Effects of Water-Cooling on the Mechanical Properties and Microstructure of 5083 Aluminum Alloy during Flame Straightening
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
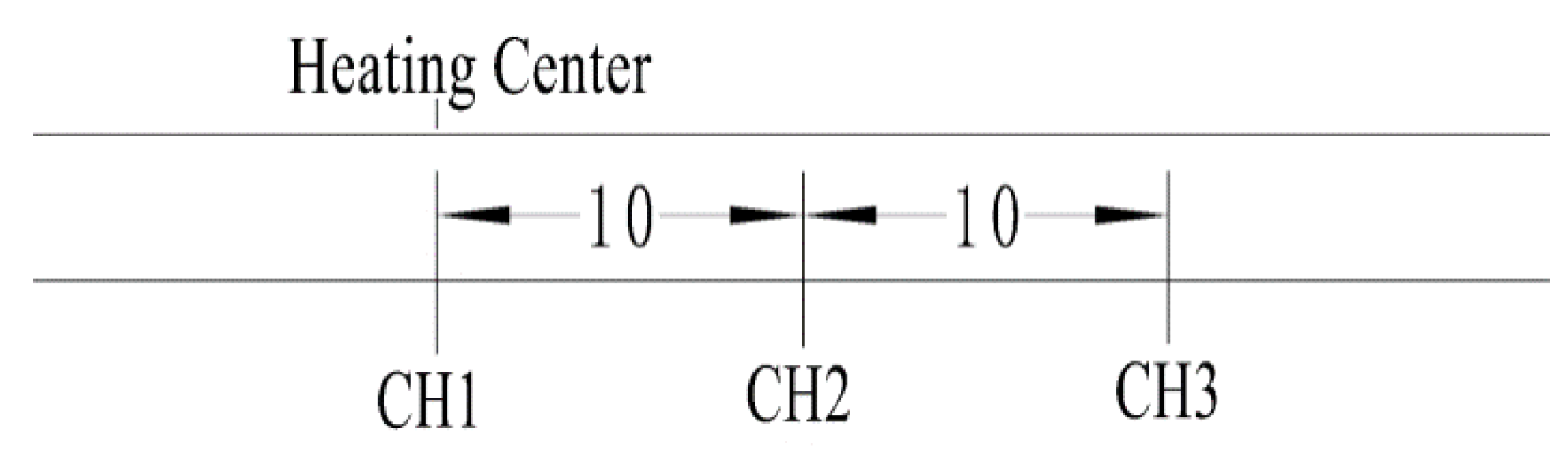
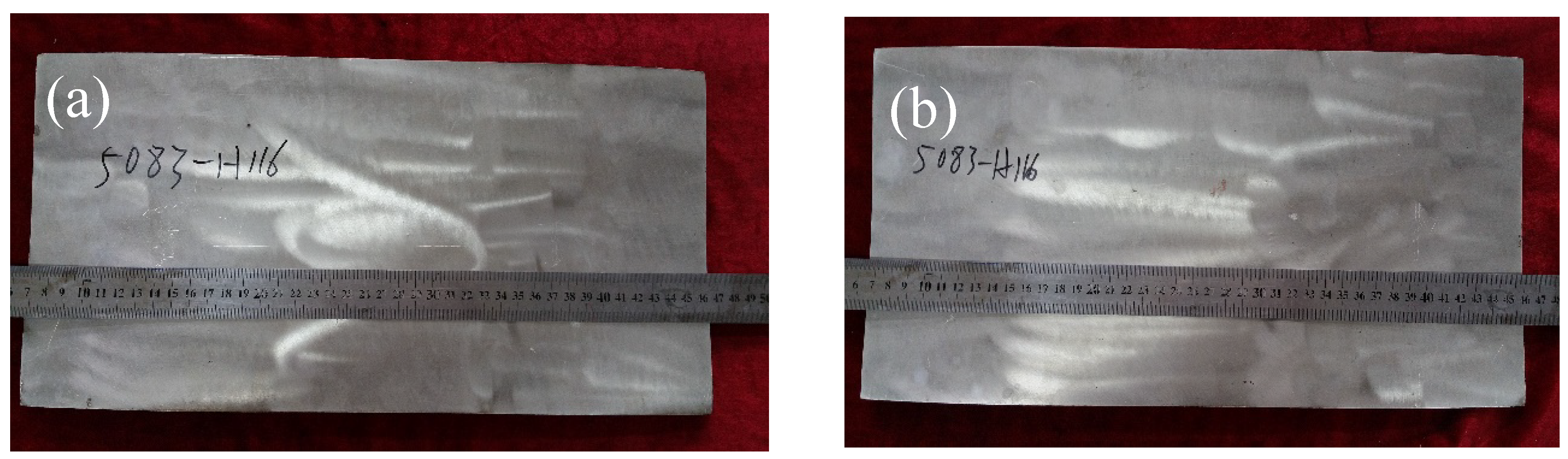
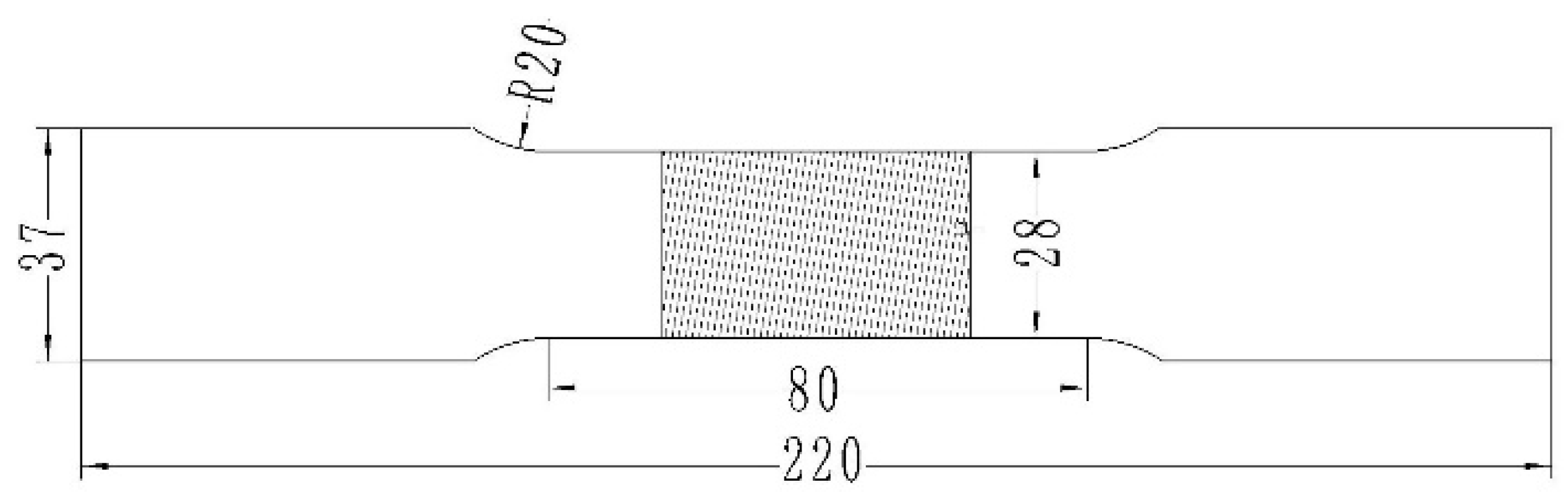
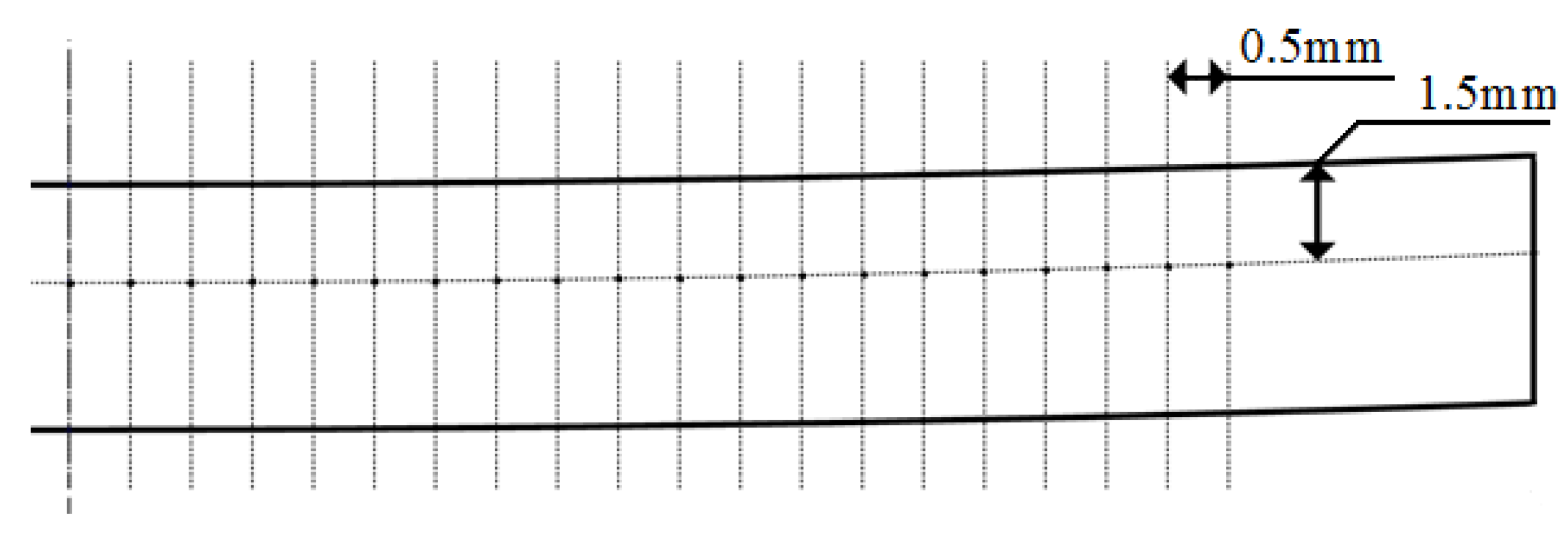
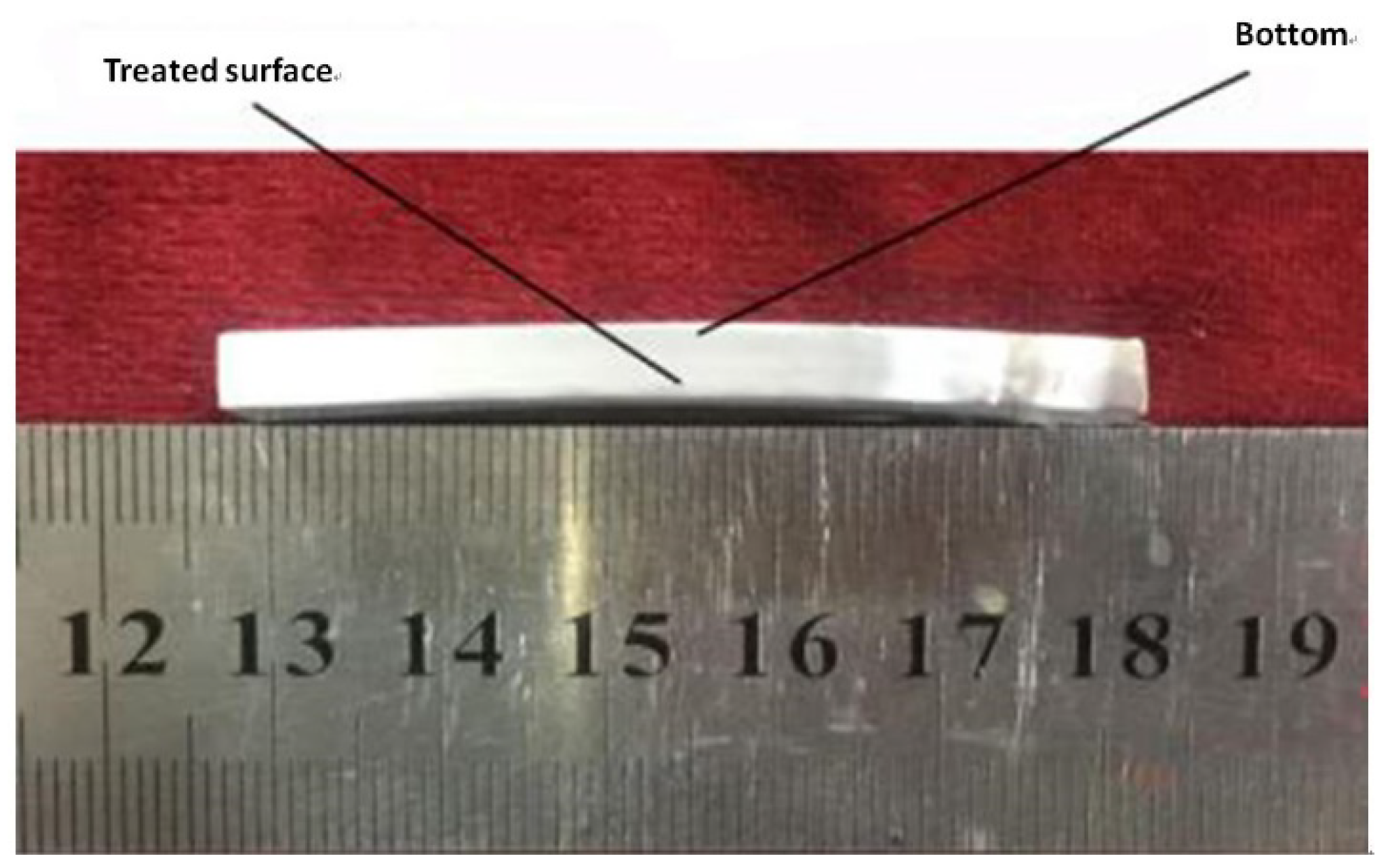
2.2. Experimental Procedure
3. Results and Discussion
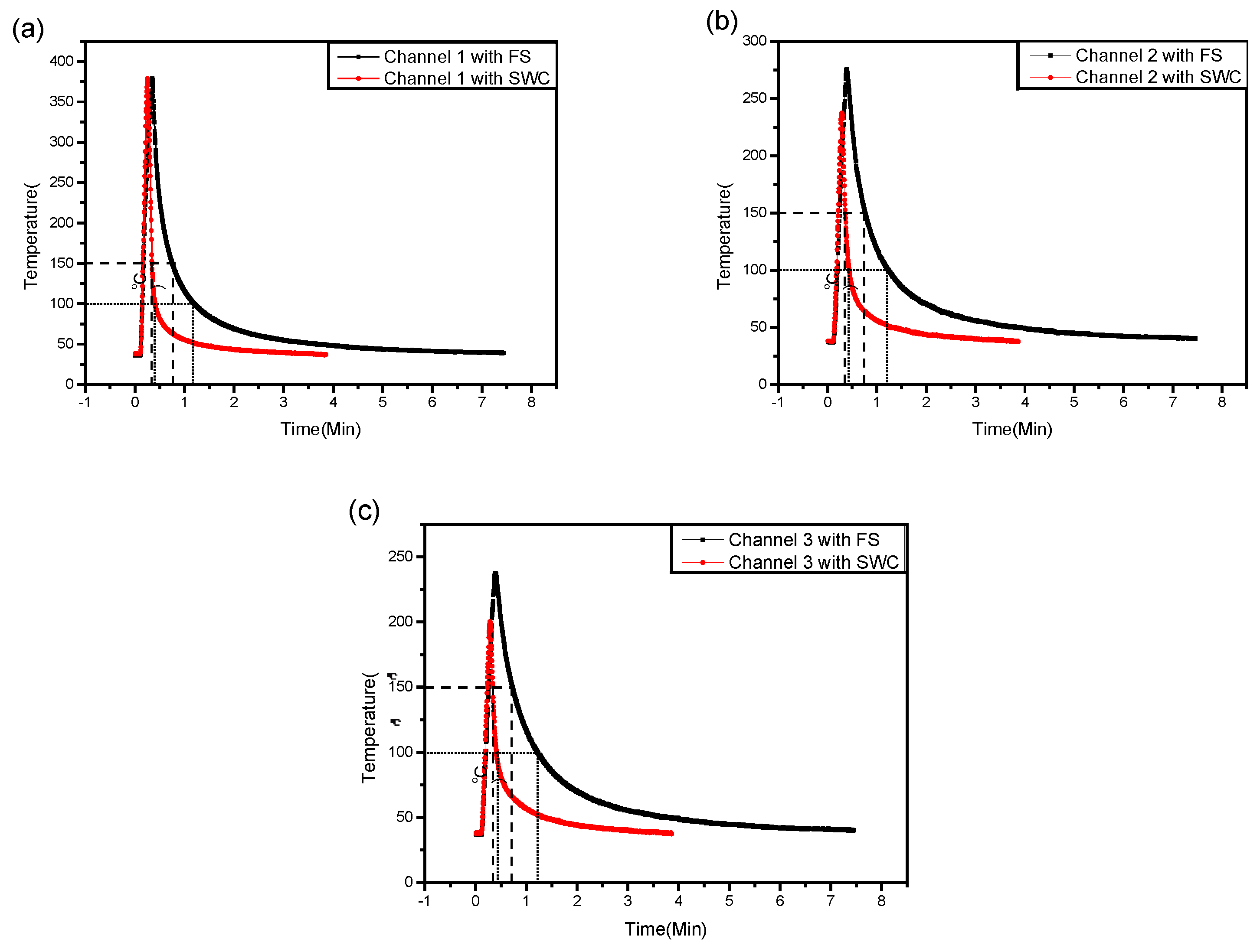
3.1. Temperature Profile
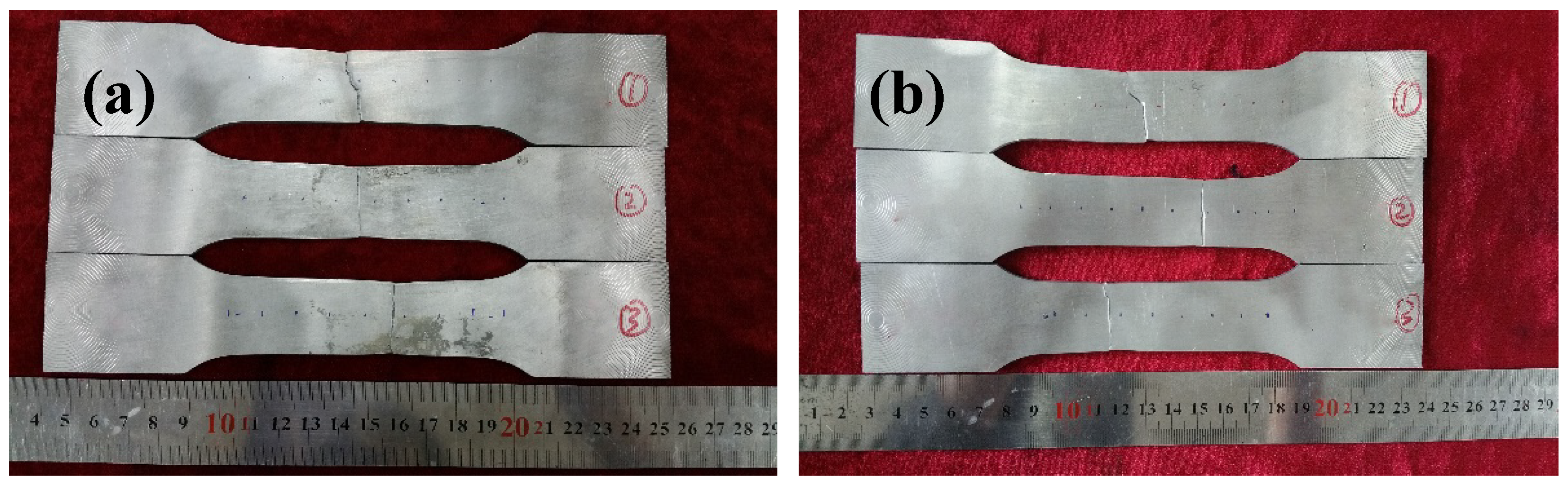
3.2. Tensile Properties
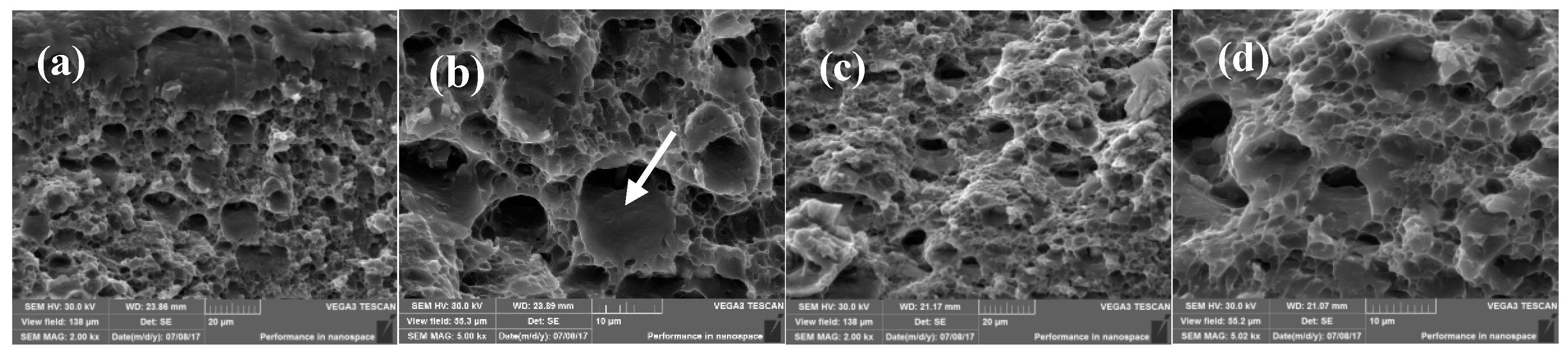
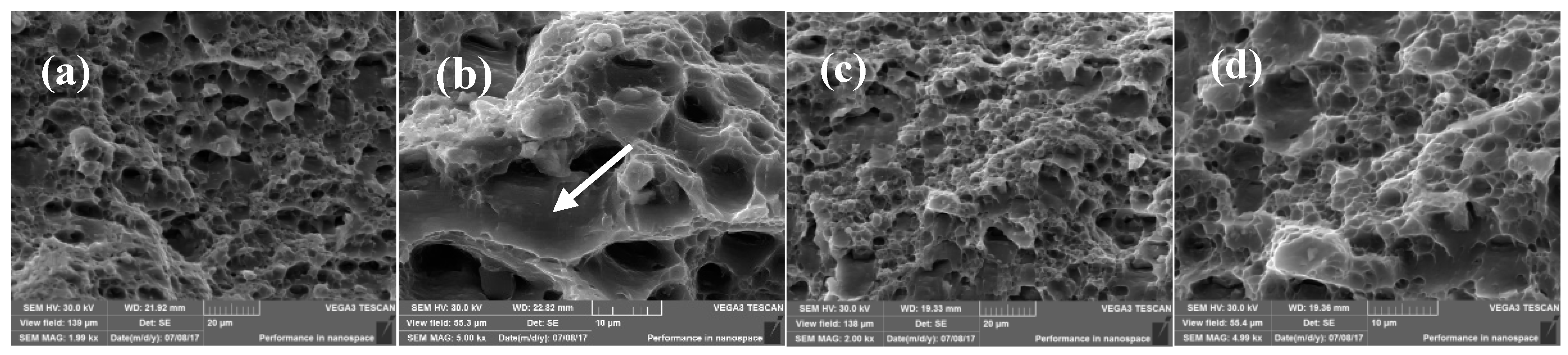
3.3. Fractography
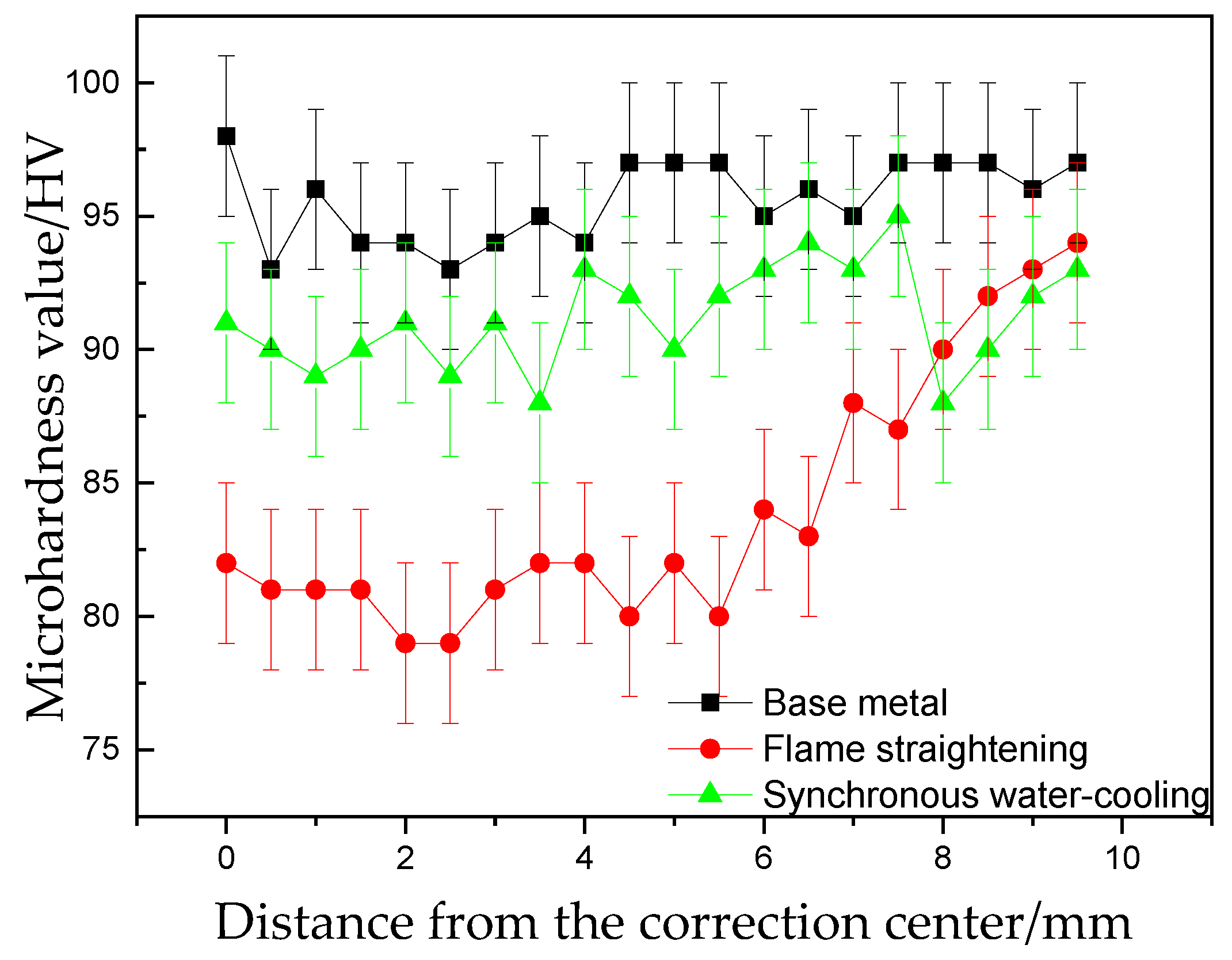
3.4. Microhardness Line
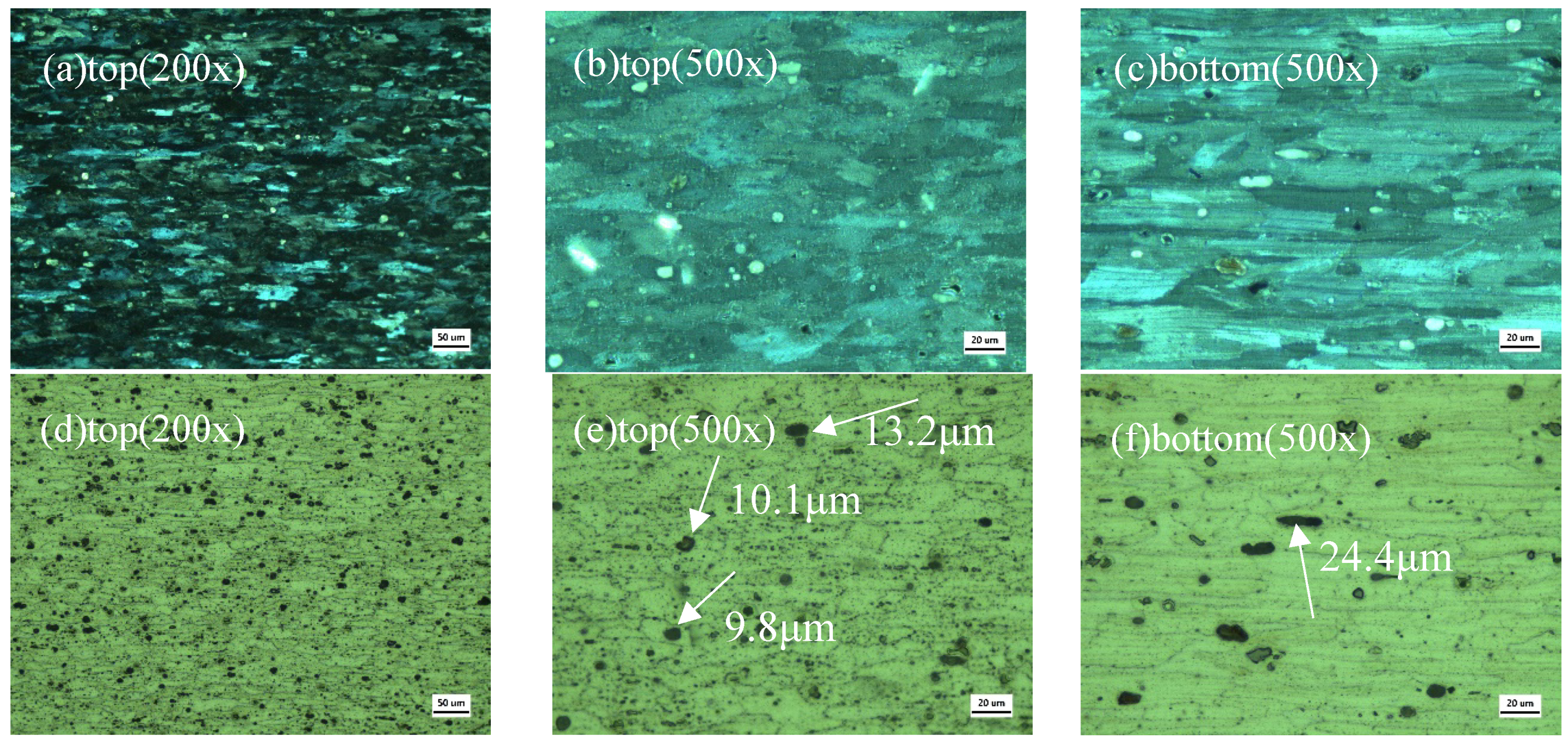
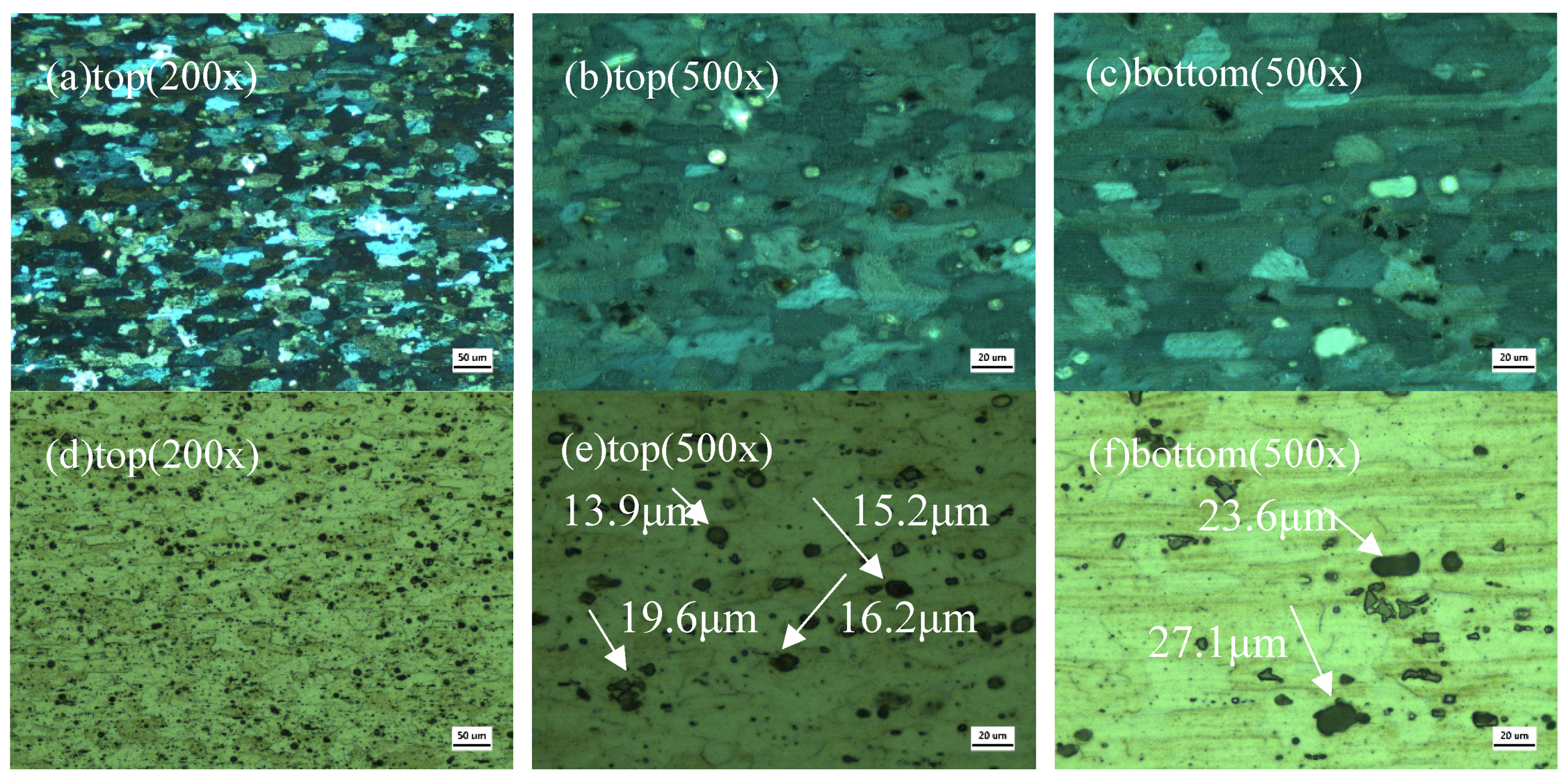
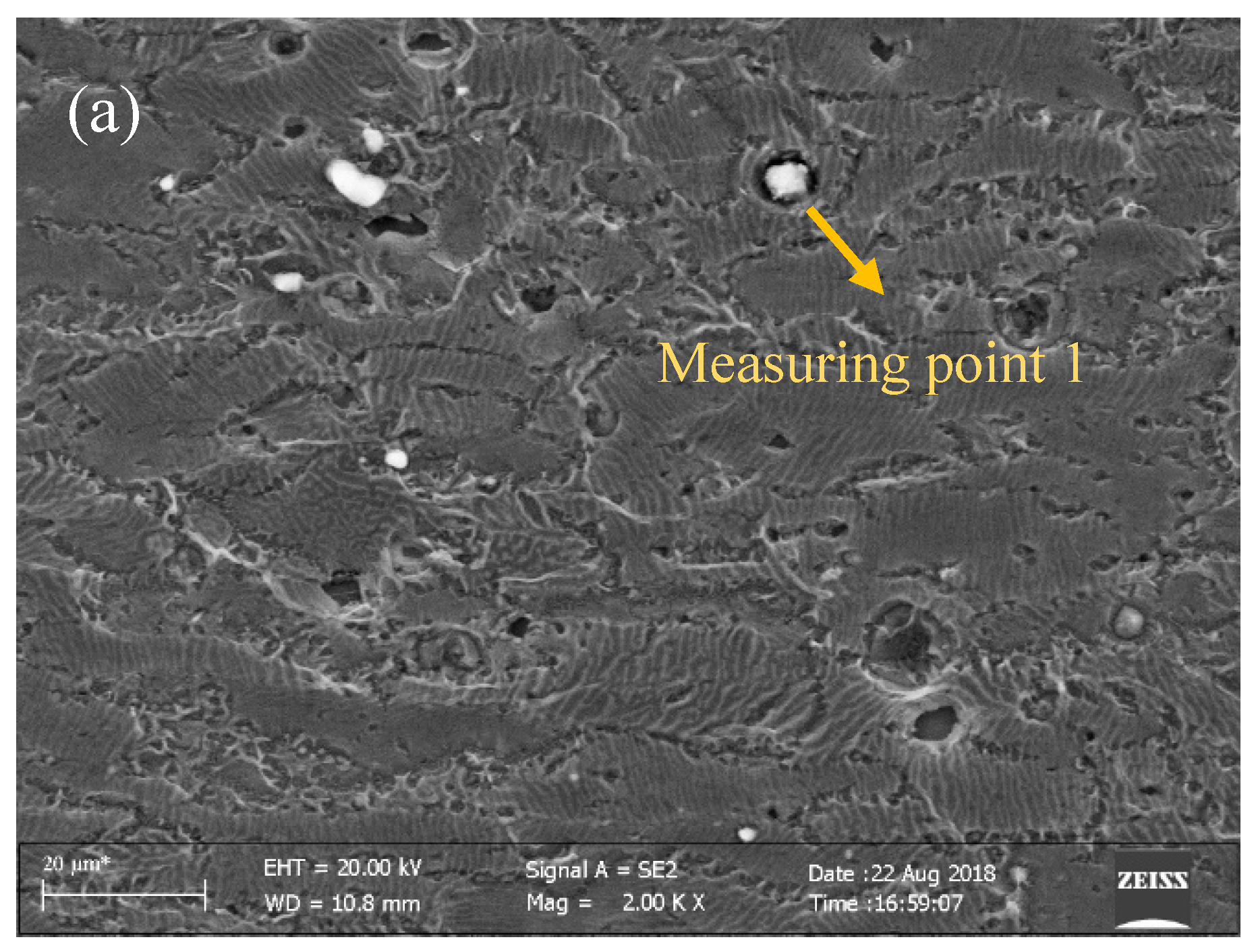
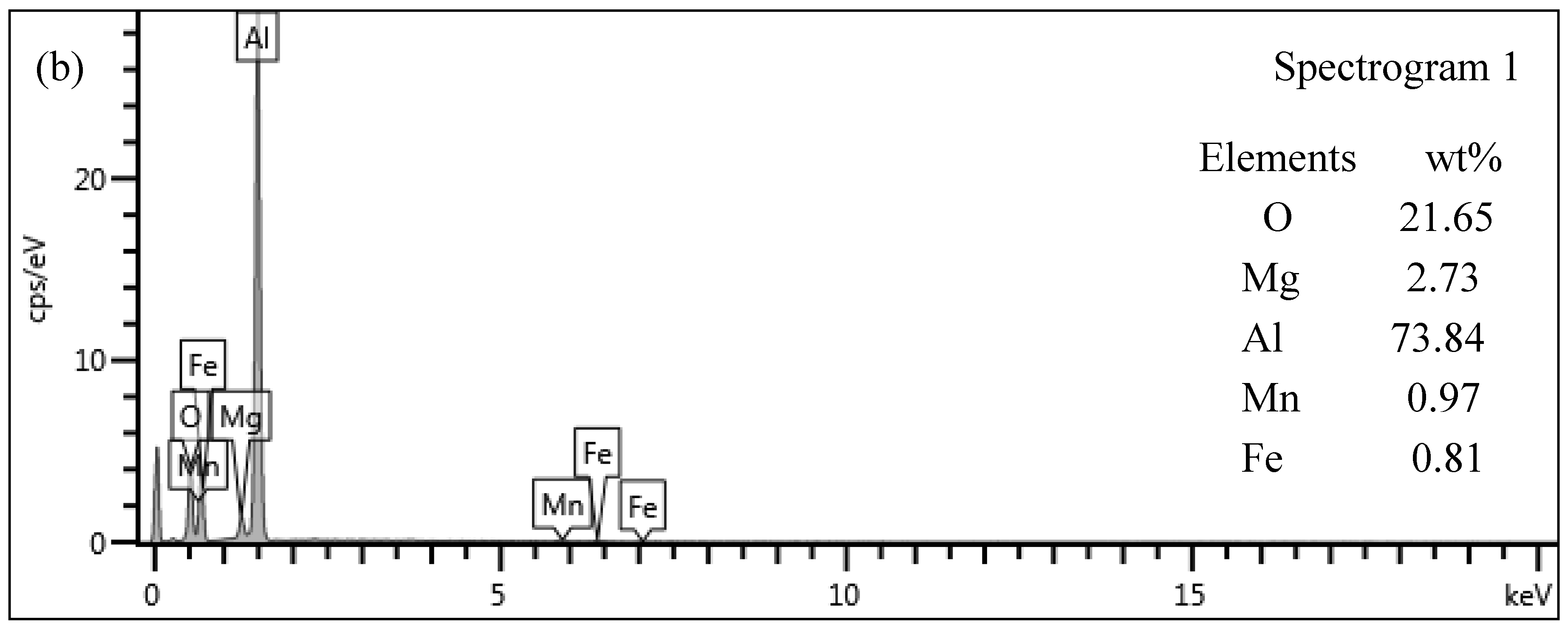
