Effects of Conservation Agriculture and Fertilization on Soil Microbial Diversity and Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site and Experimental Design
2.2. Soil Preparation and Management
2.3. Soil Sampling and Analysis
2.3.1. Soil Chemical Analysis
2.3.2. Soil Microbial Functional Diversity
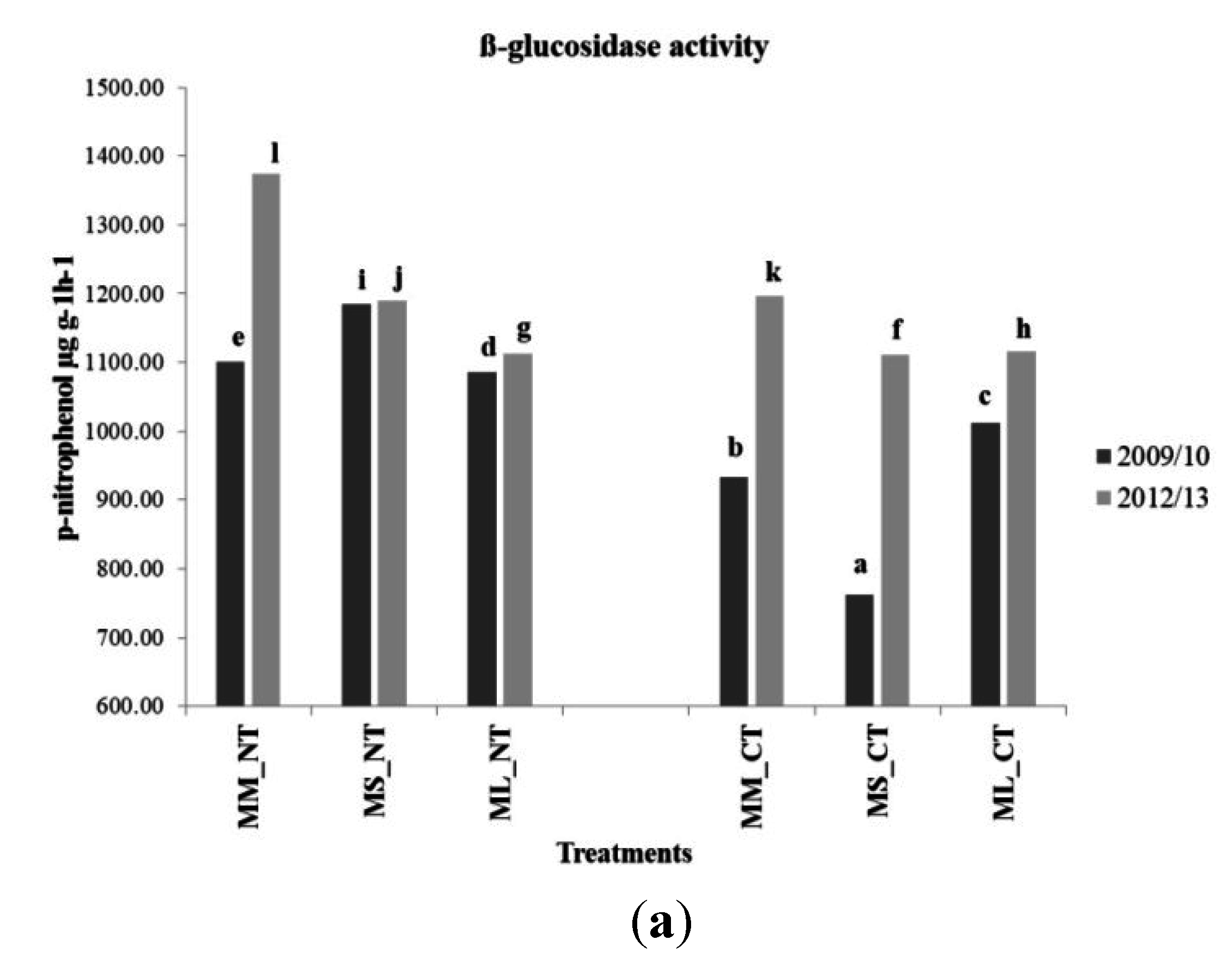
2.3.3. Soil Microbial Enzymatic Activity
2.4. Statistical Analysis
3. Results and Discussion
3.1. Climatological Observations
| Climatic Indicators | 2008/2009 | 2009/2010 | 2010/2011 | 2011/2012 | 2012/2013 |
|---|---|---|---|---|---|
| Rainfall (mm) | 708 | 1317 | 1010 | 571 | 709 |
| Temp min (°C) | 11.6 | 12.1 | 11.8 | 11.0 | 11.0 |
| Temp max (°C) | 27.5 | 27.0 | 27.1 | 27.8 | 27.6 |
3.2. Soil Nutrient Dynamics
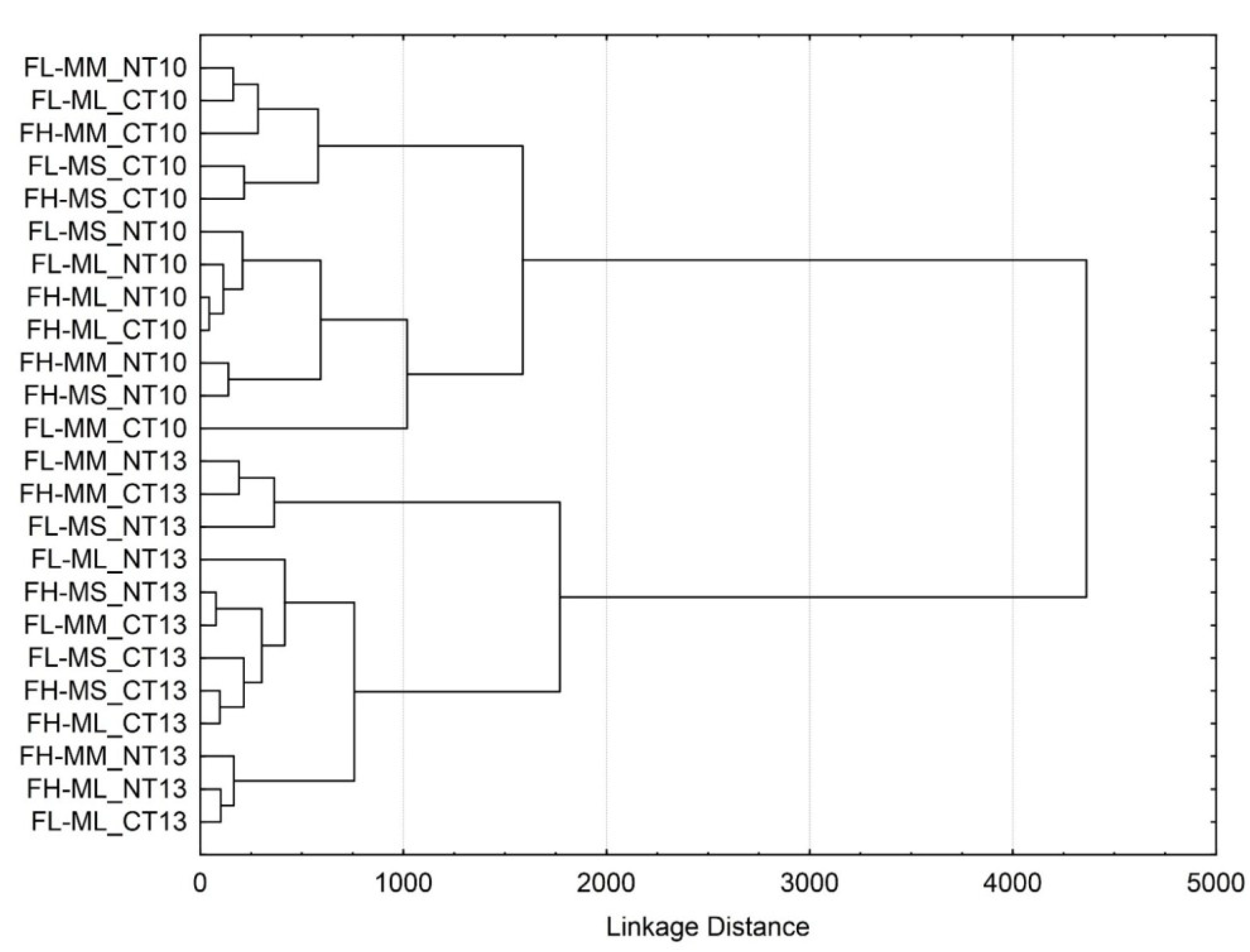
3.3. Soil Microbial Functional Diversity
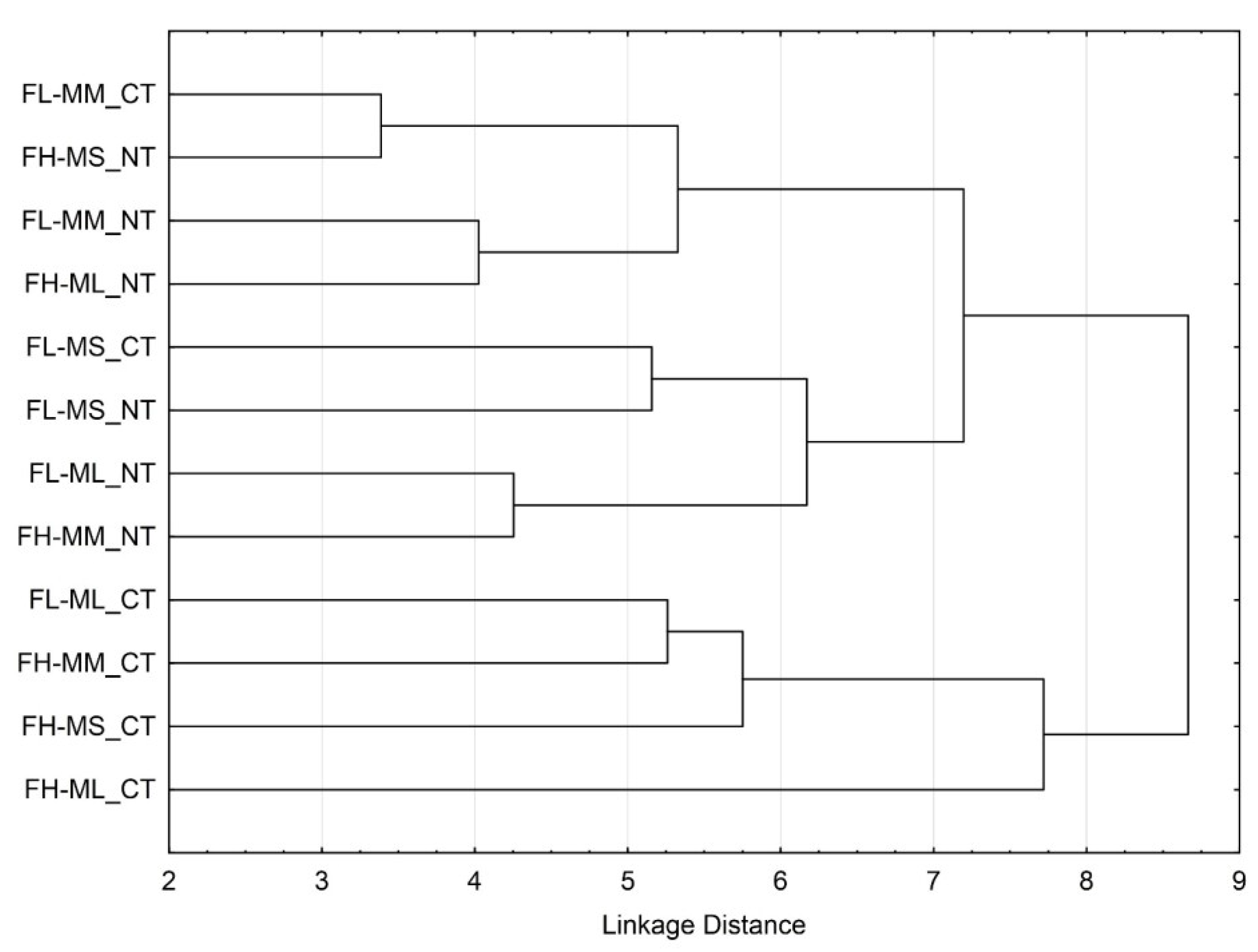
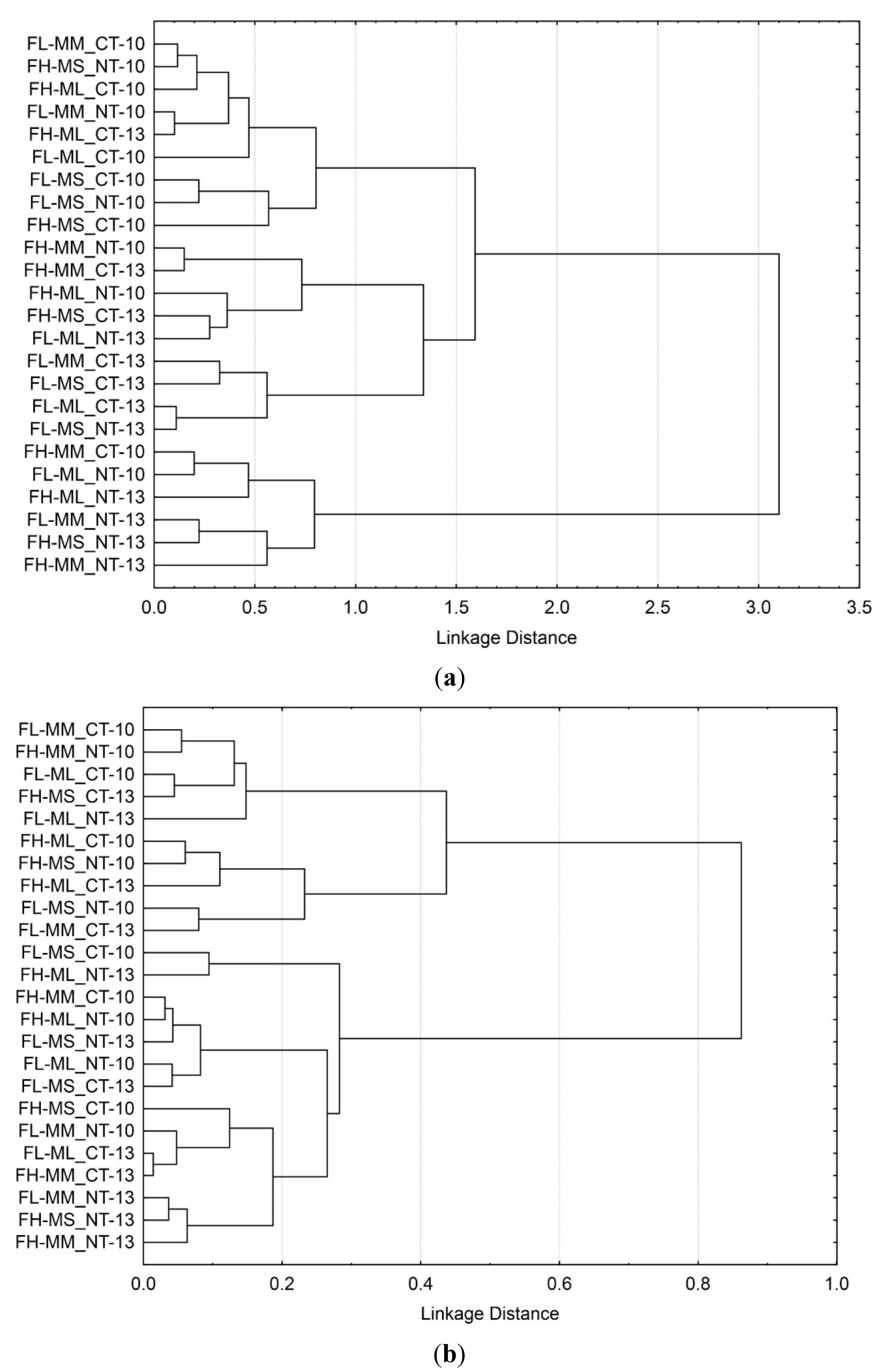

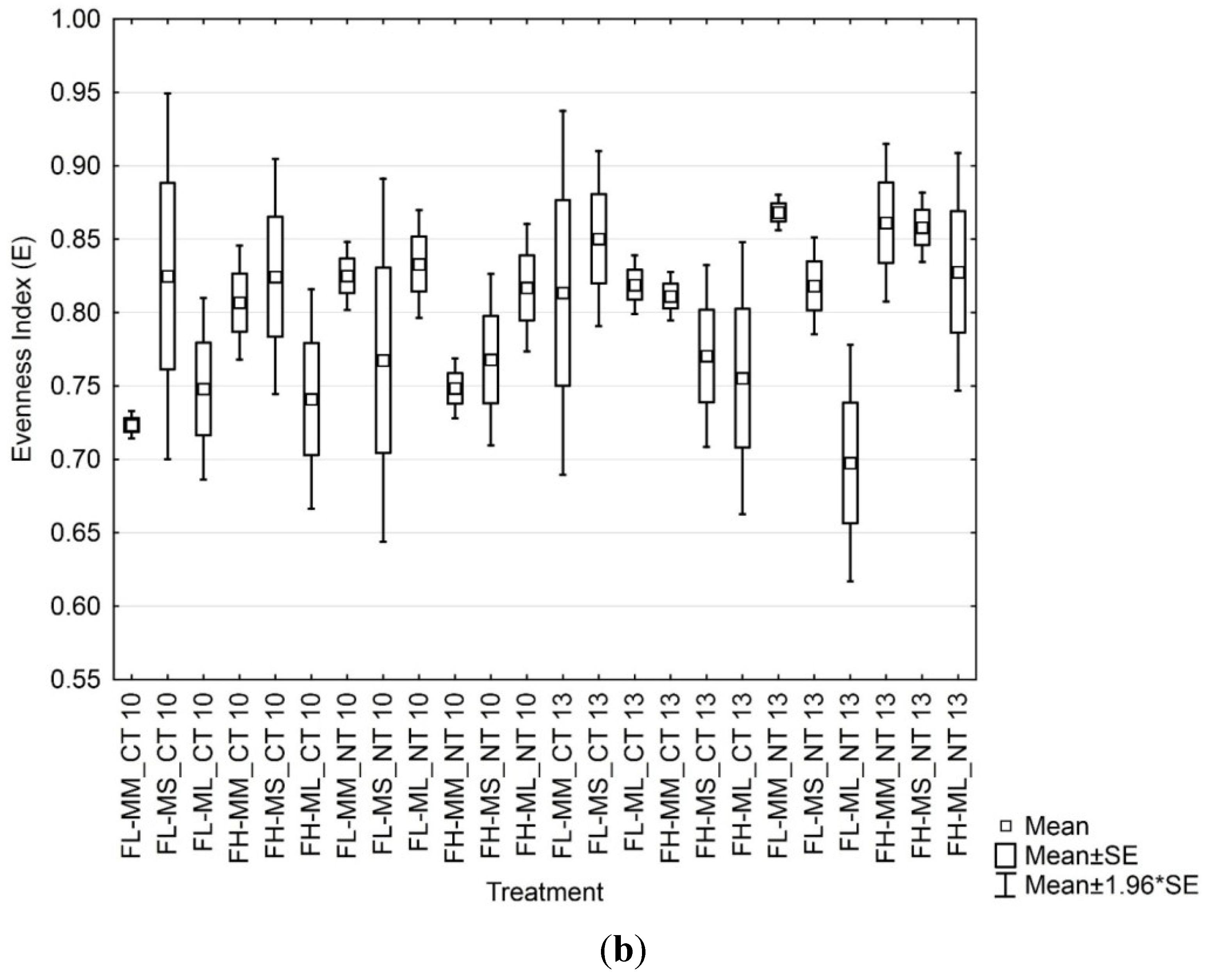
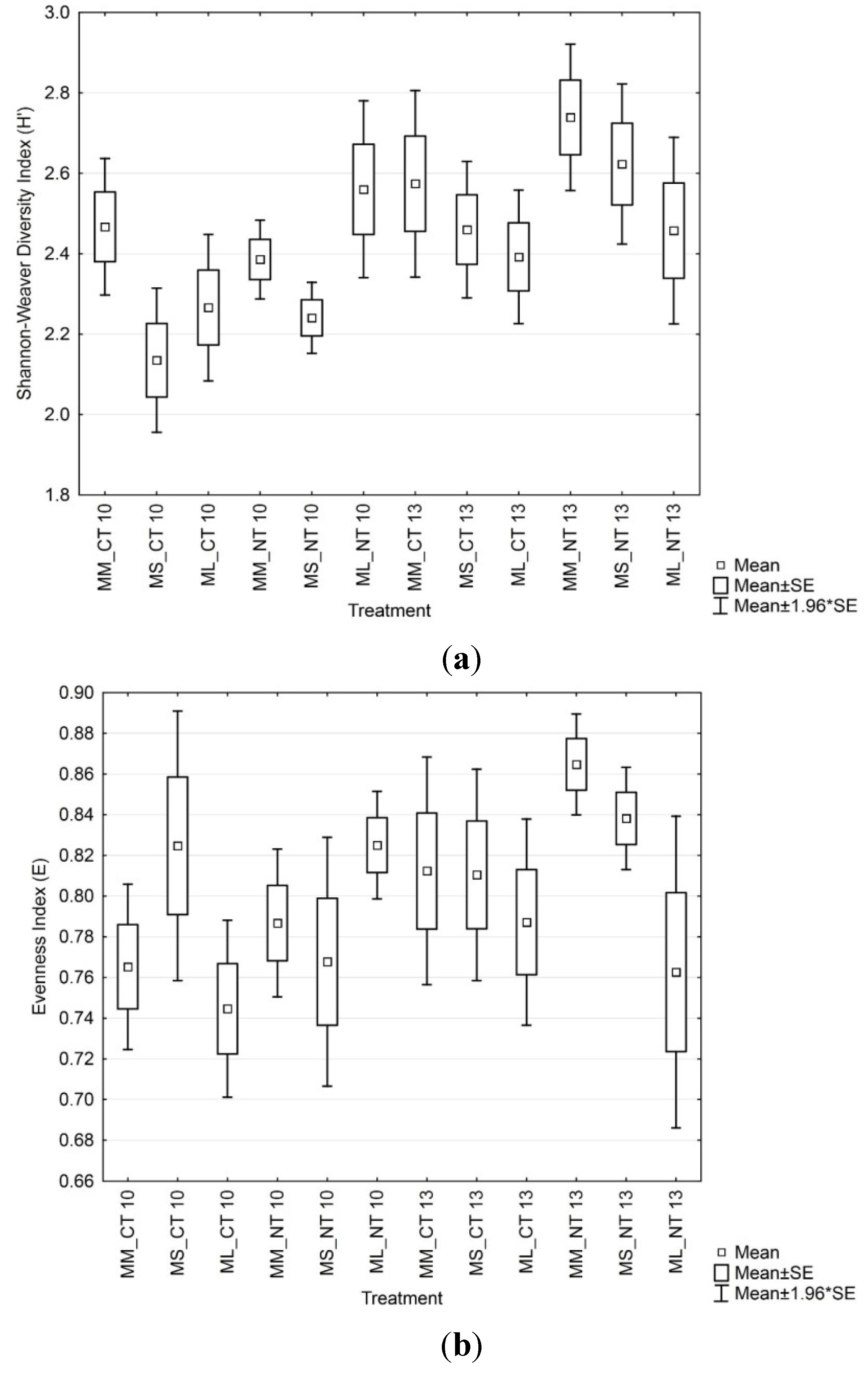
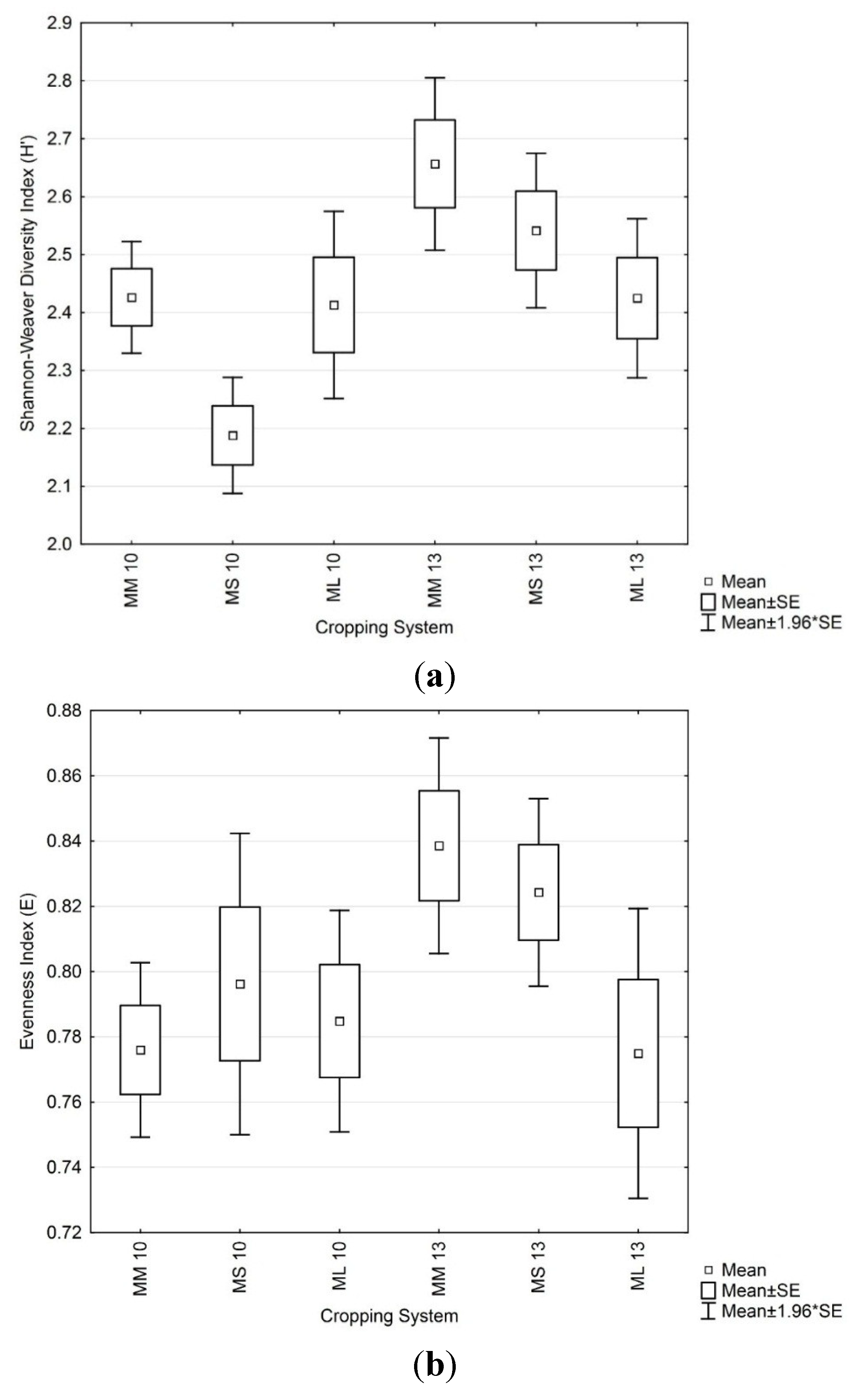
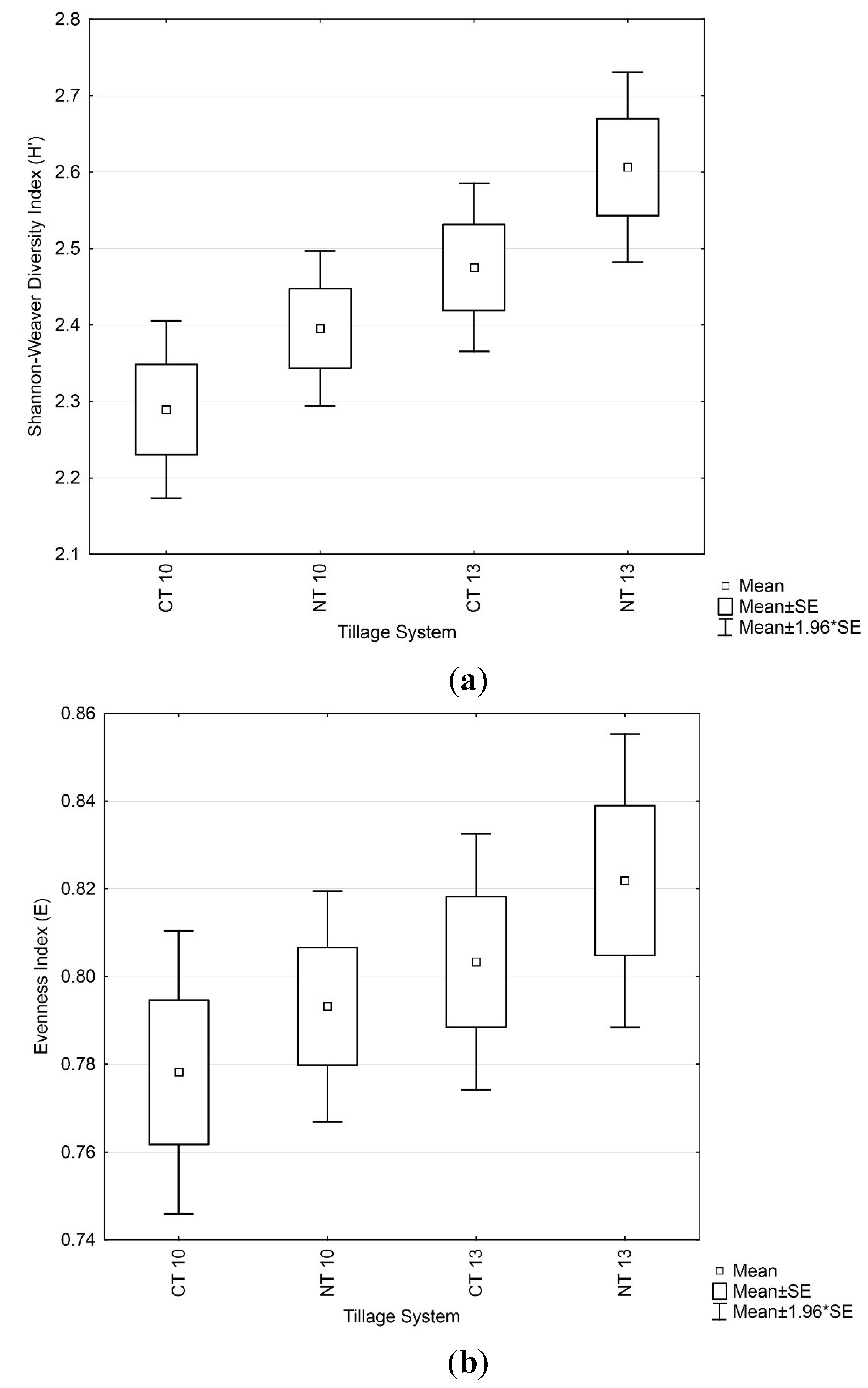
3.4. Soil Microbial Enzymatic Activity
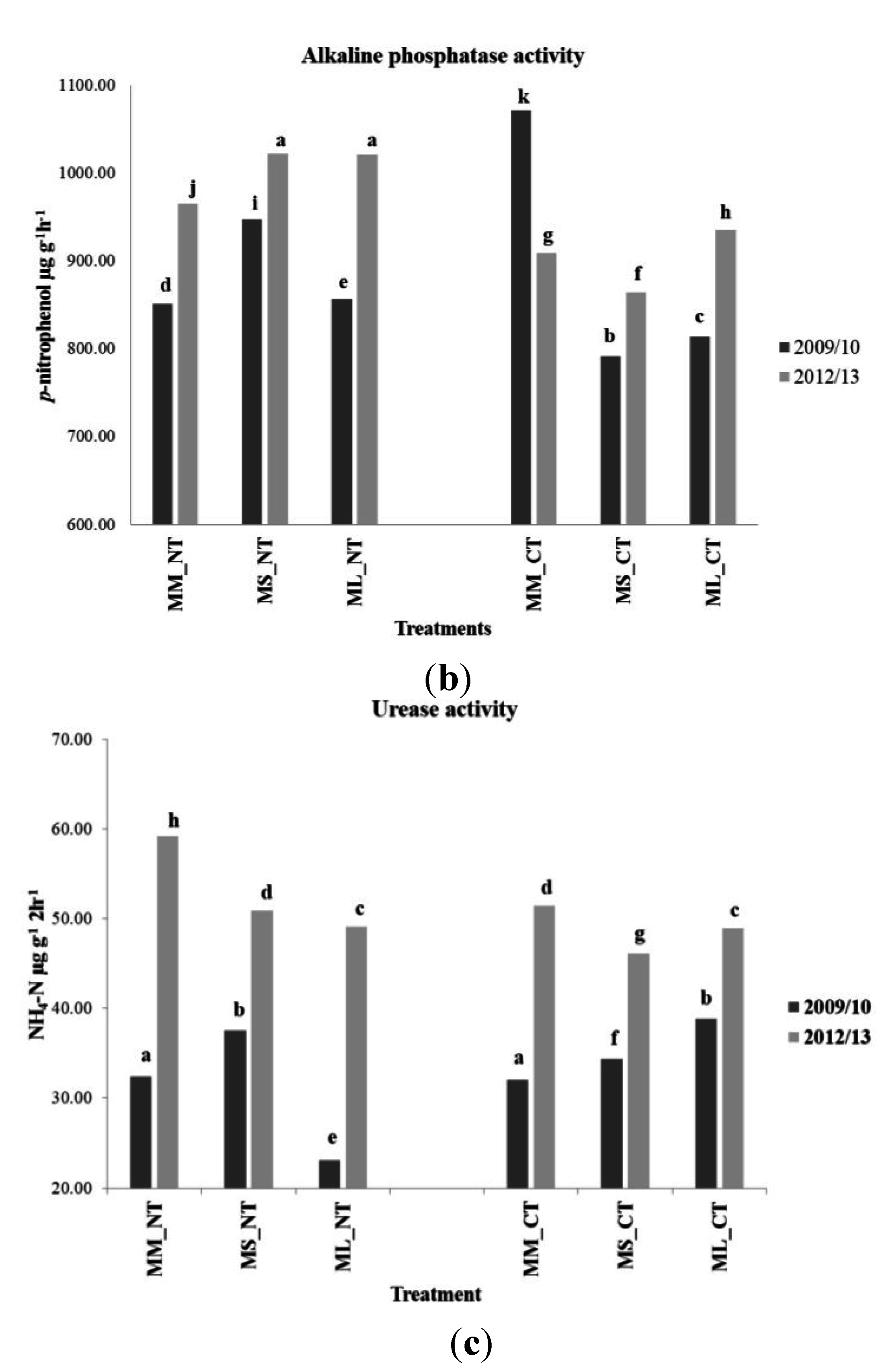
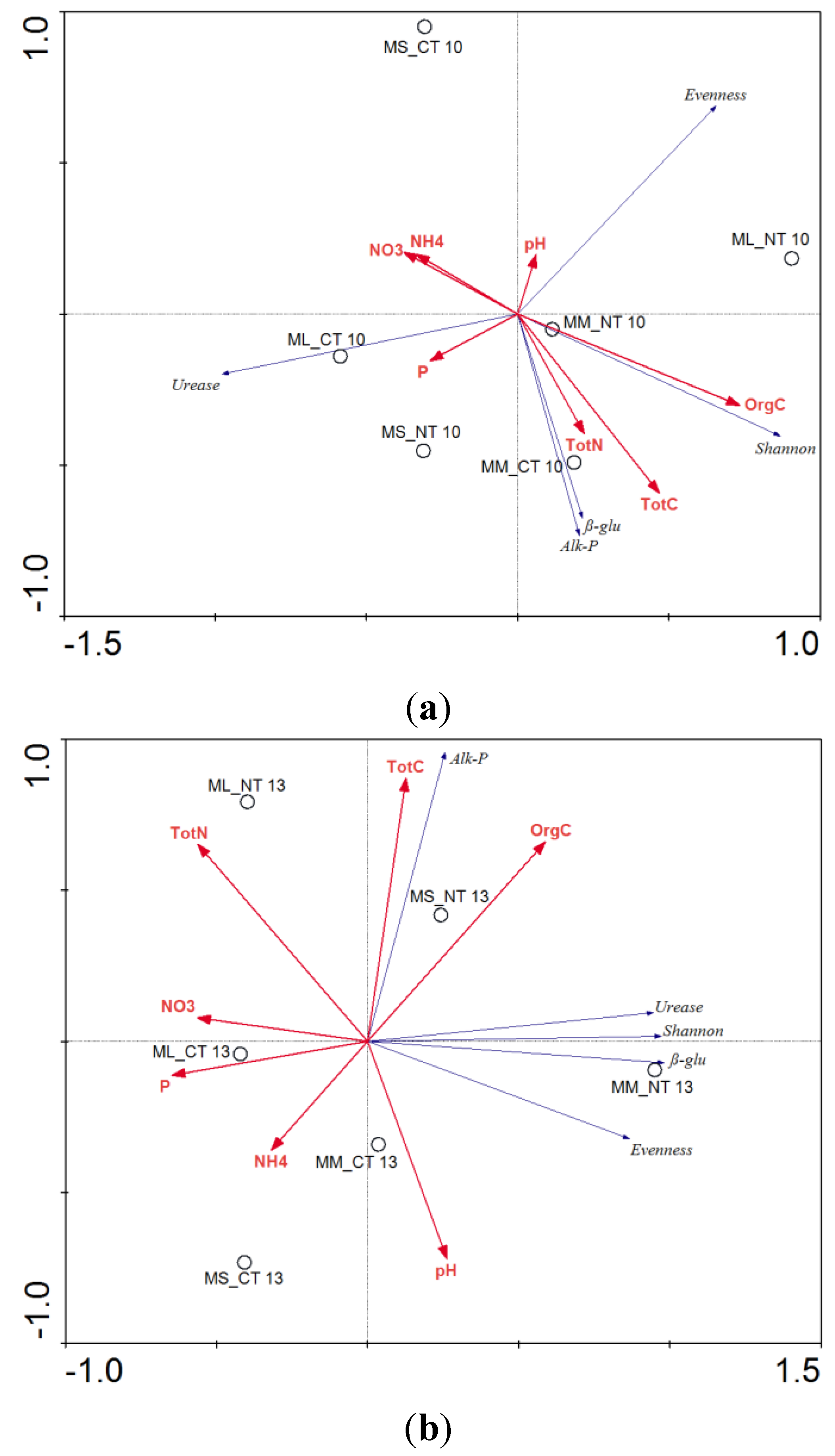
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
| Treatments | pH | P | NH4 | NO3 | Total C | Organic C | Total N |
|---|---|---|---|---|---|---|---|
| FH-ML_NT 10 | 6.327 ± 0.169 | 4.613 ± 2.540 | 6.253 ± 1.882 | 1.090 ± 0.400 | 1.350 ± 0.087 | 1.370 ± 0.101 | 0.117 ± 0.006 |
| FH-MM_NT 10 | 6.357 ± 0.214 | 4.927 ± 2.705 | 7.283 ± 2.861 | 1.313 ± 0.060 | 1.323 ± 0.029 | 1.287 ± 0.051 | 0.127 ± 0.025 |
| FH-MS_NT 10 | 6.393 ± 0.131 | 4.237 ± 1.533 | 5.873 ± 1.270 | 1.690 ± 1.331 | 1.347 ± 0.107 | 1.293 ± 0.100 | 0.113 ± 0.006 |
| FL-ML_NT 10 | 6.263 ± 0.076 | 6.163 ± 6.154 | 7.230 ± 3.482 | 1.357 ± 0.768 | 1.353 ± 0.085 | 1.327 ± 0.091 | 0.112 ± 0.007 |
| FL-MM_NT 10 | 6.340 ± 0.236 | 4.997 ± 2.289 | 7.283 ± 2.437 | 0.617 ± 0.357 | 1.373 ± 0.110 | 1.340 ± 0.108 | 0.113 ± 0.006 |
| FL-MS_NT 10 | 6.350 ± 0.115 | 5.017 ± 0.748 | 5.617 ± 0.497 | 3.323 ± 0.757 | 1.277 ± 0.025 | 1.243 ± 0.025 | 0.113 ± 0.012 |
| FH-ML_CT 10 | 6.097 ± 0.227 | 8.513 ± 9.385 | 9.980 ± 6.996 | 4.017 ± 2.746 | 1.330 ± 0.062 | 1.280 ± 0.098 | 0.113 ± 0.006 |
| FH-MM_CT 10 | 6.223 ± 0.121 | 11.223 ± 7.562 | 7.023 ± 1.825 | 1.737 ± 0.241 | 1.337 ± 0.068 | 1.260 ± 0.050 | 0.120 ± 0.000 |
| FH-MS_CT 10 | 6.220 ± 0.145 | 6.330 ± 3.683 | 8.497 ± 2.995 | 6.763 ± 2.879 | 1.313 ± 0.101 | 1.260 ± 0.089 | 0.113 ± 0.006 |
