Unusually High Incidences of Staphylococcus aureus Infection within Studies of Ventilator Associated Pneumonia Prevention Using Topical Antibiotics: Benchmarking the Evidence Base
Abstract
:1. Introduction
- to survey and visually compare the incidence of S. aureus VAP (and VAP overall) within component (control and intervention) groups decanted from these studies versus an external benchmark.
- to model the effects of various group level factors within these studies on S. aureus VAP (and VAP overall) incidence. A key factor is membership of a component group of a study which either did or did not use topical placebo to achieve observer blinding.
- To collate data on the incidence of S. aureus bacteremia and methicillin resistant S. aureus (MRSA) VAP among those studies for which this data is available.
2. Materials and Methods
2.1. Study Selection and Decant of Groups
- An electronic search of PubMed, The Cochrane database and Google Scholar for systematic reviews containing potentially eligible studies was undertaken using the following search terms; “ventilator associated pneumonia”, “mechanical ventilation”, “intensive care unit”, each combined with either “meta-analysis” or “systematic review” up to December 2015. The use of systematic reviews as the starting point for benchmarking S. aureus VAP incidence serves two purposes; they provide estimates of the apparent effect size of the interventions of interest and, they provide objective and transparent sources of VAP incidence data.
- Systematic reviews of studies of patient populations requiring prolonged (>24 h) mechanical ventilation were then streamed into one of three categories; systematic reviews of studies in which there was no intervention (observational studies), systematic reviews of infection prevention studies using topical antibiotics in any formulation [198,199,200,201,202], systematic reviews of studies of non-antibiotic interventions (non-antibiotic studies) and systematic reviews of studies of topical antiseptics. The studies of non-antibiotic methods of VAP prevention encompass a broad range of methods delivered either via the gastric route [205,206,207,208], the airway route [209,210,211,212,213,214,215,216] or via the oral care route [201,202,217,218].
- The studies within these systematic reviews were screened against the following eligibility criteria. Inclusion criteria; infection prevention studies using concurrent controls and also observational studies for which incidence data for S. aureus VAP was extractable as an incidence proportion. The denominator for this incidence proportion is the numbers of patients receiving mechanical ventilation with an ICU stay of at least 24 h. Exclusion criteria; studies limited to patients with the acute respiratory distress syndrome. Studies in a language other than English were included where these had been abstracted in an English language systematic review.
- A hand search was undertaken for additional studies not identified within systematic reviews including studies published since 2015.
- All eligible studies were then collated and any duplicate studies were removed and streamed into groups of patients from studies without a VAP prevention method (observational groups) or component groups of the studies of antibiotics, studies of anti-septics and studies of non-antibiotic interventions.
- The component groups were decanted from each study as observational, control or intervention groups.
2.2. Outcomes of Interest
2.3. Benchmarking: Visual
2.4. Benchmarking: Meta-Regression
3. Results
3.1. Characteristics of the Studies
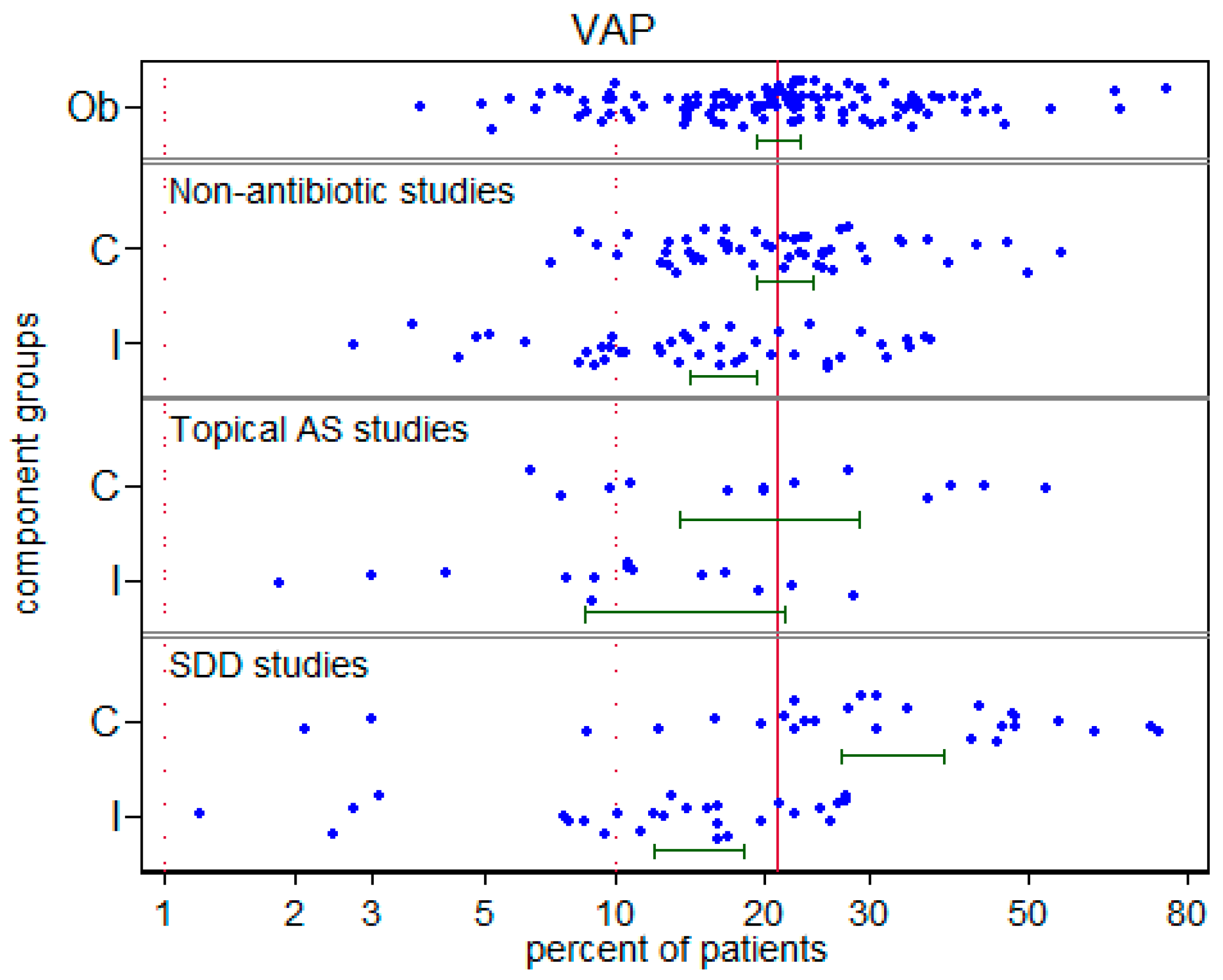
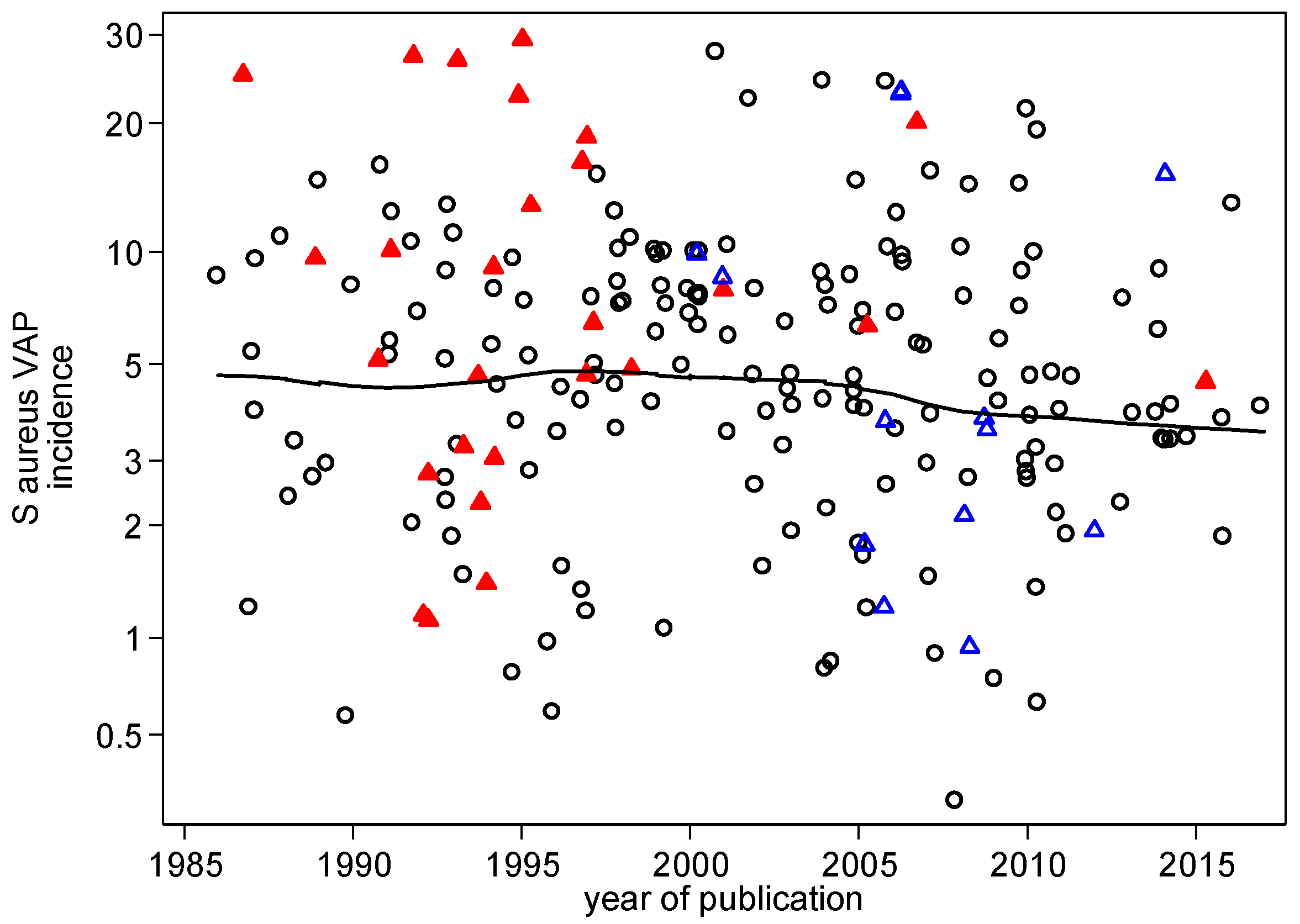
3.2. VAP Benchmarking: Visual
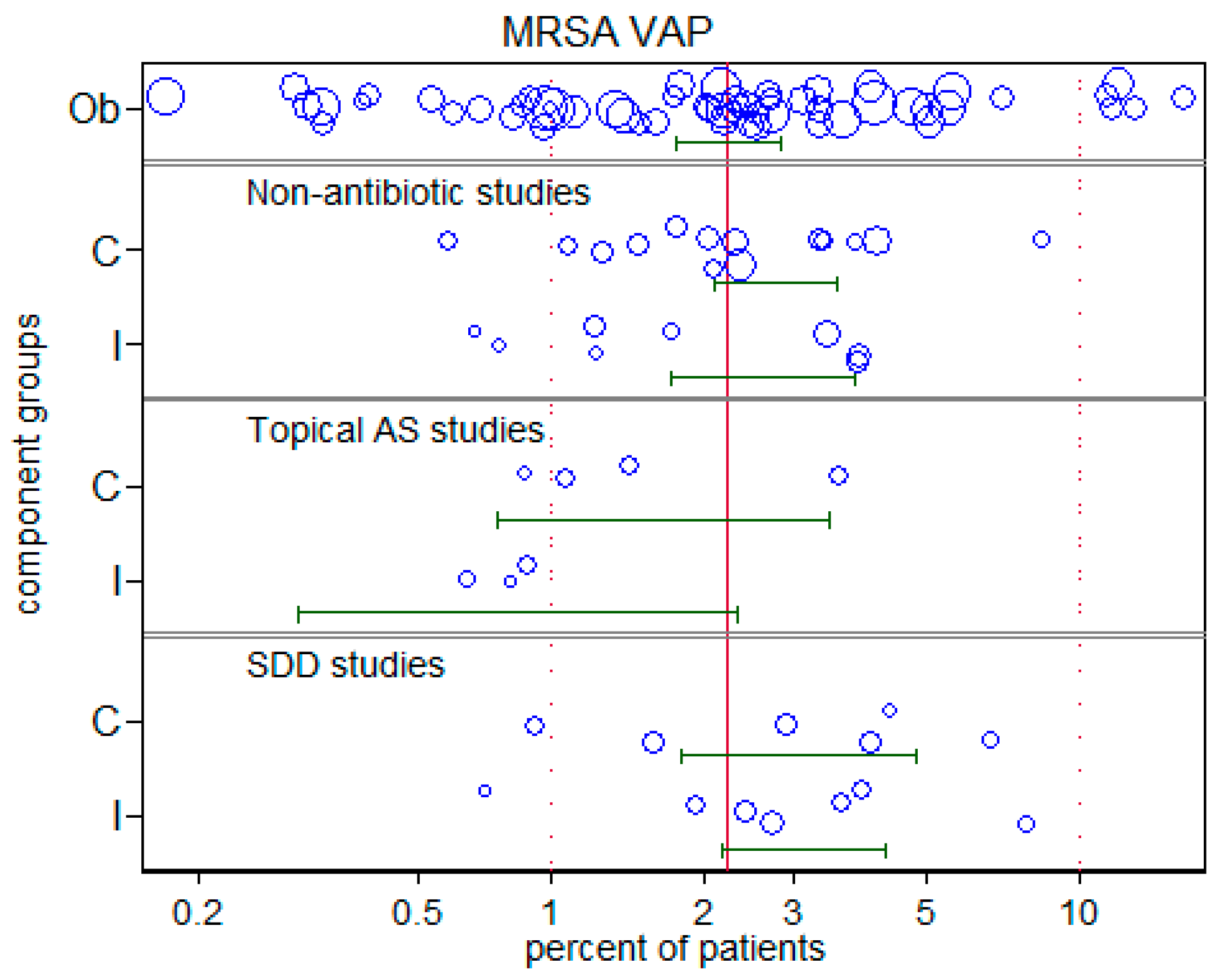
3.4. MRSA-VAP
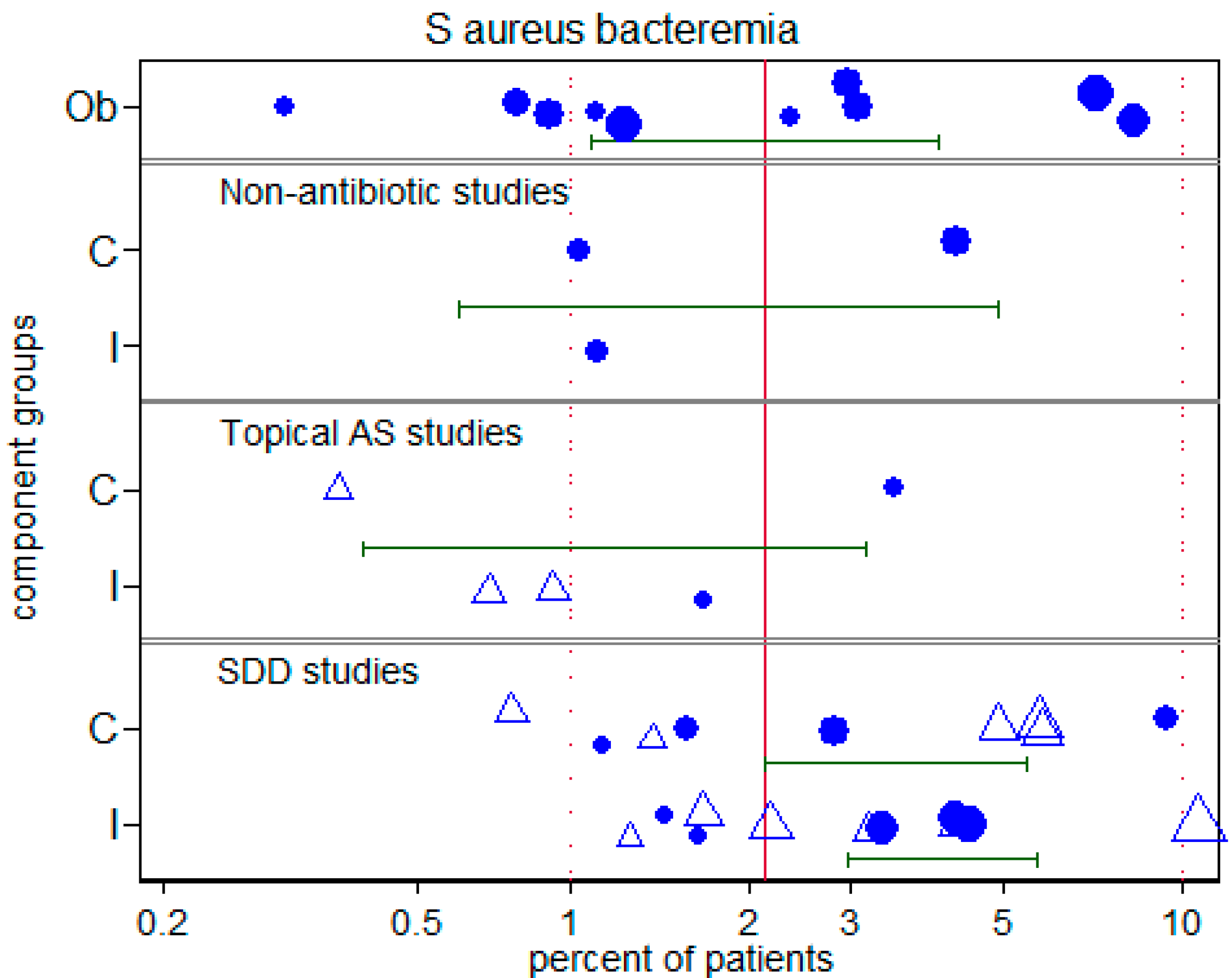
3.5. Overall and S. aureus bacteremia
4. Discussion
5. Conclusions
Supplementary Materials
Conflicts of Interest
Funding
References
- A’Court, C.H.; Garrard, C.S.; Crook, D.; Bowler, I.; Conlon, C.; Peto, T.; Anderson, E. Microbiological lung surveillance in mechanically ventilated patients, using non-directed bronchial lavage and quantitative culture. Q. J. Med. 1993, 86, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lerma, F. ICU-acquired Pneumonia Study Group. Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. Intensive Care Med. 1996, 22, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, M.; Moro, M.L.; Capelli, O.; De Blasi, R.A.; D’Errico, R.R.; Conti, G.; Bufi, M.; Gasparetto, A. Risk factors for early onset pneumonia in trauma patients. Chest 1994, 105, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulou, E.; Bakakos, P.; Katostaras, T.; Gregorakos, L. Incidence and risk factors for ventilator-associated pneumonia in 4 multidisciplinary intensive care units in Athens, Greece. Respir. Care 2003, 48, 681–688. [Google Scholar] [PubMed]
- Baker, A.M.; Meredith, J.W.; Haponik, E.F. Pneumonia in intubated trauma patients. Microbiology and outcomes. Am. J. Respir. Crit. Care Med. 1996, 153, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Bekaert, M.; Timsit, J.F.; Vansteelandt, S.; Depuydt, P.; Vésin, A.; Garrouste-Orgeas, M.; Decruyenaere, J.; Clec’h, C.; Azoulay, E.; Benoit, D. Attributable mortality of ventilator-associated pneumonia: A reappraisal using causal analysis. Am. J. Respir. Crit. Care Med. 2011, 184, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Bercault, N.; Boulain, T. Mortality rate attributable to ventilator-associated nosocomial pneumonia in an adult intensive care unit: A prospective case-control study. Crit. Care Med. 2001, 29, 2303–2309. [Google Scholar] [CrossRef] [PubMed]
- Berrouane, Y.; Daudenthun, I.; Riegel, B.; Emery, M.N.; Martin, G.; Krivosic, R.; Grandbastien, B. Early onset pneumonia in neurosurgical intensive care unit patients. J. Hosp. Infect. 1998, 40, 275–280. [Google Scholar] [CrossRef]
- Bochicchio, G.V.; Joshi, M.; Bochicchio, K.; Tracy, K.; Scalea, T.M. A time-dependent analysis of intensive care unit pneumonia in trauma patients. J. Trauma 2004, 56, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Bonten, M.J.; Gaillard, C.A.; van Tiel, F.H.; Smeets, H.G.; van der Geest, S.; Stobberingh, E.E. The stomach is not a source for colonization of the upper respiratory tract and pneumonia in ICU patients. Chest 1994, 105, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Boots, R.J.; Phillips, G.E.; George, N.; Faoagali, J.L. Surveillance culture utility and safety using low-volume blind bronchoalveolar lavage in the diagnosis of ventilator-associated pneumonia. Respirology 2008, 13, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Bornstain, C.; Azoulay, E.; De Lassence, A.; Cohen, Y.; Costa, M.A.; Mourvillier, B.; Descorps-Declere, A.; Garrouste-Orgeas, M.; Thuong, M.; Schlemmer, B.; et al. Sedation, sucralfate, and antibiotic use are potential means for protection against early-onset ventilator-associated pneumonia. Clin. Infect. Dis. 2004, 38, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Braun, S.R.; Levin, A.B.; Clark, K.L. Role of corticosteroids in the development of pneumonia in mechanically ventilated head-trauma victims. Crit. Care Med. 1986, 14, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Bregeon, F.; Papazian, L.; Visconti, A.; Gregoire, R.; Thirion, X.; Gouin, F. Relationship of microbiologic diagnostic criteria to morbidity and mortality in patients with ventilator-associated pneumonia. JAMA 1997, 277, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Bronchard, R.; Albaladejo, P.; Brezac, G.; Geffroy, A.; Seince, P.F.; Morris, W.; Branger, C.; Marty, J. Early onset pneumonia: Risk factors and consequences in head trauma patients. Anesthesiology 2004, 100, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Cade, J.F.; McOwat, E.; Siganporia, R.; Keighley, C.; Presneill, J.; Sinickas, V. Uncertain relevance of gastric colonization in the seriously ill. Intensive Care Med. 1992, 18, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, M.; Ferrer, M.; Ferrer, R.; Morforte, R.; Garnacho, A.; Torres, A. Risk and prognostic factors of ventilator-associated pneumonia in trauma patients. Crit. Care Med. 2006, 34, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Cendrero, J.A.; Solé-Violán, J.; Benitez, A.B.; Catalán, J.N.; Fernández, J.A.; Santana, P.S.; de Castro, F.R. Role of different routes of tracheal colonization in the development of pneumonia in patients receiving mechanical ventilation. Chest 1999, 116, 462–470. [Google Scholar] [CrossRef]
- Chaari, A.; El Habib, M.; Ghdhoun, H.; Algia, N.B.; Chtara, K.; Hamida, C.B.; Chelly, H.; Bahloul, M.; Bouaziz, M. Does low-dose hydrocortisone therapy prevent ventilator-associated pneumonia in trauma patients? Am. J. Ther. 2015, 22, 22–228. [Google Scholar] [CrossRef] [PubMed]