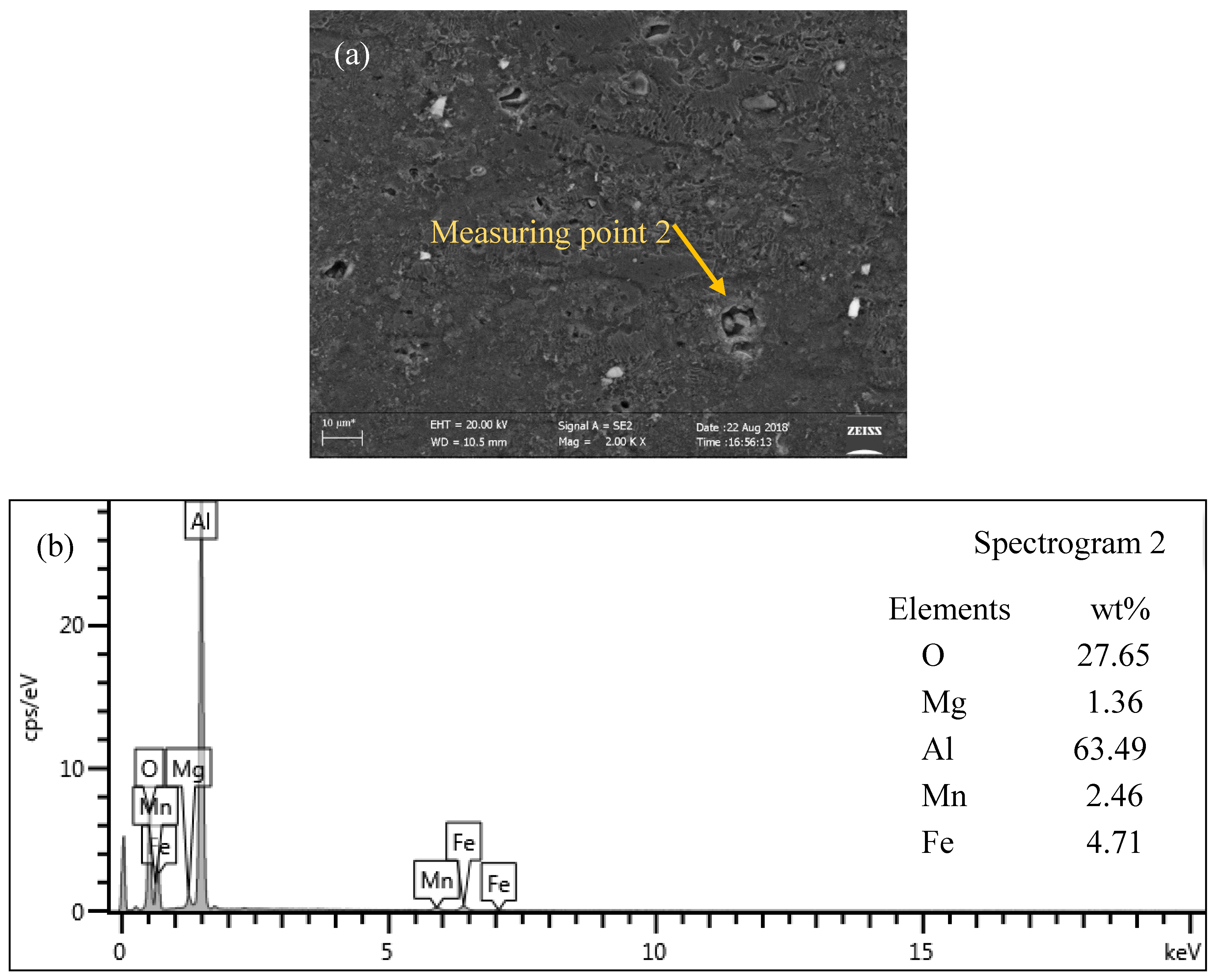
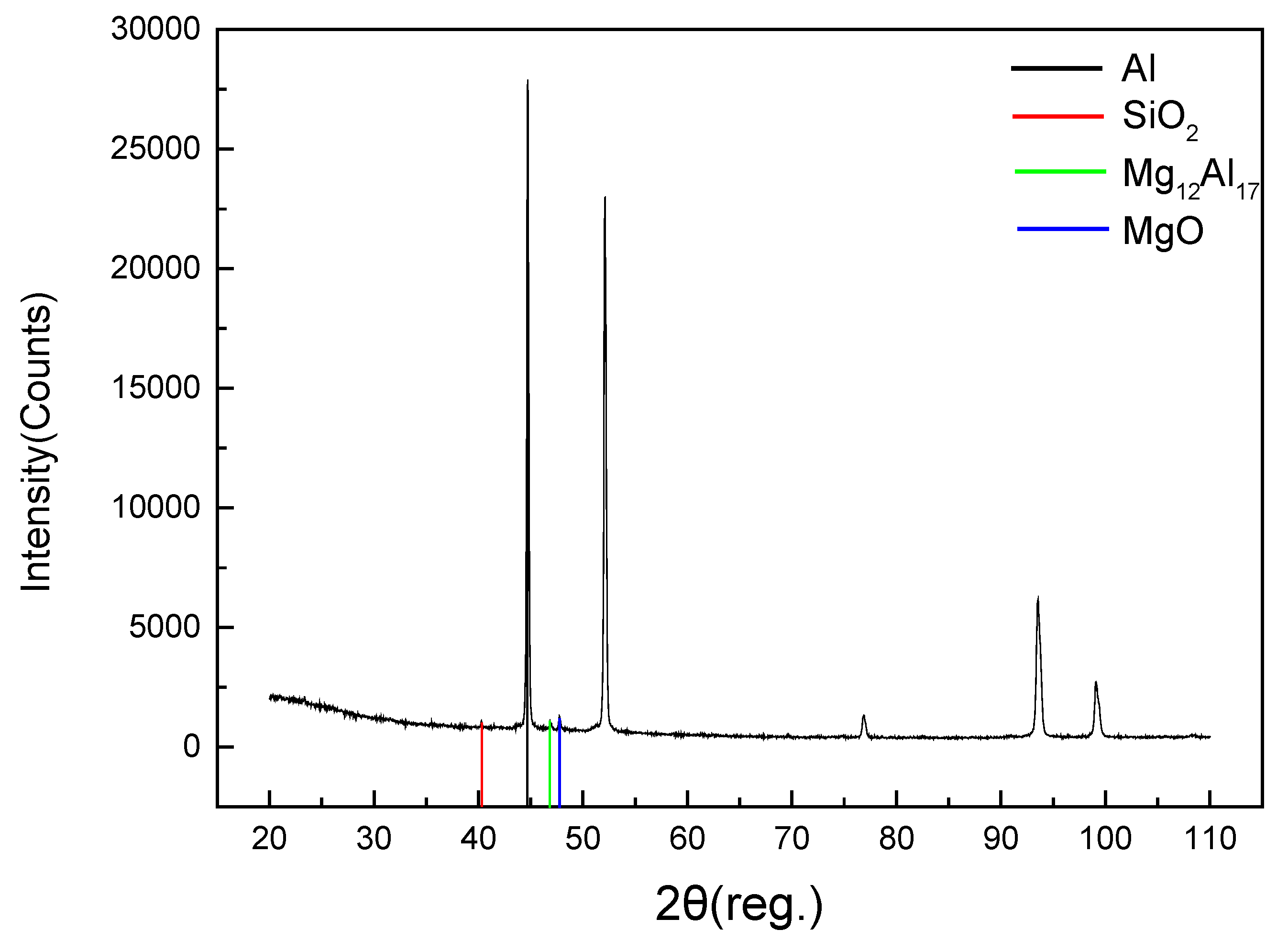
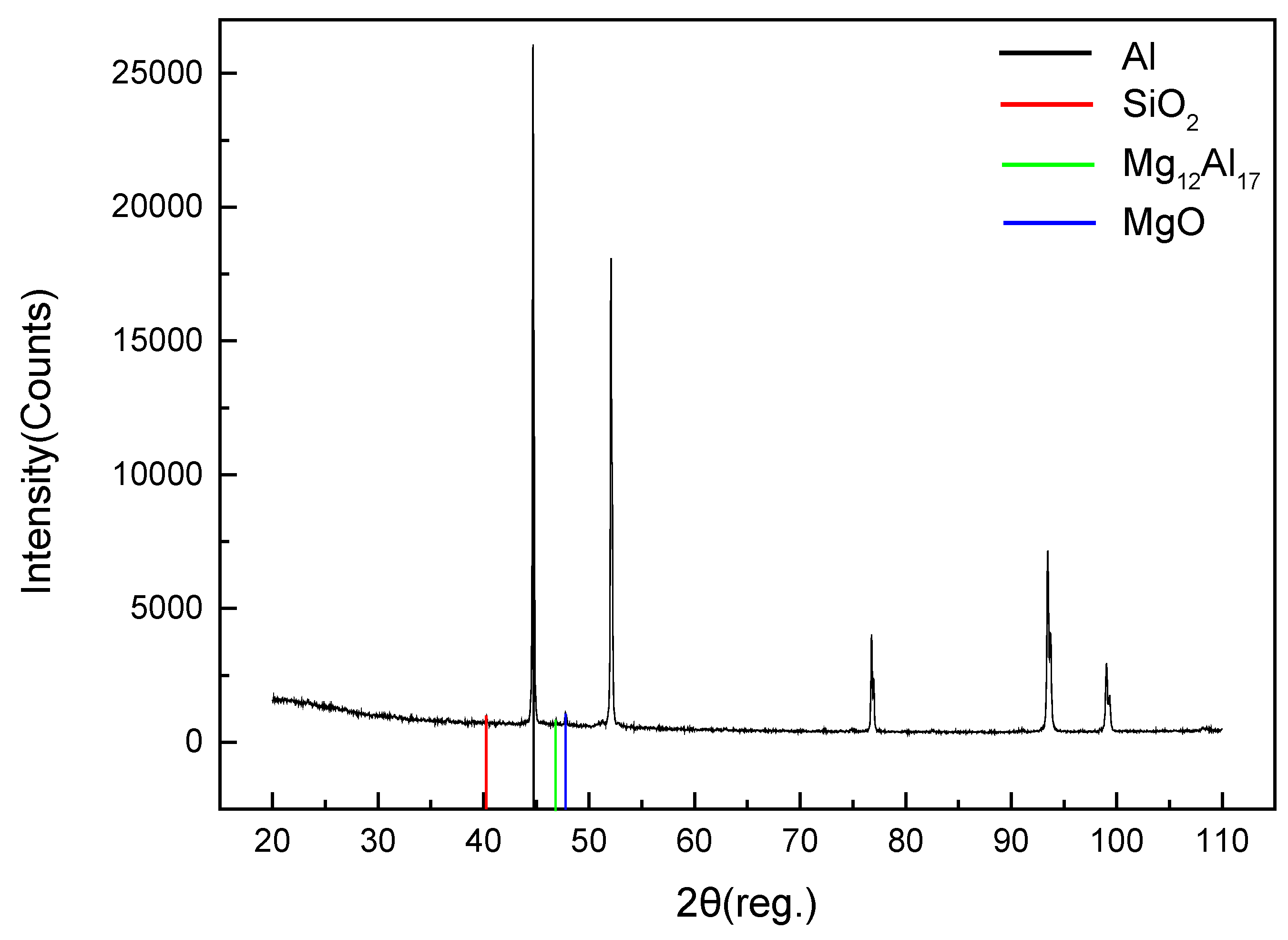
3.5. Microstructure
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stannard, D.M.; Smith, B.L. Recent advances for use of aluminum offshore. In Proceedings of the 8th International Conference on Offshore Mechanics and Arctic Engineering, Huston, TX, USA, 19–23 March 1989; pp. 299–307. [Google Scholar]
- Xue, J.; Liu, J. Research on current state and prospect of welding deformation and deformation control methods for China′s high-speed train aluminum alloy carbody. Hot Work. Technol. 2012, 41, 201–208. [Google Scholar]
- Zhang, Z.Y.; Jiang, H.B.; Yu, C.Q. Automated flame rectification process planning system in shipbuilding based on artificial intelligence. Int. J. Adv. Manuf. Technol. 2006, 30, 1119–1125. [Google Scholar] [CrossRef]