| FL-ML_CT 10 | 6.310 ± 0.118 | 8.043 ± 3.975 | 7.357 ± 2.381 | 2.687 ± 1.590 | 1.330 ± 0.075 | 1.310 ± 0.087 | 0.110 ± 0.010 |
| FL-MM_CT 10 | 6.020 ± 0.171 | 5.137 ± 2.934 | 7.990 ± 2.963 | 10.393 ± 4.096 | 1.503 ± 0.156 | 1.327 ± 0.107 | 0.117 ± 0.015 |
| FL-MS_CT 10 | 6.333 ± 0.305 | 6.037 ± 2.137 | 6.517 ± 1.362 | 5.050 ± 2.907 | 1.263 ± 0.035 | 1.227 ± 0.006 | 0.110 ± 0.010 |
| FH-ML_NT 13 | 5.997 ± 0.142 | 7.133 ± 5.036 | 2.680 ± 0.656 | 10.297 ± 3.495 | 1.318 ± 0.047 | 1.457 ± 0.205 | 0.109 ± 0.006 |
| FH-MM_NT 13 | 6.143 ± 0.131 | 5.967 ± 3.711 | 3.400 ± 0.769 | 7.800 ± 2.392 | 1.416 ± 0.079 | 1.460 ± 0.225 | 0.116 ± 0.004 |
| FH-MS_NT 13 | 6.157 ± 0.191 | 4.400 ± 1.153 | 2.870 ± 0.466 | 6.250 ± 4.235 | 1.287 ± 0.160 | 1.360 ± 0.171 | 0.102 ± 0.018 |
| FL-ML_NT 13 | 6.103 ± 0.136 | 4.867 ± 2.875 | 5.373 ± 2.352 | 17.050 ± 7.999 | 1.459 ± 0.230 | 1.397 ± 0.129 | 0.114 ± 0.019 |
| FL-MM_NT 13 | 6.193 ± 0.121 | 4.233 ± 1.528 | 3.043 ± 0.055 | 7.277 ± 2.511 | 1.208 ± 0.070 | 1.460 ± 0.141 | 0.067 ± 0.050 |
| FL-MS_NT 13 | 6.193 ± 0.099 | 4.233 ± 2.663 | 2.697 ± 1.367 | 6.910 ± 3.277 | 1.250 ± 0.149 | 1.303 ± 0.086 | 0.103 ± 0.013 |
| FH-ML_CT 13 | 6.050 ± 0.160 | 8.733 ± 2.967 | 4.950 ± 1.100 | 13.037 ± 5.846 | 1.331 ± 0.121 | 1.307 ± 0.125 | 0.110 ± 0.013 |
| FH-MM_CT 13 | 6.180 ± 0.265 | 5.700 ± 2.651 | 5.547 ± 2.039 | 13.197 ± 1.462 | 1.258 ± 0.095 | 1.297 ± 0.025 | 0.101 ± 0.013 |
| FH-MS_CT 13 | 6.097 ± 0.065 | 6.600 ± 3.905 | 3.090 ± 0.722 | 9.847 ± 4.344 | 1.166 ± 0.134 | 1.237 ± 0.103 | 0.089 ± 0.002 |
| FL-ML_CT 13 | 6.227 ± 0.304 | 5.867 ± 4.895 | 2.807 ± 1.363 | 11.300 ± 0.401 | 1.248 ± 0.162 | 1.263 ± 0.114 | 0.101 ± 0.009 |
| FL-MM_CT 13 | 6.073 ± 0.106 | 3.933 ± 1.767 | 6.253 ± 2.326 | 15.800 ± 5.909 | 1.240 ± 0.165 | 1.300 ± 0.079 | 0.099 ± 0.008 |
| FL-MS_CT 13 | 6.343 ± 0.104 | 5.567 ± 4.319 | 4.860 ± 2.120 | 8.497 ± 1.474 | 1.159 ± 0.044 | 1.180 ± 0.040 | 0.095 ± 0.002 |
| Elements | Management Practices | F-probabilities—ANOVA Output | |||
|---|---|---|---|---|---|
| 2010/2011 | 2012/2013 | ||||
| 0–5 cm | 5–10 cm | 0–5 cm | 5–10 cm | ||
| P | Fertilizer | <0.001 | <0.001 | - | - |
| K | Tillage | 0.046 | - | - | - |
| Crop | 0.038 | 0.008 | - | - | |
| Till × Fert | - | - | 0.049 | - | |
| Ca | Crop | 0.006 | - | 0.002 | 0.002 |
| Crop × Fert | - | 0.044 | 0.006 | 0.03 | |
| Mg | Tillage | 0.024 | - | - | - |
| Crop | 0.002 | - | 0.002 | 0.008 | |
| Crop × Fert | - | - | 0.03 | - | |
| Na | Fertilizer | - | 0.046 | - | - |