- Chastre, J.; Trouillet, J.L.; Vuagnat, A.; Joly-Guillou, M.L.; Clavier, H.; Dombret, M.C.; Gibert, C. Nosocomial pneumonia in patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1998, 157, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Chevret, S.; Hemmer, M.; Carlet, J. Incidence and risk factors of pneumonia acquired in intensive care units. Results from a multicenter prospective study on 996 patients. European Cooperative Group on Nosocomial Pneumonia. Intensive Care Med. 1993, 19, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.; Norwood, S.; Berne, J. Ventilator-associated pneumonia is more common and of less consequence in trauma patients compared with other critically ill patients. J. Trauma Acute Care Surg. 2010, 69, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Craven, D.E.; Kunches, L.M.; Lichtenberg, D.A.; Kollisch, N.R.; Barry, M.A.; Heeren, T.C. McCabe WR Nosocomial infection and fatality in medical and surgical intensive care unit patients. Arch. Intern. Med. 1988, 148, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Daschner, F.; Kappstein, I.; Schuster, F.; Scholz, R.; Bauer, E.; Jooβens, D.; Just, H. Influence of disposable (‘Conchapak’) and reusable humidifying systems on the incidence of ventilation pneumonia. J. Hosp. Infect. 1988, 11, 161–168. [Google Scholar] [CrossRef]
- De Latorre, F.J.; Pont, T.; Ferrer, A.; Rosselló, J.; Palomar, M.; Planas, M. Pattern of tracheal colonization during mechanical ventilation. Am. J. Respir. Crit. Care Med. 1995, 152, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Ertugrul, B.M.; Yildirim, A.; Ay, P.; Oncu, S.; Cagatay, A.; Cakar, N.; Ertekin, C.; Ozsut, H.; Eraksoy, H.; Calangu, S. Ventilator-associated pneumonia in surgical emergency intensive care unit. Saudi Med. J. 2006, 27, 52–57. [Google Scholar] [PubMed]
- Evans, H.L.; Zonies, D.H.; Warner, K.J.; Bulger, E.M.; Sharar, S.R.; Maier, R.V.; Cuschieri, J. Timing of intubation and ventilator-associated pneumonia following injury. Arch. Surg. 2010, 145, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Ewig, S.; Torres, A.; El-Ebiary, M.; Fàbregas, N.; Hernandez, C.; Gonzalez, J.; Nicolas, J.M.; Soto, L. Bacterial colonization patterns in mechanically ventilated patients with traumatic and medical head injury. Incidence, risk factors, and association with ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 1999, 159, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Fagon, J.Y.; Chastre, J.; Domart, Y.; Trouillet, J.L.; Pierre, J.; Darne, C.; Gibert, C. Nosocomial pneumonia in patients receiving continuous mechanical ventilation. Prospective analysis of 52 episodes with use of a protected specimen brush and quantitative culture techniques. Am. Rev. Respir. Dis. 1989, 139, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Gacouin, A.; Barbarot, N.; Camus, C.; Salomon, S.; Isslame, S.; Marque, S.; Lavoué, S.; Donnio, P.Y.; Thomas, R.; Le Tulzo, Y. Late-onset ventilator-associated pneumonia in nontrauma intensive care unit patients. Anesth. Analg. 2009, 109, 1584–1590. [Google Scholar] [CrossRef] [PubMed]
- Garrouste-Orgeas, M.; Chevret, S.; Arlet, G.; Marie, O.; Rouveau, M.; Popoff, N.; Schlemmer, B. Oropharyngeal or gastric colonization and nosocomial pneumonia in adult intensive care unit patients. A prospective study based on genomic DNA analysis. Am. J. Respir. Crit. Care Med. 1997, 156, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- George, D.L.; Falk, P.S.; Wunderink, R.G.; Leeper, K.V., Jr.; Meduri, G.U.; Steere, E.L.; Glen Mayhall, C. Epidemiology of ventilator-acquired pneumonia based on protected bronchoscopic sampling. Am. J. Respir. Crit. Care Med. 1998, 158, 1839–1847. [Google Scholar] [CrossRef] [PubMed]
- Georges, H.; Leroy, O.; Guery, B.; Alfandari, S.; Beaucaire, G. Predisposing factors for nosocomial pneumonia in patients receiving mechanical ventilation and requiring tracheotomy. Chest 2000, 118, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Giard, M.; Lepape, A.; Allaouchiche, B.; Guerin, C.; Lehot, J.J.; Robert, M.O.; Vanhems, P. Early-and late-onset ventilator-associated pneumonia acquired in the intensive care unit: Comparison of risk factors. J. Crit. Care 2008, 23, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Gruson, D.; Hilbert, G.; Vargas, F.; Valentino, R.; Bebear, C.; Allery, A.; Bebear, C.; Gbikpi-Benissan, G.E.; Cardinaud, J.P. Rotation and restricted use of antibiotics in a medical intensive care unit: Impact on the incidence of ventilator-associated pneumonia caused by antibiotic-resistant gram-negative bacteria. Am. J. Respir. Crit. Care Med. 2000, 162, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Gruson, D.; Hilbert, G.; Vargas, F.; Valentino, R.; Bui, N.; Pereyre, S.; Bebear, C.; Bebear, C.M.; Gbikpi-Benissan, G. Strategy of antibiotic rotation: Long-term effect on incidence and susceptibilities of Gram-negative bacilli responsible for ventilator-associated pneumonia. Crit. Care Med. 2003, 31, 1908–1914. [Google Scholar] [CrossRef] [PubMed]
- Guérin, C.; Girard, R.; Chemorin, C.; De Varax, R.; Fournier, G. Facial mask noninvasive mechanical ventilation reduces the incidence of nosocomial pneumonia. Intensive Care Med. 1997, 23, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, M.M.; Rocco, J.R. Prevalence of ventilator-associated pneumonia in a university hospital and prognosis for the patients affected. J. Bras. Pneumol. 2006, 32, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Gursel, G.; Aydogdu, M.; Nadir Ozis, T.; Tasyurek, S. Comparison of the value of initial and serial endotracheal aspirate surveillance cultures in predicting the causative pathogen of ventilator-associated pneumonia. Scand. J. Infect. Dis. 2010, 42, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Heyland, D.K.; Cook, D.J.; Schoenfeld, P.S.; Frietag, A.; Varon, J.; Wood, G. The effect of acidified enteral feeds on gastric colonization in critically ill patients: Results of a multicenter randomized trial. Canadian Critical Care Trials Group. Crit. Care Med. 1999, 27, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Hugonnet, S.; Uçkay, I.; Pittet, D. Staffing level: A determinant of late-onset ventilator-associated pneumonia. Crit. Care 2007, 11, R80. [Google Scholar] [CrossRef] [PubMed]
- Hyllienmark, P.; Gardlund, B.; Persson, J.O.; Ekdahl, K. Nosocomial pneumonia in the ICU: A prospective cohort study. Scand. J. Infect. Dis. 2007, 39, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, J.; Peñafiel, A.; Marsé, P.; Jordá, R.; Raurich, J.M.; Mata, F. Incidence of gastroesophageal reflux and aspiration in mechanically ventilated patients using small-bore nasogastric tubes. J. Parenter. Enter. Nutr. 2000, 24, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.H.; Ward, S.; Sherman, G.; Kollef, M.H. A comparative analysis of patients with early-onset vs. late-onset nosocomial pneumonia in the ICU setting. Chest 2000, 117, 1434–1442. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.; Chang, R.W.; Lee, B.; Bartlett, F.W. Continuous enteral feeding: A major cause of pneumonia among ventilated intensive care unit patients. J. Parenter. Enter. Nutr. 1990, 14, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Jaillette, E.; Nseir, S. Relationship between inhaled β2-agonists and ventilator-associated pneumonia: A cohort study. Crit. Care Med. 2011, 39, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Jaimes, F.; De La Rosa, G.; Gómez, E.; Múnera, P.; Ramírez, J.; Castrillón, S. Incidence and risk factors for ventilator-associated pneumonia in a developing country Where is the difference? Respir. Med. 2007, 101, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, P.; Torres, A.; Rodríguez-Roisin, R.; de la Bellacasa, J.P.; Aznar, R.; Gatell, J.M.; Agustí-Vidal, A. Incidence and etiology of pneumonia acquired during mechanical ventilation. Crit. Care Med. 1989, 17, 882–885. [Google Scholar] [CrossRef] [PubMed]
- Kallel, H.; Chelly, H.; Bahloul, M.; Ksibi, H.; Dammak, H.; Chaari, A.; Hamida, C.B.; Rekik, N.; Bouaziz, M. The effect of ventilator-associated pneumonia on the prognosis of head trauma patients. J. Trauma Acute Care Surg. 2005, 59, 705–710. [Google Scholar]
- Kanafani, Z.A.; Kara, L.; Hayek, S.; Kanj, S.S. Ventilator-associated pneumonia at a tertiary-care center in a developing country: Incidence, microbiology, and susceptibility patterns of isolated microorganisms. Infect. Control Hosp. Epidemiol. 2003, 24, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H. Ventilator-associated pneumonia. A multivariate analysis. JAMA 1993, 270, 1965–1970. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Silver, P.; Murphy, D.M.; Trovillion, E. The effect of late-onset ventilator-associated pneumonia in determining patient mortality. Chest 1995, 108, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Von Harz, B.; Prentice, D.; Shapiro, S.D.; Silver, P.; John, R.S.; Trovillion, E. Patient transport from intensive care increases the risk of developing ventilator-associated pneumonia. Chest 1997, 112, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Chastre, J.; Fagon, J.Y.; François, B.; Niederman, M.S.; Rello, J.; Torres, A.; Vincent, J.L.; Wunderink, R.G.; Go, K.W.; Rehm, C.; et al. Global prospective epidemiologic and surveillance study of ventilator-associated pneumonia due to Pseudomonas aeruginosa. Crit. Care Med. 2014, 42, 2178–2187. [Google Scholar] [CrossRef] [PubMed]
