; Mata-Miranda, M.M. Review of alterations in gene expression and apoptotic pathways caused in nephrotoxicity induced by cisplatin. Nefrologia 2019, 39, 362–371. [Google Scholar] [CrossRef]
- Shaikh, Z.A.; Vu, T.T.; Zaman, K. Oxidative stress as a mechanism of chronic cadmium-induced hepatotoxicity and renal toxicity and protection by antioxidants. Toxicol. Appl. Pharmacol. 1999, 154, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef] [Green Version]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.S.; Kim, K.S.; Son, J.Y.; Kim, H.R.; Park, J.H.; Lee, S.H.; Lee, D.E.; Kim, I.S.; Lee, K.Y.; Lee, B.M.; et al. Protective Effects of Dendropanax morbifera against Cisplatin-Induced Nephrotoxicity without Altering Chemotherapeutic Efficacy. Antioxidants 2019, 8, 256. https://doi.org/10.3390/antiox8080256
Kim JS, Kim KS, Son JY, Kim HR, Park JH, Lee SH, Lee DE, Kim IS, Lee KY, Lee BM, et al. Protective Effects of Dendropanax morbifera against Cisplatin-Induced Nephrotoxicity without Altering Chemotherapeutic Efficacy. Antioxidants. 2019; 8(8):256. https://doi.org/10.3390/antiox8080256
Chicago/Turabian StyleKim, Ji Su, Kyeong Seok Kim, Ji Yeon Son, Hae Ri Kim, Jae Hyeon Park, Su Hyun Lee, Da Eun Lee, In Su Kim, Kwang Youl Lee, Byung Mu Lee, and et al. 2019. "Protective Effects of Dendropanax morbifera against Cisplatin-Induced Nephrotoxicity without Altering Chemotherapeutic Efficacy" Antioxidants 8, no. 8: 256. https://doi.org/10.3390/antiox8080256