| Crop × Fert | 0.035 | 0.009 | - | - | |
| NH4 | Crop | 0.043 | 0.058 | 0.043 | 0.002 |
| Crop × Fert | 0.876 | 0.774 | - | - | |
| NO3 | Tillage | 0.025 | 0.079 | - | - |
| Crop | 0.011 | 0.064 | <0.001 | <0.001 | |
| Crop × Till | - | - | 0.001 | 0.001 | |
| Total N | Crop | 0.046 | 0.092 | - | 0.001 |
| Fertilizer | - | - | 0.04 | - | |
| Total C | Tillage | 0.040 | 0.166 | - | - |
| Crop | - | - | 0.027 | 0.007 | |
| Fertilizer | - | - | - | 0.029 | |
| Org C | Crop | 0.002 | 0.027 | 0.023 | 0.007 |
| Treatment | β-glucosidase Activity (p-nitrophenol µg·g−1·h−1) | ||||
| 2008/2009 | 2009/2010 | 2010/2011 | 2011/2012 | 2012/2013 | |
| FL-MM_NT | 559.13 | 992.94 | 2641.96 | 810.44 | 1481.33 |
| FH-MM_NT | 623.58 | 1207.95 | 2652.65 | 920.80 | 1267.99 |
| FL-MS_NT | 628.02 | 1172.31 | 2199.69 | 854.77 | 1405.49 |
| FH-MS_NT | 555.70 | 1196.94 | 2419.70 | 969.85 | 972.76 |
| FL-ML_NT | n/a ** | 1073.40 | 2538.26 | 966.65 | 1023.07 |
| FH-ML_NT | n/a ** | 1098.34 | 2327.18 | 1102.91 | 1202.67 |
| FL-MM_CT | 524.07 | 943.28 | 1429.03 | 705.17 | 1007.87 |
| FH-MM_CT | 565.50 | 923.51 | 1475.38 | 1006.95 | 1383.76 |
| FL-MS_CT | 574.03 | 818.25 | 1199.91 | 660.47 | 1143.14 |
| FH-MS_CT | 633.30 | 705.74 | 1349.17 | 639.76 | 1078.00 |
| FL-ML_CT | n/a ** | 934.13 | 1371.70 | 779.52 | 1169.21 |
| FH-ML_CT | n/a ** | 1091.05 | 1455.60 | 914.06 | 1062.63 |
| Treatment | Alkaline Phosphatase Activity (p-nitrophenol µg·g−1·h−1) | ||||
| 2008/2009 | 2009/2010 | 2010/2011 | 2011/2012 | 2012/2013 | |
| FL-MM_NT | 451.82 | 751.11 | 2268.82 | 377.07 | 981.29 |
| FH-MM_NT | 537.33 | 950.59 | 2139.82 | 700.47 | 949.23 |
| FL-MS_NT | 486.97 | 869.84 | 2395.61 | 886.62 | 1130.78 |
| FH-MS_NT | 440.10 | 1024.87 | 2121.44 | 894.73 | 911.95 |
| FL-ML_NT | n/a ** | 869.43 | 2094.44 | 905.82 | 1066.06 |
| FH-ML_NT | n/a ** | 844.24 | 1613.17 | 767.47 | 975.38 |
| FL-MM_CT | 411.89 | 1227.90 | 1432.70 | 757.46 | 885.66 |
| FH-MM_CT | 434.58 | 913.97 | 1498.62 | 836.45 | 931.45 |
| FL-MS_CT | 553.34 | 766.53 | 1275.81 | 901.16 | 839.68 |
| FH-MS_CT | 445.86 | 816.00 | 1510.01 | 698.79 | 888.42 |
| FL-ML_CT | n/a ** | 805.56 | 1267.21 | 587.45 | 929.43 |
| FH-ML_CT | n/a ** | 822.11 | 1628.90 | 654.43 | 941.26 |
| Treatment | Urease Activity (NH4-N µg g−1·2h−1) | ||||
| 2008/2009 | 2009/2010 | 2010/2011 | 2011/2012 | 2012/2013 | |
| FL-MM_NT | 36.29 | 32.52 | 57.56 | 49.88 | 64.65 |
| FH-MM_NT | 37.50 | 32.32 | 52.25 | 39.71 | 53.75 |
| FL-MS_NT | 20.91 | 30.13 | 60.14 | 56.44 | 54.82 |
