Ratio of Mercury Concentration to PCB Concentration Varies with Sex of White Sucker (Catostomus commersonii)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Methods
2.2. PCB, Hg, Methylmercury, and Age Determinations
2.3. Data Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boening, D.W. Ecological effects, transport, and fate of mercury: A general review. Chemosphere 2000, 40, 1335–1351. [Google Scholar] [CrossRef]
- Driscoll, C.T.; Mason, R.P.; Chan, H.M.; Jacob, D.J.; Pirrone, N. Mercury as a global pollutant: Sources, pathways, and effects. Environ. Sci. Technol. 2013, 47, 4967–4983. [Google Scholar] [CrossRef] [PubMed]
- Wiener, J.G.; Krabbenhoft, D.P.; Heinz, G.H.; Scheuhammer, A.M. Ecotoxicology of Mercury. In Handbook of Ecotoxicology, 2nd ed.; Hoffman, D.J., Rattner, B.A., Burton, G.A., Jr., Cairns, J., Jr., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 407–461. [Google Scholar]
- Storelli, M.M. Potential human health risks from metals (Hg, Cd, and Pb) and polychlorinated biphenyls (PCBs) via seafood consumption: Estimation of target hazard quotients (THQs) and toxic equivalents (TEQs). Food Chem. Toxicol. 2008, 46, 2782–2788. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.W.; Magos, L.; Myers, G.J. The toxicology of mercury—Current exposures and clinical manifestations. N. Engl. J. Med. 2003, 349, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Zahir, F.; Rizwi, S.J.; Haq, S.K.; Khan, R.H. Low dose mercury toxicity and human health. Environ. Toxicol. Pharmacol. 2005, 20, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Scheuhammer, A.M.; Meyer, M.W.; Sandheinrich, M.B.; Murray, M.W. Effects of environmental methylmercury on the health of wild birds, mammals, and fish. Ambio 2007, 36, 12–18. [Google Scholar] [CrossRef]
- Sandheinrich, M.B.; Bhavsar, S.P.; Bodaly, R.A.; Drevnick, P.E.; Paul, E.A. Ecological risk of methylmercury to piscivorous fish of the Great Lakes region. Ecotoxicology 2011, 20, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, B.F.; Furieri, L.B.; Peçanha, F.M.; Wiggers, G.A.; Vassallo, P.F.; Simões, M.R.; Fiorim, J.; Rossi de Batista, P.; Fioresi, M.; Rossoni, L.; et al. Toxic effects of mercury on the cardiovascular and central nervous systems. J. Biomed. Biotechnol. 2012, 2012, 949048. [Google Scholar] [CrossRef]
- Wiener, J.G.; Evers, D.C.; Gay, D.A.; Morrison, H.A.; Williams, K.A. Mercury contamination in the Laurentian Great Lakes region: Introduction and overview. Environ. Pollut. 2012, 161, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.M.; Walker, E.M., Jr.; Wu, M.; Gillette, C.; Blough, E.R. Environmental mercury and its toxic effects. J. Prev. Med. Public Health 2014, 47, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Somers, E.C.; Ganser, M.A.; Warren, J.S.; Basu, N.; Wang, L.; Zick, S.M.; Park, S.K. Mercury exposure and antinuclear antibodies among females of reproductive age in the United States: NHANES. Environ. Health Perspect. 2015, 123, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, G.L.; Toal, B.E. Development of a single-meal fish consumption advisory for methyl mercury. Risk Anal. 2000, 20, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.; Gochfeld, M.; Powers, C.W.; Waishwell, L.; Warren, C.; Goldstein, B.D. Science, policy, stakeholders, and fish consumption advisories: Developing a fish fact sheet for the Savannah River. Environ. Manag. 2001, 27, 501–514. [Google Scholar] [CrossRef]
- Pastorok, R.A.; Bartell, S.M.; Ferson, S.; Ginzburg, L.R. Ecological Modeling in Risk Assessment: Chemical Effects on Populations, Ecosystems, and Landscapes; Lewis: Boca Raton, FL, USA, 2002. [Google Scholar]
- McClain, W.C.; Chumchal, M.M.; Drenner, R.W.; Newland, L.W. Mercury concentrations in fish from Lake Meredith, Texas: Implications for the issuance of fish consumption advisories. Environ. Monit. Assess. 2006, 123, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Rooker, J.R.; Gill, G.A.; Turner, J.P. Bioaccumulation of mercury in pelagic fishes from the Northern Gulf of Mexico. Can. J. Fish. Aquat. Sci. 2007, 64, 458–469. [Google Scholar] [CrossRef]