- Koss, W.G.; Khalili, T.M.; Lemus, J.F.; Chelly, M.M.; Margulies, D.R.; Shabot, M.M. Nosocomial pneumonia is not prevented by protective contact isolation in the surgical intensive care unit. Am. Surg. 2001, 67, 1140–1144. [Google Scholar] [PubMed]
- Kunac, A.; Sifri, Z.C.; Mohr, A.M.; Horng, H.; Lavery, R.F.; Livingston, D.H. Bacteremia and Ventilator-Associated Pneumonia: A Marker for Contemporaneous Extra-Pulmonic Infection. Surg. Infect. 2014, 15, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Lepelletier, D.; Roquilly, A.; Mahe, P.J.; Loutrel, O.; Champin, P.; Corvec, S.; Naux, E.; Pinaud, M.; Lejus, C.; Asehnoune, K. Retrospective analysis of the risk factors and pathogens associated with early-onset ventilator-associated pneumonia in surgical-ICU head-trauma patients. J. Neurosurg. Anesthesiol. 2010, 22, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Luna, C.M.; Blanzaco, D.; Niederman, M.S.; Matarucco, W.; Baredes, N.C.; Desmery, P.; Palizas, F.; Menga, G.; Rios, F.; Apezteguia, C. Resolution of ventilator-associated pneumonia: Prospective evaluation of the clinical pulmonary infection score as an early clinical predictor of outcome. Crit. Care Med. 2003, 31, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Luyt, C.E.; Guérin, V.; Combes, A.; Trouillet, J.L.; Ayed, S.B.; Bernard, M.; Gibert, C.; Chastre, J. Procalcitonin kinetics as a prognostic marker of ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Magnason, S.; Kristinsson, K.G.; Stefansson, T.; Erlendsdottir, H.; Jonsdottir, K.; Kristjansson, M.; Gudmundsson, S. Risk factors and outcome in ICU-acquired infections. Acta Anaesthesiol. Scand. 2008, 52, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Magret, M.; Amaya-Villar, R.; Garnacho, J.; Lisboa, T.; Diaz, E.; DeWaele, J.; Deja, M.; Manno, E.; Rello, J. EU-VAP/CAP Study Group: Ventilator-associated pneumonia in trauma patients is associated with lower mortality: Results from EU-VAP study. J. Trauma Acute Care Surg. 2010, 69, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Mahul, P.; Auboyer, C.; Jospe, R.; Ros, A.; Guerin, C.; el Khouri, Z.; Galliez, M.; Dumont, A.; Gaudin, O. Prevention of nosocomial pneumonia in intubated patients respective role of mechanical subglottic secretions drainage and stress ulcer prophylaxis. Intensive Care Med. 1992, 18, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Makris, D.; Manoulakas, E.; Komnos, A.; Papakrivou, E.; Tzovaras, N.; Hovas, A.; Zintzaras, E.; Zakynthinos, E. Effect of pravastatin on the frequency of ventilator-associated pneumonia and on intensive care unit mortality: Open-label, randomized study. Crit. Care Med. 2011, 39, 2440–2446. [Google Scholar] [CrossRef] [PubMed]
- Markowicz, P.; Wolff, M.; Djedaini, K.; Cohen, Y.; Chastre, J.; Delclaux, C. Multicenter prospective study of ventilator-associated pneumonia during acute respiratory distress syndrome. Incidence, prognosis, and risk factors. ARDS Study Group. Am. J. Respir. Crit. Care Med. 2000, 161, 1942–1948. [Google Scholar] [CrossRef] [PubMed]
- Memish, Z.A.; Cunningham, G.; Oni, G.A.; Djazmati, W. The incidence and risk factors of ventilator-associated pneumonia in a Riyadh hospital. Infect. Control Hosp. Epidemiol. 2000, 21, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Michel, F.; Franceschini, B.; Berger, P.; Arnal, J.M.; Gainnier, M.; Sainty, J.M.; Papazian, L. Early antibiotic treatment for BAL-confirmed ventilator-associated pneumonia: A role for routine endotracheal aspirate cultures. Chest 2005, 127, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Moine, P.; Timsit, J.F.; De Lassence, A.; Troché, G.; Fosse, J.P.; Alberti, C.; Cohen, Y. Mortality associated with late-onset pneumonia in the intensive care unit: Results of a multi-center cohort study. Intensive Care Med. 2002, 28, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Myny, D.; Depuydt, P.; Colardyn, F.; Blot, S. Ventilator-associated pneumonia in a tertiary care ICU analysis of risk factors for acquisition and mortality. Acta Clin. Belg. 2005, 60, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Nguile-Makao, M.; Zahar, J.R.; Français, A.; Tabah, A.; Garrouste-Orgeas, M.; Allaouchiche, B.; Goldgran-Toledano, D.; Azoulay, E.; Adrie, C.; et al. Attributable mortality of ventilator-associated pneumonia: Respective impact of main characteristics at ICU admission and VAP onset using conditional logistic regression and multi-state models. Intensive Care Med. 2010, 36, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.L.; Røder, B.; Magnussen, P.; Engquist, A.; Frimodt-møller, N. Nosocomial pneumonia in an intensive care unit in a Danish university hospital: Incidence, mortality and etiology. Scand. J. Infect. Dis. 1992, 24, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.; Hussain, S.F. Risk factors associated with development of ventilator associated pneumonia. J. Coll. Physicians Surg. Pak. 2005, 15, 92–95. [Google Scholar] [PubMed]
- Nseir, S.; Di Pompeo, C.; Soubrier, S.; Cavestri, B.; Jozefowicz, E.; Saulnier, F.; Durocher, A. Impact of ventilator-associated pneumonia on outcome in patients with COPD. Chest 2005, 128, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Papazian, L.; Bregeon, F.; Thirion, X.; Gregoire, R.; Saux, P.; Denis, J.P.; Perin, G.; Charrel, J.; Dumon, J.F.; Affray, J.P.; Gouin, F. Effect of ventilator-associated pneumonia on mortality and morbidity. Am. J. Respir. Crit. Care Med. 1996, 154, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Potgieter, P.D.; Linton, D.M.; Oliver, S.; Forder, A. A Nosocomial infections in a respiratory intensive care unit. Crit. Care Med. 1987, 15, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Raineri, E.; Crema, L.; Dal Zoppo, S.; Acquarolo, A.; Pan, A.; Carnevale, G.; Albertario, F.; Candiani, A. Rotation of antimicrobial therapy in the intensive care unit: Impact on incidence of ventilator-associated pneumonia caused by antibiotic-resistant Gram-negative bacteria. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, P.; Lopez-Ferraz, C.; Gordon, M.; Gimeno, A.; Villarreal, E.; Ruiz, J.; Menendez, R.; Torres, A. From starting mechanical ventilation to ventilator-associated pneumonia, choosing the right moment to start antibiotic treatment. Crit. Care 2016, 20, 169. [Google Scholar] [CrossRef] [PubMed]
- Rello, J.; Quintana, E.; Ausina, V.; Castella, J.; Luquin, M.; Net, A.; Prats, G. Incidence, etiology, and outcome of nosocomial pneumonia in mechanically ventilated patients. Chest 1991, 100, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Rello, J.; Ausina, V.; Ricart, M.; Puzo, C.; Net, A.; Prats, G. Nosocomial pneumonia in critically ill comatose patients: Need for a differential therapeutic approach. Eur. Respir. J. 1992, 5, 1249–1253. [Google Scholar] [PubMed]
- Rello, J.; Sonora, R.; Jubert, P.; Artigas, A.; Rué, M.; Vallés, J. Pneumonia in intubated patients: Role of respiratory airway care. Am. J. Respir. Crit. Care Med. 1996, 154, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Rello, J.; Ollendorf, D.A.; Oster, G.; Vera-Llonch, M.; Bellm, L.; Redman, R.; Kollef, M.H.; VAP Outcomes Scientific Advisory Group. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest 2002, 122, 2115–2121. [Google Scholar] [CrossRef] [PubMed]
- Rello, J.; Lorente, C.; Diaz, E.; Bodi, M.; Boque, C.; Sandiumenge, A.; Santamaria, J.M. Incidence, etiology, and outcome of nosocomial pneumonia in ICU patients requiring percutaneous tracheotomy for mechanical ventilation. Chest 2003, 124, 2239–2243. [Google Scholar] [CrossRef] [PubMed]
- Resende, M.M.; Monteiro, S.G.; Callegari, B.; Figueiredo, P.M.; Monteiro, C.R.; Monteiro-Neto, V. Epidemiology and outcomes of ventilator-associated pneumonia in northern Brazil: An analytical descriptive prospective cohort study. BMC Infect. Dis. 2013, 13, 119. [Google Scholar] [CrossRef] [PubMed]
- Reusser, P.; Zimmerli, W.; Scheidegger, D.; Marbet, G.A.; Buser, M.; Gyr, K. Role of gastric colonization in nosocomial infections and endotoxemia: A prospective study in neurosurgical patients on mechanical ventilation. J. Infect. Dis. 1989, 160, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Rezai, M.S.; Bagheri-Nesami, M.; Nikkhah, A.; Bayg, A.H. Incidence, risk factors, and outcome of ventilator-associated Pneumonia in 18 hospitals of Iran. Running title: Ventilator-associated pneumonia in Iran. Int. J. Adv. Biotechnol. Res. 2016, 7, 936–946. [Google Scholar]
- Rincón-Ferrari, M.D.; Flores-Cordero, J.M.; Leal-Noval, S.R.; Murillo-Cabezas, F.; Cayuelas, A.; Muñoz-Sánchez, M.A.; Sánchez-Olmedo, J.I. Impact of ventilator-associated pneumonia in patients with severe head injury. J. Trauma Acute Care Surg. 2004, 57, 1234–1240. [Google Scholar] [CrossRef]