- Deng, D.; Murakawa, H.; Liang, W. Numerical simulation of welding distortion in large structures. Comput. Methods Appl. Mech. Eng. 2007, 19, 4613–4627. [Google Scholar] [CrossRef]
- Jung, G.; Tsai, C. Plasticity-based distortion analysis for fillet welded thin-plate T-joints. Weld. J. 2004, 83, 177–187. [Google Scholar]
- Davis, J.R. Corrosion of Aluminum and Aluminum Alloys; ASM International: Cleveland, OH, USA, 1999. [Google Scholar]
- Chen, X.L. Corrosion Behavior, Yield Behavior and High Temperature Deformation Behavior of Marine High Magnesium Aluminum Alloy. Master’s Thesis, Central South University, Guangzhou, China, 2009. [Google Scholar]
- Li, S.; Guo, D.; Dong, H.D. Effect of flame rectification on corrosion property of Al-Zn-Mg alloy. Trans. Nonferrous Met. Soc. China 2016, 27, 250–257. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Y.J.; Liu, A.J.; Wei, X.J. Effect of flame straightening on microstructures and properties of welded Joint of aluminum alloy for high-speed train. Trans. Mater. Heat Treat. 2003, 24, 59–61. [Google Scholar]
- Xiong, Z.L. Effect Mechanism of Heat-Straightening Temperature on Microstructure and Properties of Aluminum Alloy Joint in High-Speed Trains. Master’s Thesis, Harbin Institute of Technology, Harbin, China, 2014. [Google Scholar]
- Jiang, L.; Wei, X.J.; Yao, G.C.; Wang, D.Q. Effect of Flame Heating Temperature on Properties of Welded Joint of 6005A Aluminium Alloy. J. Northeast. Univ. 2003, 24, 365–368. [Google Scholar]
- Cao, Y.; Li, H.Y.; Liang, Z.M.; Wang, D.L. Effect of Water Cooling on the Microstructure and Mechanical Properties of 6N01 Aluminum Alloy P-MIG-Welded Joints. J. Mater. Eng. Perform. 2017, 26, 3929–3938. [Google Scholar] [CrossRef]
- Xia, S.L.; Ma, M.; Zhang, J.X.; Wang, W.X.; Liu, W.C. Effect of heating rate on the microstructure, texture and tensile properties of continuous cast AA 5083 aluminum alloy. Mater. Sci. Eng. A 2014, 609, 168–176. [Google Scholar] [CrossRef]