- Raymond, B.; Rossmann, R. Total and methyl mercury accumulation in 1994–1995 Lake Michigan lake trout and forage fish. J. Great Lakes Res. 2009, 35, 438–446. [Google Scholar] [CrossRef]
- Weis, P.; Ashley, J.T.F. Contaminants in fish of the Hackensack Meadowlands, New Jersey: Size, sex, and seasonal relationships as related to health risks. Arch. Environ. Contam. Toxicol. 2007, 52, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P.; Sullivan, K.A. Mercury in Lake Michigan. Environ. Sci. Technol. 1997, 31, 942–947. [Google Scholar] [CrossRef]
- Marvin, C.; Painter, S.; Rossmann, R. Spatial and temporal patterns in mercury contamination in sediments of the Laurentian Great Lakes. Environ. Res. 2004, 95, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Jeremiason, J.D.; Kanne, L.A.; Lacoe, T.A.; Hulting, M.; Simcik, M.F. A comparison of mercury cycling in Lakes Michigan and Superior. J. Great Lakes Res. 2009, 35, 329–336. [Google Scholar] [CrossRef]
- Lepak, R.F.; Krabbenhoft, D.P.; Ogorek, J.M.; Tate, M.T.; Bootsma, H.A.; Hurley, J.P. Influence of Cladophora-quagga mussel assemblages on nearshore methylmercury production in Lake Michigan. Environ. Sci. Technol. 2015, 49, 7606–7613. [Google Scholar] [CrossRef] [PubMed]
- Madenjian, C.P.; Rediske, R.R.; Krabbenhoft, D.P.; Stapanian, M.A.; Chernyak, S.M.; O’Keefe, J.P. Sex differences in contaminant concentrations of fish: A synthesis. Biol. Sex Differ. 2016, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Madenjian, C.P.; Blanchfield, P.J.; Hrenchuk, L.E.; Van Walleghem, J.L.A. Mercury elimination rates for adult northern pike Esox lucius: Evidence for a sex effect. Bull. Environ. Contam. Toxicol. 2014, 93, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Cooke, S.J.; Bunt, C.M.; Hamilton, S.J.; Jennings, C.A.; Pearson, M.P.; Cooperman, M.S.; Markle, D.F. Threats, conservation strategies, and prognosis for suckers (Catostomidae) in North America: Insights from regional case studies of diverse family of non-game fishes. Biol. Conserv. 2005, 121, 317–331. [Google Scholar] [CrossRef]
- Doosey, M.H.; Bart, H.L., Jr. Morphological variation of the palatal organ and chewing pad of Catostomidae (Teleostei: Cypriniformes). J. Morphol. 2011, 272, 1092–1108. [Google Scholar] [CrossRef] [PubMed]
- Childress, E.S.; Allan, J.D.; McIntyre, P.B. Nutrient subsidies from iteroparous fish migrations can enhance stream productivity. Ecosystems 2014, 17, 522–534. [Google Scholar] [CrossRef]
- Scott, W.B.; Crossman, E.J. Freshwater Fishes of Canada; Fisheries Research Board of Canada Bulletin 184: Ottawa, ON, Canada, 1973. [Google Scholar]
- Michigan Department of Natural Resources. Michigan Suckers Are Popular with Spring Anglers. 2012. Available online: http://www.michigan.gov/dnr/0,4570,7-153-275299-RSS,00.html (accessed on 1 August 2018).
- Munkittrick, K.R.; Dixon, D.G. Use of white sucker (Catostomus commersoni) populations to assess the health of aquatic ecosystems exposed to low-level contaminant stress. Can. J. Fish. Aquat. Sci. 1989, 46, 1455–1462. [Google Scholar] [CrossRef]
- Blazer, V.S.; Hoffman, J.; Walsh, H.L.; Braham, R.P.; Hahn, C.; Collins, P.; Jorgenson, Z.; Ledder, T. Health of white sucker within the St. Louis River area of concern associated with habitat usage as assessed using stable isotopes. Ecotoxicology 2014, 23, 236–251. [Google Scholar] [CrossRef] [PubMed]
- Madenjian, C.P.; Stevens, A.L.; Stapanian, M.A.; Batterman, S.A.; Chernyak, S.M.; Menczer, J.E.; McIntyre, P.B. Sex difference in PCB concentrations of a catostomid fish. J. Environ. Anal. Toxicol. 2017, 7, 515. [Google Scholar] [CrossRef]
- American Fisheries Society. Guidelines for the Use of Fishes in Research. 2004. Available online: https://fisheries.org/docs/policy_useoffishes.pdf (accessed on 1 August 2018).