| FH-MS_NT | 37.46 | 44.97 | 66.36 | 64.58 | 46.88 |
| FL-ML_NT | n/a ** | 10.25 | 49.77 | 47.12 | 47.62 |
| FH-ML_NT | n/a ** | 35.85 | 60.68 | 69.41 | 50.57 |
| FL-MM_CT | 36.67 | 32.51 | 49.79 | 55.94 | 45.74 |
| FH-MM_CT | 37.76 | 31.62 | 52.85 | 64.42 | 57.16 |
| FL-MS_CT | 41.49 | 34.95 | 48.16 | 54.16 | 50.64 |
| FH-MS_CT | 41.76 | 33.71 | 50.37 | 51.53 | 41.66 |
| FL-ML_CT | n/a ** | 38.25 | 56.61 | 57.58 | 51.93 |
| FH-ML_CT | n/a ** | 39.47 | 50.07 | 67.34 | 45.88 |
References
- IASS Vimeo Channel. Let’s Talk about Soil. 2013. Available online: https://vimeo.com/53618201 (accessed on 18 March 2015).
- Doran, J.W.; Safley, M. Defining and assessing soil health and sustainable productivity. In Biological Indicators of Soil Health; Pankhurst, C.E., Doube, B.M., Gupta, V.V.S.R., Eds.; CAB International: Wallingford, UK, 1997. [Google Scholar]
- Gugino, B.K.; Idowu, O.J.; Schindelbeck, R.R.; van Es, H.M.; Wolfe, D.W.; Moebius-Clune, B.N.; Thies, J.E.; Abawi, G.S. Cornell Soil Health Assessment Training Manual, 2nd ed.; Cornell University, College of Agriculture and Life Science: Ithaca, NY, USA, 2009. [Google Scholar]
- Doran, J.W.; Zeiss, M.R. Soil health and sustainability: Managing the biotic component of soil quality. Appl. Soil Ecol. 2000, 15, 3–11. [Google Scholar] [CrossRef]
- Nunes, J.S.; Araujo, A.S.F.; Nunes, L.A.P.L.; Lima, L.M.; Carneiro, R.F.V.; Tsai, S.M.; Salviano, A.A.C. Land degradation on soil microbial biomass and activity in Northeast Brazil. Pedosphere 2012, 22, 88–95. [Google Scholar] [CrossRef]
- Habig, J.; Hassen, A.I.; Swart, A. Application of microbiology in conservation agriculture. In Conservation Agriculture; Farooq, M., Kadambot, H.M., Eds.; Springer International Publishing: Cham (ZG), Switzerland, 2015; pp. 525–557. [Google Scholar]
- Johansson, J.F.; Paul, L.R.; Finlay, R.D. Microbial interactions in the mycorrhizosphere and their significance for sustainable agriculture (Minireview). FEMS Microbiol. Ecol. 2004, 48, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Karlen, D.L.; Eash, N.S.; Unger, P.W. Soil and crop management effects on soil quality indicators. Am. J. Altern. Agric. 1992, 7, 48–55. [Google Scholar] [CrossRef]
- Hobbs, P.R.; Sayre, K.; Gupta, R. The role of conservation agriculture in sustainable agriculture. Philos. T. Roy. Soc. B 2008, 363, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Govaerts, B.; Mezzalama, M.; Unno, Y.; Sayre, K.D.; Luna-Guido, M.; Vanherck, K.; Dendooven, L.; Deckers, J. Influence of tillage, residue management, and crop rotation on soil microbial biomass and catabolic diversity. Appl. Soil Ecol. 2007, 37, 18–30. [Google Scholar] [CrossRef]