- Rodrigues, P.M.; Neto, C.; Santos, L.R.; Knibel, M.F. Ventilator-associated pneumonia: Epidemiology and impact on the clinical evolution of ICU patients. J. Bras. Pneumol. 2009, 35, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.L.; Gibbons, K.J.; Bitzer, L.G.; Dechert, R.E.; Steinberg, S.M.; Flint, L.M. Pneumonia: Incidence, risk factors, and outcome in injured patients. J. Trauma 1991, 31, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Santana, S.; Garcia Jimenez, A.; Esteban, A.; Guerra, L.; Alvarez, B.; Corcia, S.; Gudin, J.; Martinez, A.; Quintana, E.; Armengol, S.; et al. ICU pneumonias: A multi-institutional study. Crit. Care Med. 1987, 15, 930–932. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, N.; Zafar, A.; Sukhyani, L.; Rahim, S.; Noor, M.F.; Hussain, K.; Siddiqui, S.; Islam, M.; Husain, S.J. Reducing ventilator-associated pneumonia rates through a staff education programme. J. Hosp. Infect. 2004, 57, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Salata, R.A.; Lederman, M.M.; Shlaes, D.M.; Jacobs, M.R.; Eckstein, E.; Tweardy, D.; Toossi, Z.; Chmielewski, R.; Marino, J. King CH Diagnosis of nosocomial pneumonia in intubated, intensive care unit patients. Am. Rev. Respir. Dis. 1987, 135, 426–432. [Google Scholar] [PubMed]
- Shahin, J.; Bielinski, M.; Guichon, C.; Flemming, C.; Kristof, A.S. Suspected ventilator-associated respiratory infection in severely ill patients: A prospective observational study. Crit. Care 2013, 17, R251. [Google Scholar] [CrossRef] [PubMed]
- Sofianou, D.C.; Constandinidis, T.C.; Yannacou, M.; Anastasiou, H.; Sofianos, E. Analysis of risk factors for ventilator-associated pneumonia in a multidisciplinary intensive care unit. Eur. J. Clin. Microbiol. Infect. Dis. 2000, 19, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Stéphan, F.; Mabrouk, N.; Decailliot, F.; Delclaux, C.; Legrand, P. Ventilator-associated pneumonia leading to acute lung injury after trauma: Importance of Haemophilus influenzae. Anesthesiology 2006, 104, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Zhu, S.; Yan, D.; Chen, W.; Chen, R.; Zou, J.; Yan, J.; Zhang, X.; Farmakiotis, D.; Mylonakis, E. Candida spp. airway colonization: A potential risk factor for Acinetobacter baumannii ventilator-associated pneumonia. Med. Mycol. 2016, 54, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Tejada Artigas, A.T.; Dronda, S.B.; Vallés, E.C.; Marco, J.M.; Usón, M.C.; Figueras, P.; Suarez, F.J.; Hernandez, A. Risk factors for nosocomial pneumonia in critically ill trauma patients. Crit. Care Med. 2001, 29, 304–349. [Google Scholar] [CrossRef] [PubMed]
- Timsit, J.F.; Chevret, S.; Valcke, J.; Misset, B.; Renaud, B.; Goldstein, F.W.; Vaury, P.; Carlet, J. Mortality of nosocomial pneumonia in ventilated patients: Influence of diagnostic tools. Am. J. Respir. Crit. Care Med. 1996, 154, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Aznar, R.; Gatell, J.M.; Jiménez, P.; González, J.; Ferrer, A.; Celis, R.; Rodriguez-Roisin, R. Incidence, risk, and prognosis factors of nosocomial pneumonia in mechanically ventilated patients. Am. Rev. Respir. Dis. 1990, 142, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Trouillet, J.L.; Chastre, J.; Vuagnat, A.; Joly-Guillou, M.L.; Combaux, D.; Dombret, M.C.; Gibert, C. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am. J. Respir. Crit. Care Med. 1998, 157, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Urli, T.; Perone, G.; Acquarolo, A.; Zappa, S.; Antonini, B.; Ciani, A. Surveillance of infections acquired in intensive care: Usefulness in clinical practice. J. Hosp. Infect. 2002, 52, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Valles, J.; Pobo, A.; Garcia-Esquirol, O.; Mariscal, D.; Real, J.; Fernández, R. Excess ICU mortality attributable to ventilator-associated pneumonia: The role of early vs. late onset. Intensive Care Med. 2007, 33, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Vanhems, P.; Bénet, T.; Voirin, N.; Januel, J.M.; Lepape, A.; Allaouchiche, B.; Argaud, L.; Chassard, D.; Guérin, C. Early-onset ventilator-associated pneumonia incidence in intensive care units: A surveillance-based study. BMC Infect. Dis. 2011, 11, 236. [Google Scholar] [CrossRef] [PubMed]
- Verhamme, K.M.; De Coster, W.; De Roo, L.; De Beenhouwer, H.; Nollet, G.; Verbeke, J.; Demeyer, I.; Jordens, P. Pathogens in early-onset and late-onset intensive care unit–acquired pneumonia. Infect. Control Hosp. Epidemiol. 2007, 28, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Violan, J.S.; Sanchez-Ramirez, C.; Mujica, A.P.; Cendrero, J.C.; Fernandez, J.A.; de Castro, F.R. Impact of nosocomial pneumonia on the outcome of mechanically-ventilated patients. Crit. Care 1998, 2, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Woske, H.J.; Röding, T.; Schulz, I.; Lode, H. Ventilator-associated pneumonia in a surgical intensive care unit Epidemiology, etiology and comparison of three bronchoscopic methods for microbiological specimen sampling. Crit. Care 2001, 5, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.S.; Xiong, W.; Lai, R.P.; Liu, L.; Gan, X.M.; Wang, X.H.; Wang, M.; Lou, Y.X.; Fu, X.Y.; Wang, H.F.; Xiang, H. Ventilator-associated pneumonia in intensive care units in Hubei Province, China: A multicentre prospective cohort survey. J. Hosp. Infect. 2011, 78, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Zahar, J.R.; Nguile-Makao, M.; Français, A.; Schwebel, C.; Garrouste-Orgeas, M.; Goldgran-Toledano, D.; Azoulay, E.; Thuong, M.; Jamali, S.; et al. Predicting the risk of documented ventilator-associated pneumonia for benchmarking: Construction and validation of a score. Crit. Care Med. 2009, 37, 2545–2551. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Escribano, J.; Fernández-Vivas, M.; Carmona, T.G.; Caturla-Such, J.; Garcia-Martinez, M.; Menendez-Mainer, A.; Sanchez-Payá, J. Gastric versus transpyloric feeding in severe traumatic brain injury: A prospective, randomized trial. Intensive Care Med. 2010, 36, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Bonten, M.J.; Gaillard, C.A.; Van der Geest, S.; Van Tiel, F.H.; Beysens, A.J.; Smeets, H.G.; Stobberingh, E.E. The role of intragastric acidity and stress ulcer prophylaxis on colonization and infection in mechanically ventilated ICU patients. A stratified, randomized, double-blind study of sucralfate versus antacids. Am. J. Respir. Crit. Care Med. 1995, 152, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Combes, P.; Fauvage, B.; Oleyer, C. Nosocomial pneumonia in mechanically ventilated patients, a prospective randomised evaluation of the Stericath closed suctioning system. Intensive Care Med. 2000, 26, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.; Guyatt, G.; Marshall, J.; Leasa, D.; Fuller, H.; Hall, R.; Peters, S.; Rutledge, F.; Griffith, L.; McLellan, A.; et al. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. N. Engl. J. Med. 1998, 338, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Daumal, F.; Colpart, E.; Manoury, B.; Mariani, M.; Daumal, M. Changing heat and moisture exchangers every 48 h does not increase the incidence of nosocomial pneumonia. Infect. Control Hosp. Epidemiol. 1999, 20, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Djedaini, K.; Billiard, M.; Mier, L.; Le Bourdelles, G.; Brun, P.; Markowicz, P.; Estagnasie, P.; Coste, F.; Boussougant, Y.; Dreyfuss, D. Changing heat and moisture exchangers every 48 h rather than 24 h does not affect their efficacy and the incidence of nosocomial pneumonia. Am. J. Respir. Crit. Care Med. 1995, 152, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Drakulovic, M.B.; Torres, A.; Bauer, T.T.; Nicolas, J.M.; Nogué, S.; Ferrer, M. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: A randomised trial. Lancet 1999, 354, 1851–1858. [Google Scholar] [CrossRef]
- Dreyfuss, D.; Djedaini, K.; Weber, P.; Brun, P.; Lanore, J.J.; Rahmani, J.; Coste, F. Prospective study of nosocomial pneumonia and of patient and circuit colonization during mechanical ventilation with circuit changes every 48 h versus no change. Am. Rev. Respir. Dis. 1991, 143 Pt 1, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Dreyfuss, D.; Djedaïni, K.; Gros, I.; Mier, L.; Le Bourdellés, G.; Cohen, Y.; Estagnasié, P.; Coste, F.; Boussougant, Y. Mechanical ventilation with heated humidifiers or heat and moisture exchangers: Effects on patient colonization and incidence of nosocomial pneumonia. Am. J. Respir. Crit. Care Med. 1995, 151, 986–992. [Google Scholar] [PubMed]