- USGS Mercury Research Laboratory. Methods Used for Common Analysis Types: Plant and Animal Tissue. Available online: http://wi.water.usgs.gov/mercury-lab/research/analysis-methods.html (accessed on 1 August 2018).
- United States Environmental Protection Agency. Method 7473: Mercury in Solids and Solutions by Thermal Decomposition, Amalgamation, and Atomic Absorption Spectrophotometry; USEPA, Office of Water: Washington, DC, USA, 2007.
- United States Environmental Protection Agency. Guidelines Establishing Test Procedures for the Analysis of Pollutants (Appendix B, Part 136, Definition of Procedures for the Determination of Method Detection Limit—Revision 1.11, Revised July 1, 1999); USEPA, Office of Water: Washington, DC, USA, 1990.
- Hammerschmidt, C.R.; Fitzgerald, W.F. Bioaccumulation and trophic transfer of methylmercury in Long Island Sound. Arch. Environ. Contam. Toxicol. 2006, 51, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, R.M.; Berry, C.R., Jr. Comparison of white sucker age estimates from scales, pectoral fin rays, and otoliths. N. Am. J. Fish. Manag. 2006, 26, 24–31. [Google Scholar] [CrossRef]
- Edwards, W.H.; Stapanian, M.A.; Stoneman, A.T. An evaluation of precision in age estimation of two treatments of otoliths from an aging burbot population. J. Appl. Ichthyol. 2011, 27 (Suppl. S1), 43–48. [Google Scholar] [CrossRef]
- Trippel, E.A.; Harvey, H.H. Reproductive responses of five white sucker (Catostomus commersoni) populations in relation to lake acidity. Can. J. Fish. Aquat. Sci. 1987, 44, 1018–1023. [Google Scholar] [CrossRef]
- Madenjian, C.P.; Jensen, O.P.; Krabbenhoft, D.P.; DeWild, J.F.; Ogorek, J.M.; Vastano, A.R. Mercury accumulation and the mercury-PCB-sex interaction in summer flounder. J. Mar. Sci. Res. Dev. 2016, 6, 188. [Google Scholar] [CrossRef]
- Pollock, M.S.; Dubé, M.G.; Schryer, R. Investigating the link between pulp mill effluent and endocrine disruption: Attempts to explain the presence of intersex fish in the Wabigoon River, Ontario, Canada. Environ. Toxicol. Chem. 2010, 29, 952–965. [Google Scholar] [CrossRef] [PubMed]
- Bay, K.; Asklund, C.; Skakkebaek, N.E.; Andersson, A.-M. Testicular dysgenesis syndrome: Possible role of endocrine disrupters. Best Pract. Res. Clin. Endocrinol. Metabol. 2006, 20, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Blazer, V.S.; Walsh, H.L.; Braham, R.P.; Hahn, C.M.; Mazik, P.; McIntyre, P.B. Tumours in white suckers from Lake Michigan tributaries: Ppathology and prevalence. J. Fish Dis. 2017, 40, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Madenjian, C.P.; Yule, D.L.; Chernyak, S.M.; Begnoche, L.J.; Berglund, E.K.; Isaac, E.J. Males exceed females in PCB concentrations of cisco (Coregonus artedi) from Lake Superior. Sci. Total Environ. 2014, 493, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Madenjian, C.P.; Jensen, O.P.; Rediske, R.R.; O’Keefe, J.P.; Vastano, A.R.; Pothoven, S.A. Differences in energy expenditures and growth dilution explain higher PCB concentrations in male summer flounder. PLoS ONE 2016, 11, e0147223. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.L. Mercury in Wisconsin Fishes: Pathological and Consumption Implications. Master’s Thesis, University of Wisconsin, Madison, WI, USA, 2016. [Google Scholar]
