Recovery of Valuable Metals from Lithium-Ion Batteries NMC Cathode Waste Materials by Hydrometallurgical Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Leaching
2.3. Solvent Extraction
2.4. Stripping Process
2.5. Selective Precipitation and Chemical Precipitation
3. Results and Discussion
3.1. Leaching Process
3.1.1. Effect of Acid Concentration and H2O2 Concentration
3.1.2. Effect of Liquid-Solid Mass Ratio
3.1.3. Effect of Reaction Time and Temperature
3.2. Solvent Extraction with Na-Cyanex 272
3.2.1. Effect of Equilibrium pH Value
3.2.2. Effect of Na-Cyanex 272 Concentration
3.2.3. Effect of Organic-aqueous Ratio
3.2.4. Effect of Extraction Time
3.2.5. Stripping of Co and Mn from the Organic Phase by Sulfuric Acid
3.3. Solvent Extraction with Na-D2EHPA
3.3.1. Effect of Equilibrium pH Value
3.3.2. Effect of Na-D2EHPA Concentration
3.3.3. Effect of Organic-Aqueous Ratio and Extraction Time
3.3.4. Stripping of Mn from the Organic Phase by Sulfuric Acid
3.4. Selective Precipitation with DMG
3.5. Chemical Precipitation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Gaustad, G.; Babbit, C.W.; Richa, K. Economies of scale for future lithium-ion battery recycling infrastructure. Resour. Conserv. Recycl. 2014, 83, 53–62. [Google Scholar] [CrossRef]
- Yamaji, Y.; Dodbiba, G.; Matsuo, S.; Okaya, K.; Shibayama, A.; Fujita, T. A Novel Flow Sheet for Processing of Used Lithium-ion Batteries for Recycling. Resour. Process 2011, 58, 9–11. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumari, A.; Jha, A.K.; Kumar, V.; Hait, J.; Pandey, B.D. Recovery of lithium and cobalt from waste lithium ion batteries of mobile phone. Waste Manag. 2013, 33, 1890–1897. [Google Scholar] [CrossRef] [PubMed]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Zeng, X.L.; Li, J.H.; Singh, N. Recycling of spent lithium-ion battery: A critical review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1129–1165. [Google Scholar] [CrossRef]
- Bernardes, A.M.; Espinosa, D.C.R.; Tenório, J.A.S. Recycling of batteries: A review of current processes and technologies. J. Power Sources 2004, 130, 288–293. [Google Scholar] [CrossRef]
- Shin, S.M.; Kim, N.H.; Sohn, J.S.; Yang, D.H.; Kim, Y.H. Development of a metal recovery process from Li-ion battery wastes. Hydrometallurgy 2005, 79, 172–181. [Google Scholar] [CrossRef]
- Zhao, J.M.; Shen, X.Y.; Deng, F.L.; Wang, F.C.; Wu, Y.; Liu, H.Z. Synergistic extraction and separation of valuable metals from waste cathodic material of lithium ion batteries using Cyanex272 and PC-88A. Sep. Purif. Technol. 2011, 78, 345–351. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Pai, K.V.; Santhosh, G.; Manjanna, J. Recovery of cobalt as cobalt oxalate from spent lithium ion batteries by using glycine as leaching agent. J. Environ. Chem. Eng. 2016, 4, 2378–2383. [Google Scholar] [CrossRef]
- Fouad, O.A.; Farghaly, F.I.; Bahgat, M. A novel approach for synthesis of nanocrystalline γ-LiAlO2 from spent lithium-ion batteries. J. Anal. Appl. Pyrolysis 2007, 78, 65–69. [Google Scholar] [CrossRef]
- Kang, J.G.; Senanayake, G.; Sohn, J.S.; Shin, S.M. Recovery of cobalt sulfate from spent lithium ion batteries by reductive leaching and solvent extraction with Cyanex 272. Hydrometallurgy 2010, 100, 168–171. [Google Scholar] [CrossRef]
- Paulino, J.F.; Busnardo, N.G.; Afonso, J.C. Recovery of valuable elements from spent Li-batteries. J. Hazard. Mater. 2008, 150, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: A comprehensive review. Hydrometallurgy 2014, 150, 192–208. [Google Scholar] [CrossRef]
- Xu, J.; Thomas, H.R.; Francis, R.W.; Lum, K.R.; Wang, J.; Liang, B. A review of processes and technologies for the recycling of lithium-ion secondary batteries. J. Power Sources 2008, 177, 512–527. [Google Scholar] [CrossRef]
- Diekmanna, J.; Hanisch, C.; Froböse, L.; Schälicke, G.; Loellhoeffel, T.; Fölster, A.-S.; Kwade, A. Ecological Recycling of Lithium-Ion Batteries from Electric Vehicles with Focus on Mechanical Processes. J. Electrochem. Soc. 2017, 164, A6184–A6191. [Google Scholar] [CrossRef]