- Dick, R.P. Soil enzyme activities as indicators of soil quality. In Defining Soil Quality for a Sustainable Environment; Doran, J.W., Coleman, D.C., Bezdicek, D.F., Stewart, B.A., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; Volume 35, pp. 107–124. [Google Scholar]
- Jangid, K.; Williams, M.A.; Franzluebbers, A.J.; Reeves, J.H.; Jenkins, M.B.; Endale, D.M.; Coleman, D.C.; Whitman, W.B. Relative impacts of land-use, management intensity and fertilization upon soil microbial community structure in agricultural systems. Soil Biol. Biochem. 2008, 40, 2843–2853. [Google Scholar] [CrossRef]
- Dumanski, J.; Peiretti, R.; Benites, J.R.; McGarry, D.; Pieri, C. The paradigm of conservation tillage. Proc. World Assoc. Soil Water Conserv. 2006, P1, 58–64. [Google Scholar]
- Wall, P.C. Tailoring conservation agriculture to the needs of small farmers in developing countries. J. Crop. Impr. 2008, 19, 137–155. [Google Scholar] [CrossRef]
- Quio, Y.J.; Li, Z.Z.; Wang, X.; Zhu, B.; Hu, Y.G.; Zeng, Z.H. Effect of legume-cereal mixtures on the diversity of bacteria communities in the rhizosphere. Plant Soil Environ. 2012, 58, 174–180. [Google Scholar]
- Frey, S.D.; Elliott, E.T.; Paustian, K. Bacterial and fungal abundance and biomass in conventional and no-tillage agroecosystems along two climatic gradients. Soil Biol. Biochem. 1999, 31, 573–585. [Google Scholar] [CrossRef]
- Sturz, A.V.; Christie, B.R. Beneficial microbial allelopathies in the root zone: The management of soil quality and plant disease with rhizobacteria. Soil Till. Res. 2003, 72, 107–123. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Q.; Lü, Y.; Sun, X.; Jia, S.; Zhang, X.; Liang, W. Conservation tillage positively influences the microflora and microfauna in the black soil of Northeast China. Soil Till. Res. 2015, 149, 46–52. [Google Scholar] [CrossRef]
- Kibblewhite, M.G.; Ritz, K.; Swift, M.J. Soil health in agricultural systems. Philos. Trans. R. Soc. B 2008, 363, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, G.; Jayasekara, A.P.D.A.; de Silva, M.S.D.L.; Abeysekera, U.P. Developed microbial biofilms can restore deteriorated conventional agricultural soils. Soil Biol. Biochem. 2011, 43, 1059–1062. [Google Scholar] [CrossRef]
- Degens, B.P.; Harris, J.A. Development of a physiological approach to measure the catabolic diversity of soil microbial communities. Soil Biol. Biochem. 1997, 29, 1309–1320. [Google Scholar] [CrossRef]