- Driks, M.R.; Craven, D.E.; Celli, B.R.; Manning, M.; Burke, R.A.; Garvin, G.M.; Kunches, L.M.; Farber, H.W.; Wedel, S.A.; McCabe, W.R. Nosocomial pneumonia in intubated patients given sucralfate as compared with antacids or histamine type 2 blockers. The role of gastric colonization. N. Eng. J. Med. 1987, 317, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Fabian, T.C.; Boucher, B.A.; Croce, M.A.; Kuhl, D.A.; Janning, S.W.; Coffey, B.C.; Kudsk, K.A. Pneumonia and stress ulceration in severely injured patients: A prospective evaluation of the effects of stress ulcer prophylaxis. Arch. Surg. 1993, 128, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Forestier, C.; Guelon, D.; Cluytens, V.; Guillart, T.; Sirot, J.; De Champs, C. Oral probiotic and prevention of Pseudomonas aeruginosa infections: A randomized, double-blind, placebocontrolled pilot study in intensive care unit patients. Crit. Care 2008, 12, R69. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, L.; Chevret, S.; Madinier, G.; Ohen, F.; Demingeon, G.; Coupry, A. Chaudet M Influence of long-term oro- or nasotracheal intubation on nosocomial maxillary sinusitis and pneumonia: Results of a prospective, randomized, clinical trial. Crit. Care Med. 1993, 21, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, L.; Chastang, C.; Demingeon, G.; Bohe, J.; Piralla, B.; Coupry, A. A randomized study assessing the systematic search for maxillary sinusitis in nasotracheally mechanically ventilated patients. Influence of nosocomial maxillary sinusitis on the occurrence of ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 1999, 159, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Kappstein, I.; Schulgen, G.; Friedrich, T.; Hellinger, P.; Benzing, A.; Geiger, K.; Daschner, F.D. Incidence of pneumonia in mechanically ventilated patients treated with sucralfate or cimetidine as prophylaxis for stress bleeding: Bacterial colonization of the stomach. Am. J. Med. 1991, 91, S125–S131. [Google Scholar] [CrossRef]
- Kirschenbaum, L.; Azzi, E.; Sfeir, T.; Tietjen, P.; Astiz, M. Effect of continuous lateral rotational therapy on the prevalence of ventilator-associated pneumonia in patients requiring long-term ventilatory care. Crit. Care Med. 2002, 30, 1983–1986. [Google Scholar] [CrossRef] [PubMed]
- Kirton, O.C.; DeHaven, B.; Morgan, J.; Civetta, J. A prospective, randomized comparison of an in-line heat moisture exchange filter and heated wire humidifiers: Rates of ventilator-associated early-onset (community-acquired) or late-onset (hospital-acquired) pneumonia and incidence of endotracheal tube occlusion. Chest 1997, 112, 1055–1059. [Google Scholar] [PubMed]
- Knight, D.J.; Gardiner, D.; Banks, A.; Snape, S.E.; Weston, V.C.; Bengmark, S.; Girling, K.J. Effect of synbiotic therapy on the incidence of ventilator associated pneumonia in critically ill patients: A randomised, double-blind, placebo-controlled trial. Intensive Care Med. 2009, 35, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Shapiro, S.D.; Fraser, V.J.; Silver, P.; Murphy, D.M.; Trovillion, E.; Hearns, M.L.; Richards, R.D.; Cracchilo, L.; Hossin, L. Mechanical ventilation with or without 7-day circuit changes. A randomized controlled trial. Ann. Intern. Med. 1995, 123, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Prentice, D.; Shapiro, S.D.; Fraser, V.J.; Silver, P.; Trovillion, E.; Weilitz, P.; Von Harz, B.E.; St. John, R.O. Mechanical ventilation with or without daily changes of in-line suction catheters. Am. J. Respir. Crit. Care Med. 1997, 156, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Kortbeek, J.B.; Haigh, P.I.; Doig, C. Duodenal versus gastric feeding in ventilated blunt trauma patients: A randomized controlled trial. J. Trauma 1999, 46, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Kostadima, E.; Kaditis, A.G.; Alexopoulos, E.I.; Zakynthinos, E.; Sfyras, D. Early gastrostomy reduces the rate of ventilator-associated pneumonia in stroke or head injury patients. Eur. Respir. J. 2005, 26, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Lacherade, J.C.; Auburtin, M.; Cerf, C.; Van de Louw, A.; Soufir, L.; Rebufat, Y.; Rezaiguia, S.; Ricard, J.D.; Lellouche, F.; Brun-Buisson, C.; et al. Impact of humidification systems on ventilator-associated pneumonia: A randomized multicenter trial. Am. J. Respir. Crit. Care Med. 2005, 172, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Lacherade, J.C.; De Jonghe, B.; Guezennec, P.; Debbat, K.; Hayon, J.; Monsel, A.; Bastuji-Garin, S. Intermittent subglottic secretion drainage and ventilator-associated pneumonia A multicenter trial. Am. J. Respir. Crit. Care Med. 2010, 182, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Launey, Y.; Nesseler, N.; Le Cousin, A.; Feuillet, F.; Garlantezec, R.; Mallédant, Y.; Seguin, P. Effect of a fever control protocol-based strategy on ventilator-associated pneumonia in severely brain-injured patients. Crit. Care 2014, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Lorente, L.; Lecuona, M.; Málaga, J.; Revert, C.; Mora, M.L.; Sierra, A. Bacterial filters in respiratory circuits: An unnecessary cost? Crit. Care Med. 2003, 31, 2126–2130. [Google Scholar] [CrossRef] [PubMed]
- Lorente, L.; Lecuona, M.; Galván, R.; Ramos, M.J.; Mora, M.L.; Sierra, A. Periodically changing ventilator circuits is not necessary to prevent ventilator-associated pneumonia when a heat and moisture exchanger is used. Infect. Control Hosp. Epidemiol. 2004, 25, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Lorente, L.; Lecuona, M.; Martín, M.M.; García, C.; Mora, M.L.; Sierra, A. Ventilator-associated pneumonia using a closed versus an open tracheal suction system. Crit. Care Med. 2005, 33, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Lorente, L.; Lecuona, M.; Jiménez, A.; Mora, M.L.; Sierra, A. Tracheal suction by closed system without daily change versus open system. Intensive Care Med. 2006, 32, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Lorente, L.; Lecuona, M.; Jimenez, A.; Mora, M.L.; Sierra, A. Ventilator-associated pneumonia using a heated humidifier or a heat and moisture exchanger: A randomized controlled trial [ISRCTN88724583]. Crit. Care 2006, 10, R116. [Google Scholar] [CrossRef] [PubMed]
- Lorente, L.; Lecuona, M.; Jimenez, A.; Mora, M.L. Sierra: Influence of an endotracheal tube with polyurethane cuff and subglottic secretion drainage on pneumonia. Am. J. Respir. Crit. Care Med. 2007, 176, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Lorente, L.; Lecuona, M.; Jiménez, A.; Lorenzo, L.; Roca, I.; Cabrera, J.; Llanos, C.; Mora, M.L. Continuous endotracheal tube cuff pressure control system protects against ventilator-associated pneumonia. Crit. Care 2014, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Manzano, F.; Fernandez-Mondejar, E.; Colmenero, M.; Poyatos, M.E.; Rivera, R.; Machado, J.; Catalan, I.; Artigas, A. Positive-end expiratory pressure reduces incidence of ventilator-associated pneumonia in nonhypoxemic patients. Crit. Care Med. 2008, 36, 2225–2231. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Perrin, G.; Gevaudan, M.J.; Saux, P.; Gouin, F. Heat and moisture exchangers and vaporizing humidifiers in the intensive care unit. Chest 1990, 97, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Morrow, L.E.; Kollef, M.H.; Casale, T.B. Probiotic prophylaxis of ventilator-associated pneumonia: A blinded, randomized, controlled trial. Am. J. Respir. Crit. Care Med. 2010, 182, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Nseir, S.; Zerimech, F.; Fournier, C.; Lubret, R.; Ramon, P.; Durocher, A.; Balduyck, M. Continuous control of tracheal cuff pressure and microaspiration of gastric contents in critically ill patients. Am. J. Respir. Crit. Care Med. 2011, 184, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Pickworth, K.K.; Falcone, R.E.; Hoogeboom, J.E.; Santanello, S.A. Occurrence of nosocomial pneumonia in mechanically ventilated trauma patients: A comparison of sucralfate and ranitidine. Crit. Care Med. 1993, 21, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Pneumatikos, I.; Konstantonis, D.; Tsagaris, I.; Theodorou, V.; Vretzakis, G.; Danielides, V.; Bouros, D. Prevention of nosocomial maxillary sinusitis in the ICU: The effects of topically applied alpha-adrenergic agonists and corticosteroids. Intensive Care Med. 2006, 32, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Prod’hom, G.; Leuenberger, P.; Koerfer, J.; Blum, A.; Chiolero, R.; Schaller, M.D.; Perret, C.; Spinnler, O.; Blondel, J.; Siegrist, H.; et al. Nosocomial pneumonia in mechanically ventilated patients receiving antacid, ranitidine, or sucralfate as prophylaxis for stress ulcer. A randomized controlled trial. Ann. Intern. Med. 1994, 120, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Reignier, J.; Mercier, E.; Le Gouge, A.; Boulain, T.; Desachy, A.; Bellec, F.; Lascarrou, J.B. Effect of Not Monitoring Residual Gastric Volume on Risk of Ventilator-Associated Pneumonia in Adults Receiving Mechanical Ventilation and Early Enteral Feeding. A Randomized Controlled Trial. JAMA 2013, 309, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Rumbak, M.J.; Truncale, T.; Newton, M.N.; Adams, B.; Hazard, P. A Prospective, Randomized Study Comparing Early Versus Delayed Percutaneous Tracheostomy In Critically Ill Medical Patients Requiring Prolonged Mechanical Ventilation. Chest 2000, 118, 97S–98S. [Google Scholar]
- Ryan, P.; Dawson, J.; Teres, D.; Celoria, G.; Navab, F. Nosocomial pneumonia during stress ulcer prophylaxis with cimetidine and sucralfate. Arch. Surg. 1993, 128, 1353–1357. [Google Scholar] [CrossRef] [PubMed]