- Pagnanelli, F.; Moscardini, E.; Altimari, P.; Atia, T.A.; Toro, L. Leaching of electrodic powders from lithium ion batteries: Optimization of operating conditions and effect of physical pretreatment for waste fraction retrieval. Waste Manag. 2017, 60, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Barik, S.P.; Prabaharan, G.; Kumar, B. An innovative approach to recover the metal values from spent lithium-ion batteries. Waste Manag. 2016, 51, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xue, Q.; Li, L.; Fan, E.; Wu, F.; Chen, R. Sustainable Recycling and Regeneration of Cathode Scraps from Industrial Production of Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2016, 4, 7041–7049. [Google Scholar] [CrossRef]
- Nayl, A.A.; Elkhashab, R.A.; Badawy, S.M.; El-Khateeb, M.A. Acid leaching of mixed spent Li-ion batteries. Arab. J. Chem. 2014, 10, 3632S–S3639. [Google Scholar] [CrossRef]
- Takacova, Z.; Havlik, T.; Kukurugya, F.; Orac, D. Cobalt and lithium recovery from active mass of spent Li-ion batteries: Theoretical and experimental approach. Hydrometallurgy 2016, 163, 9–17. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R.; Deveci, H. Acid baking of spent lithium ion batteries for selective recovery of major metals: A two-step process. J. Ind. Eng. Chem. 2016, 43, 117–126. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, Y.; Cao, H.; Nawaz, F.; Zhang, Y. A novel process for recycling and resynthesizing LiNi1/3Co1/3Mn1/3O2 from the cathode scraps intended for lithium-ion batteries. Waste Manag. 2014, 34, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Pant, D.; Dolker, T. Green and facile method for the recovery of spent Lithium Nickel Manganese Cobalt Oxide (NMC) based Lithium ion batteries. Waste Manag. 2017, 60, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Nayl, A.A.; Hamed, M.M.; Rizk, S.E. Selective extraction and separation of metal values from leach liquor of mixed spent Li-ion batteries. J. Taiwan Inst. Chem. Eng. 2015, 55, 119–125. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Lee, J.C.; Jeong, J.; Kim, B.S.; Pandey, B.D. Selective recovery of cobalt, nickel and lithium from sulfate leachate of cathode scrap of Li-ion batteries using liquid-liquid extraction. Met. Mater. Int. 2014, 20, 357–365. [Google Scholar] [CrossRef]
- Chiu, K.L.; Chen, W.S. Recovery and Separation of Valuable Metals from Cathode Materials of Spent Lithium-Ion Batteries (LIBs) by Ion Exchange. Sci. Adv. Mater. 2017, 9, 2155–2160. [Google Scholar] [CrossRef]
- Song, D.; Wang, X.; Zhou, E.; Hou, P.Y.; Guo, F.X.; Zhang, L.Q. Recovery and heat treatment of the Li(Ni1/3Co1/3Mn1/3)O2 cathode scrap material for lithium ion battery. J. Power Sources 2013, 232, 348–352. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Hydrometallurgical processing of spent lithium ion batteries (LIBs) in the presence of a reducing agent with emphasis on kinetics of leaching. Chem. Eng. J. 2015, 281, 418–427. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, H.B.; Xie, Y.B.; Ning, P.G.; An, H.J.; You, H.X.; Nawaz, F. A closed-loop process for recycling LiNi1/3Co1/3Mn1/3O2 from the cathode scraps of lithium-ion batteries: Process optimization and kinetics analysis. Sep. Purif. Technol. 2015, 150, 186–195. [Google Scholar] [CrossRef]
- He, L.P.; Sun, S.Y.; Song, X.F.; Yu, J.G. Leaching process for recovering valuable metals from the LiNi1/3Co1/3Mn1/3O2 cathode of lithium-ion batteries. Waste Manag. 2017, 64, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gaustad, G.; Babbitt, C.W.; Bailey, C.; Ganter, M.J.; Landi, B.J. Economic and environmental characterization of an evolving Li-ion battery waste stream. J. Environ. Manag. 2014, 135, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.A.; Prados, L.M.Z.; Majuste, D.; Mansur, M.B. Hydrometallurgical separation of aluminium, cobalt, copper and lithium from spent Li-ion batteries. J. Power Sources 2009, 187, 238–246. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Recovery of valuable metals from cathodic active material of spent lithium ion batteries: Leaching and kinetic aspects. Waste Manag. 2015, 45, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Sun, R.; Xu, J.; Chen, Z.; Kang, M. Recovery of cobalt from spent lithium ion batteries using sulphuric acid leaching followed by solid-liquid separation and solvent extraction. RSC Adv. 2016, 88, 85303–85313. [Google Scholar] [CrossRef]