- Degens, B.P.; Schippers, L.A.; Sparling, G.P.; Vojvodic-Vukovic, M. Decreases in organic C reserves in soils can reduce the catabolic diversity of soil microbial communities. Soil Biol. Biochem. 2000, 32, 189–196. [Google Scholar] [CrossRef]
- Elliot, E.T. Rationale for developing bioindicators of soil health. In Biological Indicators of Soil Health; Pankhurst, C.E., Doube, B.M., Gupta, V.V.S.R., Eds.; CAB International: Wallingford, UK, 1997; pp. 49–78. [Google Scholar]
- Tikhonovich, I.A.; Provorov, N.A. Microbiology is the basis of sustainable agriculture: An opinion. Ann. Appl. Biol. 2011, 159, 155–168. [Google Scholar] [CrossRef]
- Njira, K.O.W.; Nabwami, J. Soil management practices that improve soil health: Elucidating their implications on biological indicators. J. Anim. Plant Sci. 2013, 18, 2750–2760. [Google Scholar]
- Garland, J.L.; Mills, A.L. Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilisation. Appl. Environ. Microb. 1991, 57, 2351–2359. [Google Scholar]
- Bossio, D.A.; Girvan, M.S.; Verchot, L.; Bullimore, J.; Borelli, T.; Albrecht, A.; Scow, K.M.; Ball, A.S.; Pretty, J.N.; Osborn, A.M. Soil microbial community response to land use change in an agricultural landscape of Western Kenya. Microb. Ecol. 2005, 49, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Doi, R.; Ranamukhaarachchi, S.L. Community-level physiological profiling in monitoring rehabilitative effects of Acacia auriculiformis plantation on degraded land in Sakaerat, Thailand. Silva Fenn. 2009, 43, 739–754. [Google Scholar] [CrossRef]
- Caldwell, B.A. Enzyme activities as a component of soil biodiversity: A review. Pedobiologia 2005, 49, 637–644. [Google Scholar] [CrossRef]
- Aon, M.A.; Colaneri, A.C. Temporal and spatial evolution of enzymatic activities and physicochemical properties in an agricultural soil. Appl. Soil Ecol. 2001, 18, 255–270. [Google Scholar] [CrossRef]
- Garcia, C.; Hernandez, T.; Roldan, A.; Martin, A. Effect of plant cover decline on chemical and microbiological parameters under Mediterranean climate. Soil Biol. Biochem. 2002, 34, 635–642. [Google Scholar] [CrossRef]
- Dick, R.P. Soil enzyme activities as integrative indicators of soil health. In Biological Indicators of Soil Health; Pankhurst, C.E., Doube, B.M., Gupta, V.V.S.R., Eds.; CAB International: Wallingford, UK, 1997; pp. 121–156. [Google Scholar]