- Lacalle, R.; AÁlvarez, J.; Ferreño, D.; Portilla, J.; Ruiz, E.; Arroyo, B.; Gutiérrez-Solana, F. Influence of the flame straightening process on microstructural, mechanical and fracture properties of S235 JR, S460 ML and S690 QL structural steels. Exp. Mech. 2013, 53, 893–909. [Google Scholar] [CrossRef]
- Li, S.; Dong, H.G.; Shi, L.; Li, P.; Ye, F. Corrosion behavior and mechanical properties of Al-Zn-Mg aluminum alloy weld. Corros. Sci. 2017, 123, 243–255. [Google Scholar] [CrossRef]
- Shankar, K.; Wu, W.D. Effect of welding and weld repair on crack propagation behaviour in aluminium alloy 5083 plates. Mater. Des. 2002, 23, 201–208. [Google Scholar] [CrossRef]
- Hamana, D.; Bouchear, M.; Betrouche, M.; Derafa, A.; Rokhmanov, N.Y. Comparative study of formation and transformation of transition phases in Al–12 wt.% Mg alloy. J. Alloys Compd. 2001, 320, 93–102. [Google Scholar] [CrossRef]
- Carroll, M.C. Improvements to the Strength and Corrosion Resistance of Al-Mg-Mn Alloys of Near-AA5083 Chemistry. Ph.D. Thesis, The Ohio State of USA University, Columbus, OH, USA, 2001. [Google Scholar]
- Sheppard, T.; Parson, N.C.; Zaidi, M.A. Dynamic recrystallization in Al-7Mg alloy. Met. Sci. 1983, 17, 481–490. [Google Scholar] [CrossRef]
- Takeda, M.; Ohkubo, F.; Shirai, T.; Fukui, K. Stability of Metastable Phases and Microstructures in the Ageing Process of Al-Mg-Si Ternary Alloys. Mater. Sci. 1998, 33, 2385–2390. [Google Scholar] [CrossRef]
- Lu, H.; Shi, L.; Dong, H.G.; Li, S.; Guo, D.; Tao, C. Influence of flame rectification on mechanical properties of Al-Zn-Mg alloy. J. Alloys Compd. 2016, 689, 278–286. [Google Scholar] [CrossRef]