- Smulders, K.; van der Hoeven, H.; Weers-Pothoff, I.; Vandenbroucke-Grauls, C. A randomized clinical trial of intermittent subglottic secretion drainage in patients receiving mechanical ventilation. Chest 2002, 121, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Staudinger, T.; Bojic, A.; Holzinger, U.; Meyer, B.; Rohwer, M.; Mallner, F.; Locker, G.J. Continuous lateral rotation therapy to prevent ventilator-associated pneumonia. Crit. Care Med. 2010, 38, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Thomachot, L.; Viviand, X.; Arnaud, S.; Boisson, C.; Martin, C.D. Comparing two heat and moisture exchangers, one hydrophobic and one hygroscopic, on humidifying efficacy and the rate of nosocomial pneumonia. Chest 1998, 114, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Thomachot, L.; Leone, M.; Razzouk, K.; Antonini, F.; Vialet, R.; Martin, C. Do the components of heat and moisture exchanger filters affect humidifying efficacy and the incidence of nosocomial pneumonia? Crit. Care Med. 1999, 27, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Thomachot, L.; Leone, M.; Razzouk, K.; Antonini, F.; Vialet, R.; Martin, C. Randomized Clinical Trial of Extended Use of a Hydrophobic Condenser Humidifier: 1 vs. 7 Days. Crit. Care Med. 2002, 30, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Valencia, M.; Ferrer, M.; Farre, R.; Navajas, D.; Badia, J.R.; Nicolas, J.M.; Torres, A. Automatic control of tracheal tube cuff pressure in ventilated patients in semirecumbent position: A randomized trial. Crit. Care Med. 2007, 35, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Wang, C.T.; Zhang, F.S.; Qi, F.; Wang, S.F.; Ma, S.; Wu, T.J.; Tian, H.; Tian, Z.T.; Zhang, S.L.; et al. Effect of probiotics on the incidence of ventilator-associated pneumonia in critically ill patients: A randomized controlled multicenter trial. Intensive Care Med. 2016, 42, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Kantorova, I.; Svoboda, P.; Scheer, P.; Doubek, J.; Rehorkova, D.; Bosakova, H.; Ochmann, J. Stress ulcer prophylaxis in critically ill patients: A randomized controlled trial. Hepatogastroenterology 2004, 51, 757–761. [Google Scholar] [PubMed]
- Ćabov, T.; Macan, D.; Husedžinović, I.; Škrlin-Šubić, J.; Bošnjak, D.; Šestan-Crnek, S.; Perić, B.; Kovač, Z.; Golubović, V. The impact of oral health and 0.2% chlorhexidine oral gel on the prevalence of nosocomial infections in surgical intensive-care patients: A randomized placebo-controlled study. Wien. Klin. Wochenschr. 2010, 122, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Caruso, P.; Denari, S.; Ruiz, S.A.; Demarzo, S.E.; Deheinzelin, D. Saline instillation before tracheal suctioning decreases the incidence of ventilator-associated pneumonia. Crit. Care Med. 2009, 37, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Fourrier, F.E.; Cau-Pottier, H.; Boutigny, M.; Roussel-Delvallez, M.; Jourdain; Chopin, C. Effects of dental plaque antiseptic decontamination on bacterial colonization and nosocomial infections in critically ill patients. Intensive Care Med. 2000, 26, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Fourrier, F.; Dubois, D.; Pronnier, P.; Herbecq, P.; Leroy, O.; Desmettre, T.; Roussel-Delvallez, M. Effect of gingival and dental plaque antiseptic decontamination on nosocomial infections acquired in the intensive care unit a double-blind placebo-controlled multicenter study. Crit. Care Med. 2005, 33, 1728–1735. [Google Scholar] [CrossRef] [PubMed]
- Genuit, T.; Bochicchio, G.; Napolitano, L.M.; McCarter, R.J.; Roghman, M.C. Prophylactic chlorhexidine oral rinse decreases ventilator-associated pneumonia in surgical ICU patients. Surg. Infect. 2001, 2, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Koeman, M.; van der Ven, A.J.; Hak, E.; Joore, H.C.; Kaasjager, K.; de Smet, A.G.; Ramsay, G.; Dormans, T.P.; Aarts, L.P.; de Bel, E.E.; et al. Oral decontamination with chlorhexidine reduces the incidence of ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 2006, 173, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Afessa, B.; Anzueto, A.; Veremakis, C.; Kerr, K.M.; Margolis, B.D.; Schinner, R. Silver-coated endotracheal tubes and incidence of ventilator-associated pneumonia: The NASCENT randomized trial. JAMA 2008, 300, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Lorente, L.; Lecuona, M.; Jiménez, A.; Palmero, S.; Pastor, E.; Lafuente, N.; Ramos, M.J.; Mora, M.L.; Sierra, A. Ventilator-associated pneumonia with or without toothbrushing a randomized controlled trial. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Hirasawa, H.; Oda, S.; Shiga, H.; Matsuda, K.; Nakamura, M. Oral care reduces incidence of ventilator-associated pneumonia in ICU populations. Intensive Care Med. 2006, 32, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Panchabhai, T.S.; Dangayach, N.S.; Krishnan, A.; Kothari, V.M.; Karnad, D.R. Oropharyngeal cleansing with 0.2% chlorhexidine for prevention of nosocomial pneumonia in critically ill patients: An open-label randomized trial with 0.01% potassium permanganate as control. Chest 2009, 135, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Seguin, P.; Tanguy, M.; Laviolle, B.; Tirel, O.; Malledant, Y. Effect of oropharyngeal decontamination by povidone-iodine on ventilator-associated pneumonia in patients with head trauma. Crit. Care Med. 2006, 34, 1514–1519. [Google Scholar] [CrossRef] [PubMed]
- Seguin, P.; Laviolle, B.; Dahyot-Fizelier, C.; Dumont, R.; Veber, B.; Gergaud, S.; Asehnoune, K.; Mimoz, O.; Donnio, P.Y.; Bellissant, E.; et al. Effect of oropharyngeal povidone-iodine preventive oral care on ventilator-associated pneumonia in severely brain-injured or cerebral hemorrhage patients: A multicenter, randomized controlled trial. Crit. Care Med. 2014, 42, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tantipong, H.; Morkchareonpong, C.; Jaiyindee, S.; Thamlikitkul, V. Randomized controlled trial and meta-analysis of oral decontamination with 2% chlorhexidine solution for the prevention of ventilator-associated pneumonia. Infect. Control Hosp. Epidemiol. 2008, 29, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Pobo, A.; Lisboa, T.; Rodriguez, A.; Sole, R.; Magret, M.; Trefler, S.; Gómez, F.; Rello, J. A randomized trial of dental brushing for preventing ventilator-associated pneumonia. Chest 2009, 136, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Abele-Horn, M.; Dauber, A.; Bauernfeind, A.; Russwurm, W.; Seyfarth-Metzger, I.; Gleich, P.; Ruckdeschel, G. Decrease in nosocomial pneumonia in ventilated patients by selective oropharyngeal decontamination (SOD). Intensive Care Med. 1997, 23, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Aerdts, S.J.; van Dalen, R.; Clasener, H.A.; Festen, J.; van Lier, H.J.; Vollaard, E.J. Antibiotic prophylaxis of respiratory tract infection in mechanically ventilated patients. A prospective, blinded, randomized trial of the effect of a novel regimen. Chest 1991, 100, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Bergmans, D.C.; Bonten, M.J.; Gaillard, C.A.; Paling, J.C.; van der Geest, S.; van Tiel, F.H.; Beysens, A.J.; De Leeuw, P.W.; Stobberingh, E.E. Prevention of ventilator-associated pneumonia by oral decontamination: A prospective, randomized, double-blind, placebo-controlled study. Am. J. Respir. Crit. Care Med. 2001, 164, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.; Rowlands, B.J.; Lowry, K.; Webb, H.; Armstrong, P.; Smilie, J. Selective decontamination of the digestive tract: A stratified, randomized, prospective study in a mixed intensive care unit. Surgery 1991, 110, 303–309. [Google Scholar] [PubMed]
- Bonten, M.J.; Gaillard, C.A.; Johanson, W.G., Jr.; Van Tiel, F.H.; Smeets, H.G.; Van Der Geest, S.; Stobberingh, E.E. Colonization in patients receiving and not receiving topical antimicrobial prophylaxis. Am. J. Respir. Crit. Care Med. 1994, 150, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Camus, C.; Salomon, S.; Bouchigny, C.; Gacouin, A.; Lavoué, S.; Donnio, P.Y.; Bellissant, E. Short-Term Decline in All-Cause Acquired Infections With the Routine Use of a Decontamination Regimen Combining Topical Polymyxin, Tobramycin, and Amphotericin B With Mupirocin and Chlorhexidine in the ICU: A Single-Center Experience. Crit. Care Med. 2014, 42, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Torres, A.; Gonzalez, J.; Puig de la Bellacasa, J.; El-Ebiary, M.; Roca, M.; Gatell, J.M.; Rodriguez-Roisin, R. Utility of selective digestive decontamination in mechanically ventilated patients. Ann. Intern. Med. 1994, 120, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Godard, J.; Guillaume, C.; Reverdy, M.E.; Bachmann, P.; Bui-Xuan, B.; Nageotte, A.; Motin, J. Intestinal decontamination in a polyvalent ICU. A double-blind study. Intensive Care Med. 1990, 16, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Georges, B.; Mazerolles, M.; Decun, J.-F.; Rouge, P.; Pomies, S.; Cougot, P.; Andrieu, P.; Virenque, C. Décontamination digestive sélective résultats d'une étude chez le polytraumatisé. Réanim. Urgence 1994, 3, 621–627. [Google Scholar] [CrossRef]