- Stapanian, M.A.; Madenjian, C.P.; Batterman, S.A.; Chernyak, S.M.; Edwards, W.H.; McIntyre, P.B. Distributions of PCB congeners and homologues in white sucker and coho salmon from Lake Michigan. Environ. Sci. Technol. 2018, 52, 4393–4401. [Google Scholar] [CrossRef] [PubMed]
- Madenjian, C.P.; Stapanian, M.A.; Cott, P.A.; Krabbenhoft, D.P.; Edwards, W.H.; Ogilvie, L.M.; Mychek-Londer, J.G.; DeWild, J.F. Females exceed males in mercury concentrations of burbot Lota lota. Arch. Environ. Contam. Toxicol. 2015, 68, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Madenjian, C.P.; Ebener, M.P.; Krabbenhoft, D.P. Mercury accumulation, and the mercury-PCB-sex interaction, in lake whitefish (Coregonus clupeaformis). Environments 2016, 3, 7. [Google Scholar] [CrossRef]
- Bloom, N.S. On the chemical form of mercury in edible fish and marine invertebrate tissue. Can. J. Fish. Aquat. Sci. 1992, 49, 1010–1017. [Google Scholar] [CrossRef]
- Scott, D.P. Mercury concentration of white muscle in relation to age, growth, and condition in four species of fishes from Clay Lake, Ontario. J. Fish. Res. Board Can. 1974, 31, 1723–1729. [Google Scholar] [CrossRef]
- Becker, D.S.; Bigham, G.N. Distribution of mercury in the aquatic food web of Onondaga Lake, New York. Water Air Soil Pollut. 1995, 80, 563–571. [Google Scholar] [CrossRef]
- Surette, C.; Lucotte, M.; Tremblay, A. Influence of intensive fishing on the partitioning of mercury and methylmercury in three lakes of Northern Québec. Sci. Total Environ. 2006, 368, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Donald, D.B.; Wissel, B.; Anas, M.U.M. Species-specific mercury bioaccumulation in a diverse fish community. Environ. Toxicol. Chem. 2015, 34, 2846–2855. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.A. Biological and environmental control of mercury accumulation by fish in lakes and reservoirs of Northern Manitoba, Canada. Can. J. Fish. Aquat. Sci. 1991, 48, 2449–2470. [Google Scholar] [CrossRef]
- Bodaly, R.A.; Fudge, R.J.P. Uptake of mercury by fish in an experimental boreal reservoir. Arch. Environ. Contam. Toxicol. 1999, 37, 103–109. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Water Quality Criterion for the Protection of Human Health: Methylmercury; EPA-823-R-01-001; USEPA, Office of Water: Washington, DC, USA, 2001.
- Stevens, A.L.; Baird, I.G.; McIntyre, P.B. Differences in mercury exposure among Wisconsin anglers arising from fish consumption preferences and advisory awareness. Fisheries 2018, 43, 31–41. [Google Scholar] [CrossRef]
- A Protocol for Mercury-Based Fish Consumption Advice: An Addendum to the 1993 Protocol for a Uniform Great Lakes Sport Fish Consumption Advisory. 2007. Available online: https://www.in.gov/isdh/files/Mercury_Protocol.pdf (accessed on 1 August 2018).