- Deng, S.P.; Tabatabai, M.A. Effects of crop residue management on soil enzyme activities in soils. III. Phosphatases and arylsulphatase. Biol. Fertil. Soils 1997, 24, 141–146. [Google Scholar] [CrossRef]
- Soil Classification Working Group. Soil Classification; a Taxonomic System for South Africa; Soil and Irrigation Research Institute, Department of Agricultural Development: Pretoria, South Africa, 1991.
- Little, T.M.; Hills, F.J. Agricultural Experimentation Design and Analysis; John Wiley: New York, NY, USA, 1978; pp. 125–137. [Google Scholar]
- FSSA. Fertilizer Handbook 2007; Fertilizer Society of South Africa: Pretoria, South Africa, 2007. [Google Scholar]
- Buyer, J.S.; Drinkwater, L.E. Comparison of substrate utilization assay and fatty acid analysis of soil microbial communities. J. Microbiol. Meth. 1997, 30, 3–11. [Google Scholar] [CrossRef]
- Winding, A.; Hendriksen, N.B. BIOLOG® substrate utilization assay for metabolic fingerprints of soil bacteria: Incubation effects. In Microbial Communities: Functional versus Structural Approaches; Insam, I., Rangger, A., Eds.; Springer-Verlag: Berlin, Germany; Heidelberg, Germany, 1997; pp. 195–205. [Google Scholar]
- Magurran, A.E. Ecological Diversity and its Measurement; Princeton University Press: Princeton, NJ, USA, 1988. [Google Scholar]
- Lupwayi, N.Z.; Arshad, M.A.; Rice, W.A.; Clayton, G.W. Bacterial diversity in water-stable aggregates of soils under conventional and zero tillage management. Appl. Soil Ecol. 2001, 16, 251–261. [Google Scholar] [CrossRef]
- Zak, J.C.; Willig, M.R.; Moorhead, D.L.; Wildman, H.G. Functional diversity of microbial communities: A quantitative approach. Soil Biol. Biochem. 1994, 26, 1101–1108. [Google Scholar] [CrossRef]
- Dick, R.P.; Breakwell, D.P.; Turco, R.F. Soil enzyme activities and biodiversity measurements as integrative microbiological indicators. In Methods for Assessing Soil Quality; Doran, J.W., Jones, A.J., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; SSSA Special Publication 49; pp. 247–271. [Google Scholar]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- TerBraak, C.F.; Smilauer, P. Canoco Reference Manual for User’s Guide to Canoco Community Ordination, Ver. 4; Microcomputer Power: New York, NY, USA, 1998. [Google Scholar]
- ARC-ISCW. Agro-Climatology Long-term Reports. In ARC-ISCW Climate Information System; Agricultural Research Council—Institute for Soil, Climate and Water: Pretoria, South Africa, 2006. [Google Scholar]