- Hammond, J.M.; Potgieter, P.D.; Saunders, L.G. Selective decontamination of the digestive tract in multiple trauma patients-Is there a role? Results of a prospective, double-blind, randomized trial. Crit. Care Med. 1994, 22, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.; Foweraker, J.E.; Roberts, S.E. Effectiveness of selective decontamination of the digestive tract (SDD) in an ICU with a policy encouraging a low gastric pH. Clin. Intensive Med. 1992, 3, 52–58. [Google Scholar]
- Karvouniaris, M.; Makris, D.; Zygoulis, P.; Triantaris, A.; Xitsas, S.; Mantzarlis, K.; Petinaki, E.; Zakynthinos, E. Nebulised colistin for ventilator-associated pneumonia prevention. Eur. Respir. J. 2015, 46, 1544–1547. [Google Scholar] [CrossRef] [PubMed]
- Korinek, A.M.; Laisne, M.J.; Nicolas, M.H.; Raskine, L.; Deroin, V.; Sanson-lepors, M.J. Selective decontamination of the digestive tract in neurosurgical intensive care unit patients: A double-blind, randomized, placebo-controlled study. Crit. Care Med. 1993, 21, 1466–1473. [Google Scholar] [CrossRef] [PubMed]
- Laggner, A.N.; Tryba, M.; Georgopoulos, A.; Lenz, K.; Grimm, G.; Graninger, W.; Schneeweiss, B.; Druml, W. Oropharyngeal decontamination with gentamicin for long-term ventilated patients on stress ulcer prophylaxis with sucralfate? Wien. Klin. Wochenschr. 1994, 106, 15–19. [Google Scholar] [PubMed]
- Langlois-Karaga, A.; Bues-Charbit, M.; Davignon, A.; Albanese, J.; Durbec, O.; Martin, C.; Morati, N.; Balansard, G. Selective digestive decontamination in multiple trauma patients: Cost and efficacy. Pharm. World Sci. 1995, 17, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Palomar, M.; Alvarez-Lerma, F.; Jorda, R.; Bermejo, B.; Catalan Study Group of Nosocomial Pneumonia Prevention. Prevention of nosocomial infection in mechanically ventilated patients: Selective digestive decontamination versus sucralfate. Clin. Intensive Care 1997, 8, 228–235. [Google Scholar] [CrossRef]
- Quinio, B.; Albanese, J.; Bues-Charbit, M.; Viviand, X.; Martin, C. Selective decontamination of the digestive tract in multiple trauma patients. A prospective double-blind, randomized, placebo-controlled study. Chest 1996, 109, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.A.; Martin, M.J.; Pita, S.; Paz, J.; Seco, C.; Margusino, L.; Villanueva, R.; Duran, M.T. Prevention of nosocomial infection in critically ill patients by selective decontamination of the digestive tract. A randomized, double blind, placebo-controlled study. Intensive Care Med. 1992, 18, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Rolando, N.; Gimson, A.; Wade, J.; Philpott-Howard, J.; Casewell, M.; Williams, R. Prospective controlled trial of selective parenteral and enteral antimicrobial regimen in fulminant liver failure. Hepatology 1993, 17, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Garcia, M.; Cambronero, J.A.; Lopez-Diaz, J.; Cerdá Cerdá, E.; Rubio Blasco, J.; Gómez Aguinaga, M.A.; Núnez Reiz, A.; Rogero Marín, S.; Onoro Canaveral, J.J.; Sacristán del Castillo, J.A. Effectiveness and cost of selective decontamination of the digestive tract in critically ill intubated patients. A randomized, double-blind, placebo-controlled multicenter trial. Am. J. Respir. Crit. Care Med. 1998, 158, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Stoutenbeek, C.P.; van Saene, H.K.F.; Little, R.A.; Whitehead, A. The effect of selective decontamination of the digestive tract on mortality in multiple trauma patients: A multicenter randomized controlled trial. Intensive Care Med. 2007, 33, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Unertl, K.; Ruckdeschel, G.; Selbmann, H.K.; Jensen, U.; Forst, H.; LenhartK, F.P. Peter Prevention of colonization and respiratory infections in long-term ventilated patients by local antimicrobial prophylaxis. Intensive Care Med. 1987, 13, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, C.; Harinck-deWeerd, J.E.; Bakker, N.C.; Jacz, K.; Doornbos, L.; De Ridder, V.A. Selective decontamination of the digestive tract with norfloxacin in the prevention of ICU-acquired infections: A prospective randomized study. Intensive Care Med. 1989, 15, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Verwaest, C.; Verhaegen, J.; Ferdinande, P.; Schetz, M.; Van den Berghe, G.; Verbist, L.; Lauwers, P. Randomized, controlled trial of selective digestive decontamination in 600 mechanically ventilated patients in a multidisciplinary intensive care unit. Crit. Care Med. 1997, 25, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Wiener, J.; Itokazu, G.; Nathan, C.; Kabins, S.A.; Weinstein, R.A. A randomized, double-blind, placebo-controlled trial of selective digestive decontamination in a medical-surgical intensive care unit. Clin. Infect. Dis. 1995, 20, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Winter, R.; Humphreys, H.; Pick, A.; MacGowan, A.P.; Willatts, S.M.; Speller, D.C. A controlled trial of selective decontamination of the digestive tract in intensive care and its effect on nosocomial infection. J. Antimicrob. Chemother. 1992, 30, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Chastre, J.; Fagon, J.Y. Ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 2002, 165, 867–903. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; D’Amico, R.; Pifferi, S.; Torri, V.; Brazzi, L.; Parmelli, E. Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database Syst. Rev. 2009, CD000022. [Google Scholar] [CrossRef]
- Hurley, J.C. Prophylaxis with enteral antibiotics in ventilated patients: Selective decontamination or selective cross-infection? Antimicrob. Agents Chemother. 1995, 39, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, L.; Van Saene, H.K.; Milanese, M.; Gregori, D.; Gullo, A. Selective decontamination of the digestive tract reduces bacterial bloodstream infection and mortality in critically ill patients. Systematic review of randomized, controlled trials. J. Hosp. Infect. 2007, 65, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Pileggi, C.; Bianco, A.; Flotta, D.; Nobile, C.G.; Pavia, M. Prevention of ventilator-associated pneumonia, mortality and all intensive care unit acquired infections by topically applied antimicrobial or antiseptic agents: A meta-analysis of randomized controlled trials in intensive care units. Crit. Care 2011, 15, R155. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.Y.; Ruest, A.; Meade, M.O.; Cook, D.J. Oral decontamination for prevention of pneumonia in mechanically ventilated adults: Systematic review and meta-analysis. BMJ 2007, 334, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Saunders, G.L.; Hammond, J.M.; Potgieter, P.D.; Plumb, H.A.; Forder, A.A. Microbiological surveillance during selective decontamination of the digestive tract (SDD). J. Antimicrob. Chemother. 1994, 34, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Nardi, G.; Valentinis, U.; Proietti, A.; De Monte, A.; Di Silvestre, A.; Muzzi, R.; Giordano, F. Epidemiological impact of prolonged systematic use of topical SDD on bacterial colonization of the tracheobronchial tree and antibiotic resistance. A three year study. Intensive Care Med. 1993, 19, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Messori, A.; Trippoli, S.; Vaiani, M.; Gorini, M.; Corrado, A. Bleeding and pneumonia in intensive care patients given ranitidine and sucralfate for prevention of stress ulcer: Meta-analysis of randomised controlled trials. BMJ 2000, 321, 1103–1106. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Cao, Y.; Liao, C.; Wu, L.; Gao, F. Effect of histamine-2-receptor antagonists versus sucralfate on stress ulcer prophylaxis in mechanically ventilated patients: A meta-analysis of 10 randomized controlled trials. Crit. Care 2010, 14, R194. [Google Scholar] [CrossRef] [PubMed]
- Alhazzani, W.; Almasoud, A.; Jaeschke, R.; Lo, B.W.; Sindi, A.; Altayyar, S.; Fox-Robichaud, A. Small bowel feeding and risk of pneumonia in adult critically ill patients: A systematic review and meta-analysis of randomized trials. Crit. Care 2013, 17, R127. [Google Scholar] [CrossRef] [PubMed]
- Melsen, W.G.; Rovers, M.M.; Bonten, M.J.M. Ventilator-associated pneumonia and mortality: A systematic review of observational studies. Crit. Care Med. 2009, 37, 2709–2718. [Google Scholar] [PubMed]
- Safdar, N.; Dezfulian, C.; Collard, H.R.; Saint, S. Clinical and economic consequences of ventilator-associated pneumonia: A systematic review. Crit. Care Med. 2005, 33, 2184–2193. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Liu, Y. Effect of ventilator circuit changes on ventilator-associated pneumonia: A systematic review and meta-analysis. Respir. Care 2010, 55, 467–474. [Google Scholar] [PubMed]
- Subirana, M.; Solà, I.; Benito, S. Closed tracheal suction systems versus open tracheal suction systems for mechanically ventilated adult patients. Cochrane Database Syst. Rev. 2007, 4, CD004581. [Google Scholar] [CrossRef]
- Siempos, I.I.; Vardakas, K.Z.; Kopterides, P.; Falagas, M.E. Impact of passive humidification on clinical outcomes of mechanically ventilated patients: A meta-analysis of randomized controlled trials. Crit. Care Med. 2007, 35, 2843–2851. [Google Scholar] [PubMed]