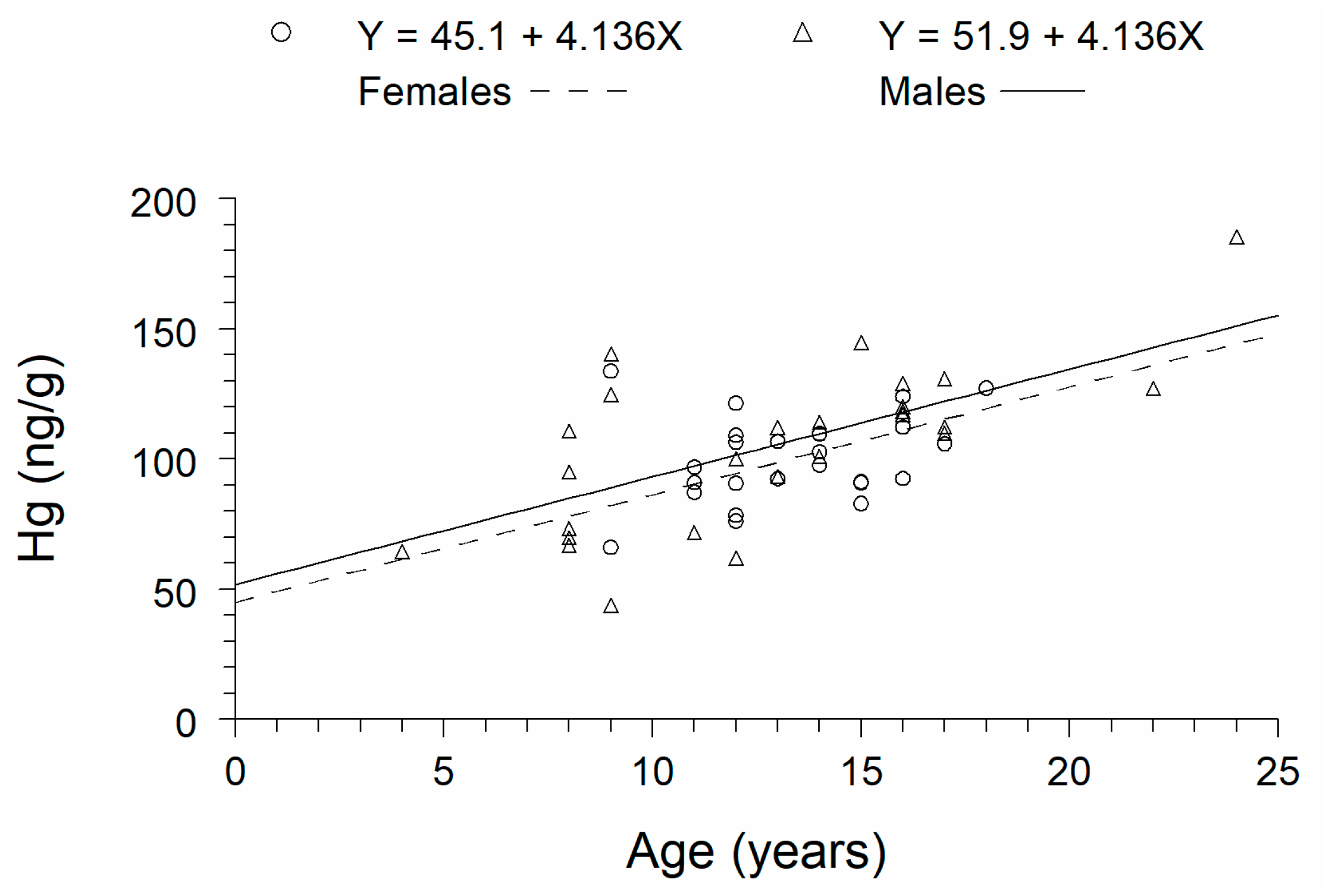
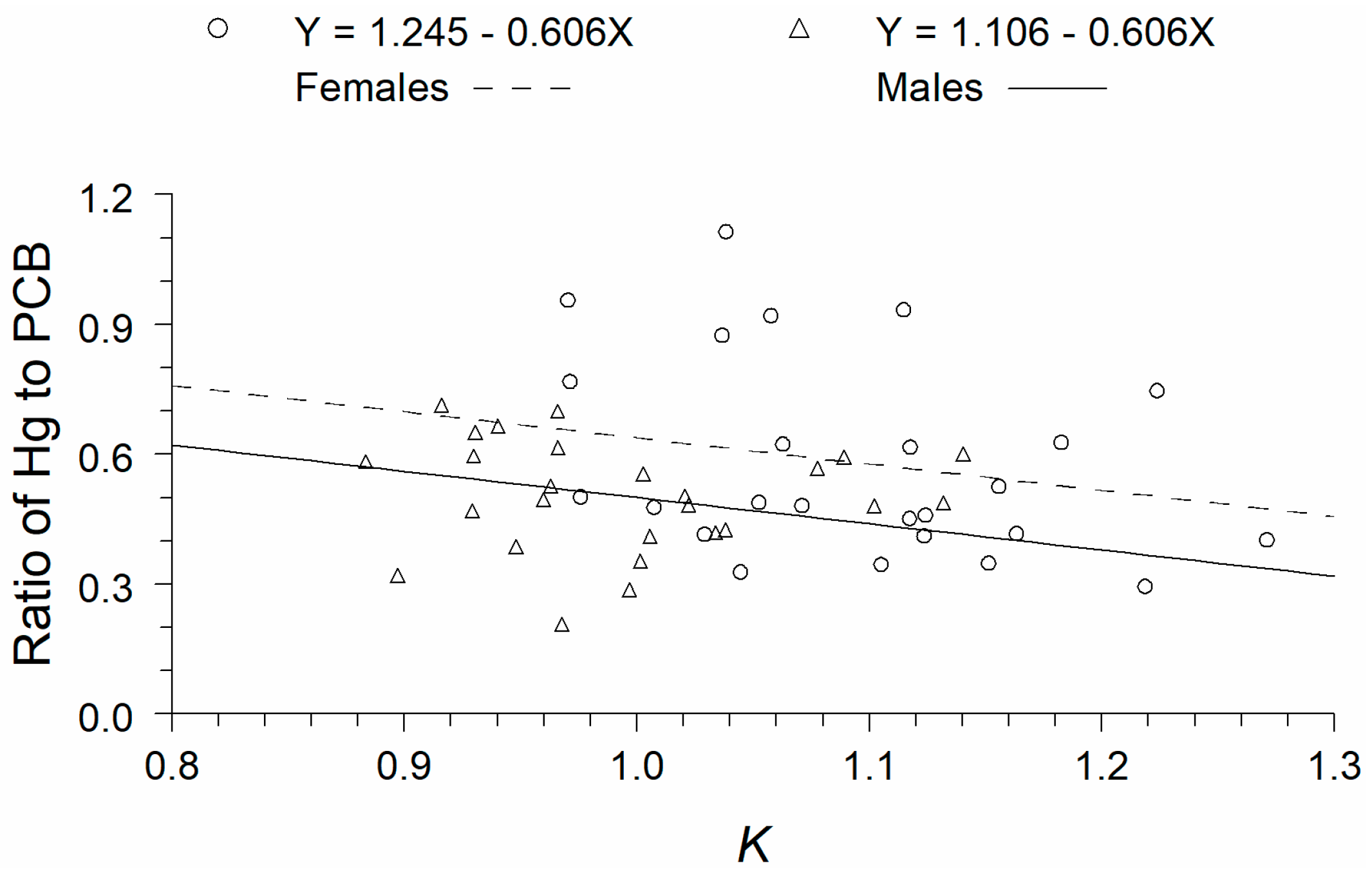
| Attribute | Females | Males |
|---|---|---|
| Total length (mm) | 490 (5) {450–540} | 442 (6) {375–510} |
| Weight (g) | 1298 (43) {885–1726} | 868 (33) {490–1215} |
| K | 1.095 (0.016) {0.970–1.271} | 0.995 (0.014) {0.833–1.140} |
| Age (years) | 13.3 (0.5) {9–18} | 12.9 (0.9) {4–24} |
| PCB concentration (ng/g) | 192 (12) {104–310} | 213 (9) {93–311} |
| Hg concentration (ng/g) | 100 (3) {66–134} | 105 (6) {44–185} |
| MeHg concentration (ng/g) | 90 (4) {65–122} | 100 (8) {40–150} |
| Proportion of Hg represented by MeHg | 0.972 (0.014) {0.893–1.058} | 0.981 (0.010) {0.924–1.052} |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madenjian, C.P.; Stevens, A.L.; Stapanian, M.A.; Krabbenhoft, D.P.; DeWild, J.F.; Ogorek, J.M.; Edwards, W.H.; Ogilvie, L.M.; McIntyre, P.B. Ratio of Mercury Concentration to PCB Concentration Varies with Sex of White Sucker (Catostomus commersonii). Environments 2018, 5, 94. https://doi.org/10.3390/environments5090094
Madenjian CP, Stevens AL, Stapanian MA, Krabbenhoft DP, DeWild JF, Ogorek JM, Edwards WH, Ogilvie LM, McIntyre PB. Ratio of Mercury Concentration to PCB Concentration Varies with Sex of White Sucker (Catostomus commersonii). Environments. 2018; 5(9):94. https://doi.org/10.3390/environments5090094
Chicago/Turabian StyleMadenjian, Charles P., Andrew L. Stevens, Martin A. Stapanian, David P. Krabbenhoft, John F. DeWild, Jacob M. Ogorek, William H. Edwards, Lynn M. Ogilvie, and Peter B. McIntyre. 2018. "Ratio of Mercury Concentration to PCB Concentration Varies with Sex of White Sucker (Catostomus commersonii)" Environments 5, no. 9: 94. https://doi.org/10.3390/environments5090094