- Guo, D. Influence of Flame Correction of Microstructure and Properties of Al-Zn-Mg Alloy. Master’s Thesis, Dalian University of Technology, Dalian, China, 2015. [Google Scholar]

| Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Al |
|---|---|---|---|---|---|---|---|---|
| 0.4 | 0.1 | 0.1 | 0.4–1.0 | 4.0–4.9 | 4.0–4.9 | 0.25 | 0.15 | 88.2–90.6 |
| Tensile Data State | Tensile Strength (Mpa) | 0.2% Yield Strength (Mpa) | Elongation (%) | |
|---|---|---|---|---|
| Base Metal | 1 | 338.7 | 293.4 | 13.9 |
| 2 | 339.5 | 295.0 | 12.0 | |
| 3 | 334.8 | 290.3 | 12.1 | |
| Average | 337.7 | 292.9 | 12.7 | |
| Standard deviation | 2.0 | 2.0 | 0.9 | |
| Synchronous Water-Cooling | 1 | 340.7 | 232.0 | 17.4 |
| 2 | 338.0 | 231.3 | 17.4 | |
| 3 | 335.9 | 232.8 | 17.7 | |
| Average | 338.2 | 232.0 | 17.5 | |
| Standard deviation | 2.0 | 0.6 | 0.1 | |
| Flame Straightening | 1 | 337.2 | 219.3 | 20.7 |
| 2 | 334.1 | 224.4 | 17.3 | |
| 3 | 338.3 | 225.0 | 20.3 | |
| Average | 336.5 | 222.9 | 19.4 | |
| Standard deviation | 1.8 | 2.6 | 1.5 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, C.; Wang, X.; Liang, Z.; Rao, Y.; Wang, H.; Yan, D. Effects of Water-Cooling on the Mechanical Properties and Microstructure of 5083 Aluminum Alloy during Flame Straightening. Metals 2018, 8, 692. https://doi.org/10.3390/met8090692
Cai C, Wang X, Liang Z, Rao Y, Wang H, Yan D. Effects of Water-Cooling on the Mechanical Properties and Microstructure of 5083 Aluminum Alloy during Flame Straightening. Metals. 2018; 8(9):692. https://doi.org/10.3390/met8090692
Chicago/Turabian StyleCai, Congwei, Xue Wang, Zhimin Liang, Yuzhong Rao, Hongbo Wang, and Dejun Yan. 2018. "Effects of Water-Cooling on the Mechanical Properties and Microstructure of 5083 Aluminum Alloy during Flame Straightening" Metals 8, no. 9: 692. https://doi.org/10.3390/met8090692