- Fisk, M.C.; Fahey, T.J. Microbial biomass and nitrogen cycling responses to fertilization and litter removal in young northern hardwood forests. Biogeochemistry 2001, 53, 201–223. [Google Scholar] [CrossRef]
- De Quardos, P.D.; Zhalnina, K.; Davis-Richardson, A.; Fagen, J.R.; Drew, J.; Bayer, C.; Camargo, F.A.O.; Triplett, E.W. The effect of tillage system and crop rotation on soil microbial diversity and composition in a subtropical acrisol. Diversity 2012, 4, 375–395. [Google Scholar]
- Vargas, L.K.; Selbach, P.A.; de Sá, E.L.S. Microbial changes in soil during a maize crop season in no-till and conventional systems. Pesqui. Agropecu. Bras. 2004, 39, 749–755. [Google Scholar]
- Ceja-Navarro, J.A.; Rivera, F.N.; Patiño-Zuñiga, L.; Govaerts, B.; Marsch, R.; Vila-Sanjurjo, A.; Dendooven, L. Molecular characterization of soil bacterial communities in contrasting zero tillage systems. Plant Soil 2010, 329, 127–137. [Google Scholar] [CrossRef]
- Silva, A.P.; Babujia, L.C.; Matsumoto, L.S.; Guimarães, M.F.; Hungria, M. Bacterial diversity under different tillage and crop rotation systems in an Oxisol of Southern Brazil. Open Agric. J. 2013, 7 (Suppl. 1-M6), 40–47. [Google Scholar] [CrossRef]
- Swanepoel, C.M.; Marais, M.; Swart, A.; Habig, J.; Koch, S.; Sekgota, W.M.; Mampana, R.; Trytsman, G.; Beukes, D.J. Quantifying the Effects of Conservation Agriculture (CA) Practices on Soil and Plant Properties; ARC-ISCW Report No.: GW/A/2014/37; Agricultural Research Council-Institute for Soil, Climate and Water: Pretoria, South Africa, 2014. [Google Scholar]
- Gianinazzi, S.; Gollotte, A.; Binet, M.N.; van Tuinen, D.; Redecker, D.; Wipf, D. Agroecology: The key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 2010, 20, 519–530. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.A.; Thomas, G.W. Anion leaching in two Kentucky soils under conventional and no-till culture. Agron. J. 1976, 68, 437–442. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habig, J.; Swanepoel, C. Effects of Conservation Agriculture and Fertilization on Soil Microbial Diversity and Activity. Environments 2015, 2, 358-384. https://doi.org/10.3390/environments2030358
Habig J, Swanepoel C. Effects of Conservation Agriculture and Fertilization on Soil Microbial Diversity and Activity. Environments. 2015; 2(3):358-384. https://doi.org/10.3390/environments2030358
Chicago/Turabian StyleHabig, Johan, and Corrie Swanepoel. 2015. "Effects of Conservation Agriculture and Fertilization on Soil Microbial Diversity and Activity" Environments 2, no. 3: 358-384. https://doi.org/10.3390/environments2030358