- Muscedere, J.; Rewa, O.; McKechnie, K.; Jiang, X.; Laporta, D.; Heyland, D.K. Subglottic secretion drainage for the prevention of ventilator-associated pneumonia: A systematic review and meta-analysis. Crit. Care Med. 2011, 39, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Delaney, A.; Gray, H.; Laupland, K.B.; Zuege, D.J. Kinetic bed therapy to prevent nosocomial pneumonia in mechanically ventilated patients: A systematic review and meta-analysis. Crit. Care 2006, 10, R70. [Google Scholar] [CrossRef] [PubMed]
- Sud, S.; Friedrich, J.O.; Taccone, P.; Polli, F.; Adhikari, N.K.; Latini, R.; Gattinoni, L. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: Systematic review and meta-analysis. Intensive Care Med. 2010, 36, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Siempos, I.I.; Vardakas, K.Z.; Falagas, M.E. Closed tracheal suction systems for prevention of ventilator-associated pneumonia. Br. J. Anaesth. 2008, 100, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, L.; Weir, I.; Gregori, D.; Taylor, D.; Van Saene, J.; Van Saene, H. Effectiveness of oral chlorhexidine on nosocomial pneumonia, causative microorganisms and mortality in critically ill patients: A systematic review and meta-analysis. Minerva Anestesiol. 2014, 80, 805–820. [Google Scholar] [PubMed]
- Labeau, S.O.; Van de Vyver, K.; Brusselaers, N.; Vogelaers, D.; Blot, S.I. Prevention of ventilator-associated pneumonia with oral antiseptics: A systematic review and meta-analysis. Lancet Infect. Dis. 2011, 11, 845–854. [Google Scholar] [CrossRef]
- Hurley, J.C. Profound effect of study design factors on ventilator-associated pneumonia incidence of prevention studies: Benchmarking the literature experience. J. Antimicrob. Chemother. 2008, 61, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.C. Topical antibiotics as a major contextual hazard toward bacteremia within selective digestive decontamination studies: A meta-analysis. BMC Infect. Dis. 2014, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.C. Impact of selective digestive decontamination on respiratory tract Candida among patients with suspected ventilator-associated pneumonia. A meta-analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.C. ICU-acquired candidemia within selective digestive decontamination studies: A meta-analysis. Intensive Care Med. 2015, 41, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.C. Ventilator Associated Pneumonia prevention methods using topical antibiotics: Herd protection or herd peril? Chest 2014, 146, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.C. The perfidious effect of topical placebo: A calibration of Staphylococcus aureus Ventilator Associated Pneumonia incidence within Selective Digestive Decontamination (SDD) studies versus the broader evidence base. Antimicrob Agents Chemother. Antimicrob. Agents Chemother. 2013, 57, 4524–4531. [Google Scholar] [CrossRef] [PubMed]
- Hróbjartsson, A.; Gøtzsche, P.C. Placebo interventions for all clinical conditions. Cochrane Database Syst. Rev. 2010, CD003974. [Google Scholar] [CrossRef]
- Oostdijk, E.A.N.; Kesecioglu, J.; Schultz, M.J.; Visser, C.E.; de Jonge, E.; van Essen, E.H.R.; Bernards, A.T.; Purmer, I.; Brimicombe, R.; Bergmans, D.; van Tiel, F.; et al. Notice of Retraction and Replacement: Oostdijk et al. Effects of Decontamination of the Oropharynx and Intestinal Tract on Antibiotic Resistance in ICUs: A Randomized Clinical Trial. JAMA 2014, 312, 1429–1437, JAMA 2017, 317, 1583–1584. [Google Scholar] [CrossRef] [PubMed]
- Noto, M.J.; Domenico, H.J.; Byrne, D.W.; Talbot, T.; Rice, T.W.; Bernard, G.R.; Wheeler, A.P. Chlorhexidine bathing and health care–associated infections: A randomized clinical trial. JAMA 2015, 313, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Septimus, E.; Kleinman, K.; Moody, J.; Hickok, J.; Avery, T.R.; Lankiewicz, J.; Gombosev, A.; Terpstra, L.; Hartford, F.; et al. Targeted versus universal decolonization to prevent ICU infection. N. Engl. J. Med. 2013, 368, 2255–2265. [Google Scholar] [CrossRef] [PubMed]
- Laupland, K.B.; Kirkpatrick, A.W.; Church, D.L.; Ross, T.; Gregson, D.B. Intensive-care-unit-acquired bloodstream infections in a regional critically ill population. J. Hosp. Infect. 2004, 58, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.H.; Sherman, G.; Ward, S.; Fraser, V.J.; Kollef, M.H. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 2000, 118, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Stoutenbeek, C.P.; van Saene, H.K.; Miranda, D.R.; Zandstra, D.F. The effect of selective decontamination of the digestive tract on colonisation and infection rate in multiple trauma patients. Intensive Care Med. 1984, 10, 185–192. [Google Scholar] [CrossRef] [PubMed]


| Variable | Observational (No Intervention) | Non-Antibiotic Studies | Topical Antiseptic Studies | Topical Antibiotic Studies |
|---|---|---|---|---|
| Supplemental material | Table S1 | Table S2 | Table S3 | Table S4 |
| Number of studies a | 106 | 50 | 14 | 26 |
| Publication year (range) | 1987–2016 | 1987–2016 | 2000–2014 | 1987–2015 |
| Studies sourced from systematic reviews b | 45 | 32 | 8 | 24 |
| Topical placebo used in study | NA | 8 | 7 | 14 |
| Bronchoscopic sampling for VAP diagnosis (n) c | 58 | 22 | 5 | 8 |
| Trauma ICU’s (n) d | 21 | 11 | 3 | 8 |
| North American ICU’s (n) e | 21 | 13 | 2 | 1 |
| Patients per study group median (IQR) f | 279 135–618 | 75 61–149 | 114 52–147 | 52 36–126 |
| VAP incidence per 100 patients | ||||
| Observational (mean) 95% CI n | 20.8 19.0–22.8 115 | |||
| Control (mean) 95% CI n | 21.6 19.2–24.1 50 | 20.1 13.6–28.9 14 | 32.7 26.9–38.9 27 | |
| Intervention (mean) 95% CI n | 16.5 14.2–19.2 43 | 13.8 8.6–21.4 14 | 14.8 12.0–18.1 26 | |
| S. aureus VAP incidence per 100 patients | ||||
| Observational (mean) 95% CI n | 4.8 4.2–5.6 115 | |||
| Control (mean) 95% CI n | 7.2 6.3–8.3 50 | 5.1 2.7–9.3 14 | 9.6 6.9–13.2 27 | |
| Intervention (mean) 95% CI n | 5.3 4.1–6.6 43 | 3.1 1.6–6.1 14 | 6.4 4.8–8.3 26 | |
| VAP | S. aureus VAP | |||||
|---|---|---|---|---|---|---|
| Factor | Coefficient b | 95% CI | p | Coefficient b | 95% CI | p |
| Groups from observational studies (reference group) | −1.30 | −1.62 to −0.97 | −2.70 | −3.01 to −2.29 | ||
| Control groups | ||||||
| Non- antibiotic studies, no placebo | +0.04 | −0.21 to +0.29 | 0.77 | +0.10 | −0.15 to +0.33 | 0.42 |
| Non- antibiotic studies; with topical placebo | −0.08 | −0.60 to +0.45 | 0.77 | +0.33 | −0.14 to +0.81 | 0.17 |
| Topical antiseptic studies; no placebo | −0.11 | −0.66 to +0.44 | 0.69 | +0.01 | −0.55 to +0.57 | 0.97 |
| Topical antiseptic studies; with topical placebo | −0.20 | −0.81 to +0.41 | 0.53 | +0.07 | −0.48 to +0.62 | 0.81 |
| Topical antibiotic studies; no placebo | +0.50 c | +0.05 to +0.95 | 0.03 | +0.31 d,e | −0.08 to +0.69 | 0.12 |
| Topical antibiotic studies; with topical placebo | +0.38 c | −0.04 to +0.79 | 0.08 | +0.48 d,e | +0.15 to +0.82 | 0.004 |
| Intervention groups | ||||||
| Non-antibiotic studies | −0.34 | −0.60 to −0.09 | 0.008 | −0.14 | −0.39 to +0.12 | 0.30 |
| Topical antiseptic studies; | −0.77 | −1.18 to −0.35 | 0.001 | −0.53 | −1.03 to −0.03 | 0.039 |
| Topical antibiotic studies; | −0.57 | −0.90 to −0.23 | 0.001 | −0.21 | −0.53 to +0.11 | 0.20 |
| Trauma ICU f | +0.39 | +0.18 to +0.59 | 0.001 | +0.86 | +0.64 to +1.08 | 0.001 |
| Mode of diagnosis g | −0.03 | −0.21 to +0.14 | 0.73 | +0.08 | −0.11 to +0.28 | 0.41 |
| North American study h | −0.31 | −0.56 to −0.06 | 0.01 | −0.28 | −0.54 to −0.02 | 0.04 |
| Year of publication i | +0.01 | −0.01 to +0.01 | 0.88 | −0.01 | −0.03 to +0.001 | 0.08 |
| S. aureus bacteremia Incidence Proportion | |||
|---|---|---|---|
| Factor | Per 100 Patients | 95% CI | Number of Groups |
| Groups from observational studies | 2.1 | 1.1–4.1 | 10 |
| Non-antibiotic studies | |||
| Control and Intervention groups | 1.8 | 0.6–5.1 | 3 |
| Topical antiseptic studies; | |||
| Control and Intervention groups | 1.2 | 0.4–3.1 | 5 |
| Topical antibiotic studies; | |||
| Control groups | 3.8 | 2.1–5.7 | 9 |
| Intervention groups | 4.2 | 2.9–5.9 | 11 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hurley, J.C. Unusually High Incidences of Staphylococcus aureus Infection within Studies of Ventilator Associated Pneumonia Prevention Using Topical Antibiotics: Benchmarking the Evidence Base. Microorganisms 2018, 6, 2. https://doi.org/10.3390/microorganisms6010002
Hurley JC. Unusually High Incidences of Staphylococcus aureus Infection within Studies of Ventilator Associated Pneumonia Prevention Using Topical Antibiotics: Benchmarking the Evidence Base. Microorganisms. 2018; 6(1):2. https://doi.org/10.3390/microorganisms6010002
Chicago/Turabian StyleHurley, James C. 2018. "Unusually High Incidences of Staphylococcus aureus Infection within Studies of Ventilator Associated Pneumonia Prevention Using Topical Antibiotics: Benchmarking the Evidence Base" Microorganisms 6, no. 1: 2. https://doi.org/10.3390/microorganisms6010002