- Hung, S.H.; Lin, C.F.; Chiang, P.C.; Tsai, T.H.; Peng, C.Y. Recovery of metal ions from spent Lithium Ion Batteries (LIBs) using sodium salts of D2EHPA or P507: Performance evaluation and life cycle assessment. Res. J. Chem. Environ. 2014, 18, 39–47. [Google Scholar] [CrossRef]
- Swain, B.; Mishra, C.; Jeong, J.; Lee, J.C.; Hong, H.S.; Pandey, B.D. Separation of Co(II) and Li(I) with Cyanex 272 using hollow fiber supported liquid membrane: A comparison with flat sheet supported liquid membrane and dispersive solvent extraction process. Chem. Eng. J. 2015, 271, 61–70. [Google Scholar] [CrossRef]
- Mantuano, D.P.; Dorella, G.; Elias, R.C.A.; Mansur, M.B. Analysis of a hydrometallurgical route to recover base metals from spent rechargeable batteries by liquid-liquid extraction with Cyanex 272. J. Power Sources 2006, 159, 1510–1518. [Google Scholar] [CrossRef]
- Chen, X.P.; Xu, B.; Zhou, T.; Liu, D.; Hu, H.; Fan, S.Y. Separation and recovery of metal values from leaching liquor of mixed-type of spent lithium-ion batteries. Sep. Purif. Technol. 2015, 144, 197–205. [Google Scholar] [CrossRef]
- Wang, R.C.; Lin, Y.C.; Wu, S.H. A novel recovery process of metal values from the cathode active materials of the lithium-ion secondary batteries. Hydrometallurgy 2009, 99, 194–201. [Google Scholar] [CrossRef]
- Chen, X.P.; Zhou, T.; Kong, J.R.; Fang, H.X.; Chen, Y.B. Separation and recovery of metal values from leach liquor of waste lithium nickel cobalt manganese oxide based cathodes. Sep. Purif. Technol. 2015, 141, 76–83. [Google Scholar] [CrossRef]
- Joo, S.H.; Shin, D.J.; Oh, C.H.; Wang, J.P.; Park, J.T.; Shin, S.M. Application of Co and Mn for a Co-Mn-Br or Co-Mn-C2H3O2 Petroleum Liquid Catalyst from the Cathode Material of Spent Lithium Ion Batteries by Hydrometallurgical Route. Metals 2017, 7, 439. [Google Scholar] [CrossRef]
- Devi, N.B.; Nathsarma, K.C.; Chakravortty, V. Separation and recovery of cobalt (II) and nickel (II) from sulphate solutions using sodium salts of D2EHPA, PC 88A and Cyanex 272. Hydrometallurgy 1998, 49, 47–61. [Google Scholar] [CrossRef]
- Mohapatra, D.; Kim, H.I.; Nam, C.W.; Park, K.H. Liquid-liquid extraction of aluminium(III) from mixed sulphate solutions using sodium salts of Cyanex 272 and D2EHPA. Sep. Purif. Technol. 2007, 56, 311–318. [Google Scholar] [CrossRef]
- Sarangi, K.; Reddy, B.R.; Das, R.P. Extraction studies of cobalt(II) and nickel(II) from chloride solutions using Na-Cyanex 272. Separation of Co(II)/Ni(II) by the sodium salts of D2EHPA, PC88A and Cyanex 272 and their mixtures. Hydrometallurgy 1999, 52, 253–265. [Google Scholar] [CrossRef]
- Park, K.H.; Kim, H.I.; Parhi, P.K.; Mishra, D.; Nam, C.W.; Park, J.T.; Kim, D.J. Extraction of metals from Mo-Ni/Al2O3 spent catalyst using H2SO4 baking-leaching-solvent extraction technique. J. Ind. Eng. Chem. 2012, 18, 2036–2045. [Google Scholar] [CrossRef]
- Mubarok, M.Z.; Hanif, L.I. Cobalt and Nickel Separation in Nitric Acid Solution by Solvent Extraction Using Cyanex 272 and Versatic 10. Procedia Chem. 2016, 19, 743–750. [Google Scholar] [CrossRef]
- Flett, D.S. Cobalt-Nickel Separation in Hydrometallurgy: A Review. Chem. Sustain. Dev. 2004, 12, 81–91. [Google Scholar]
- Lewis, A.; Van Hille, R. An exploration into the sulphide precipitation method and its effect on metal sulphide removal. Hydrometallurgy 2006, 81, 197–204. [Google Scholar] [CrossRef]
- Hammack, R.W.; Edenborn, H.M. The removal of nickel from mine waters using bacterial sulfate reduction. Appl. Microbiol. Biotechnol. 1992, 37, 674–678. [Google Scholar] [CrossRef]
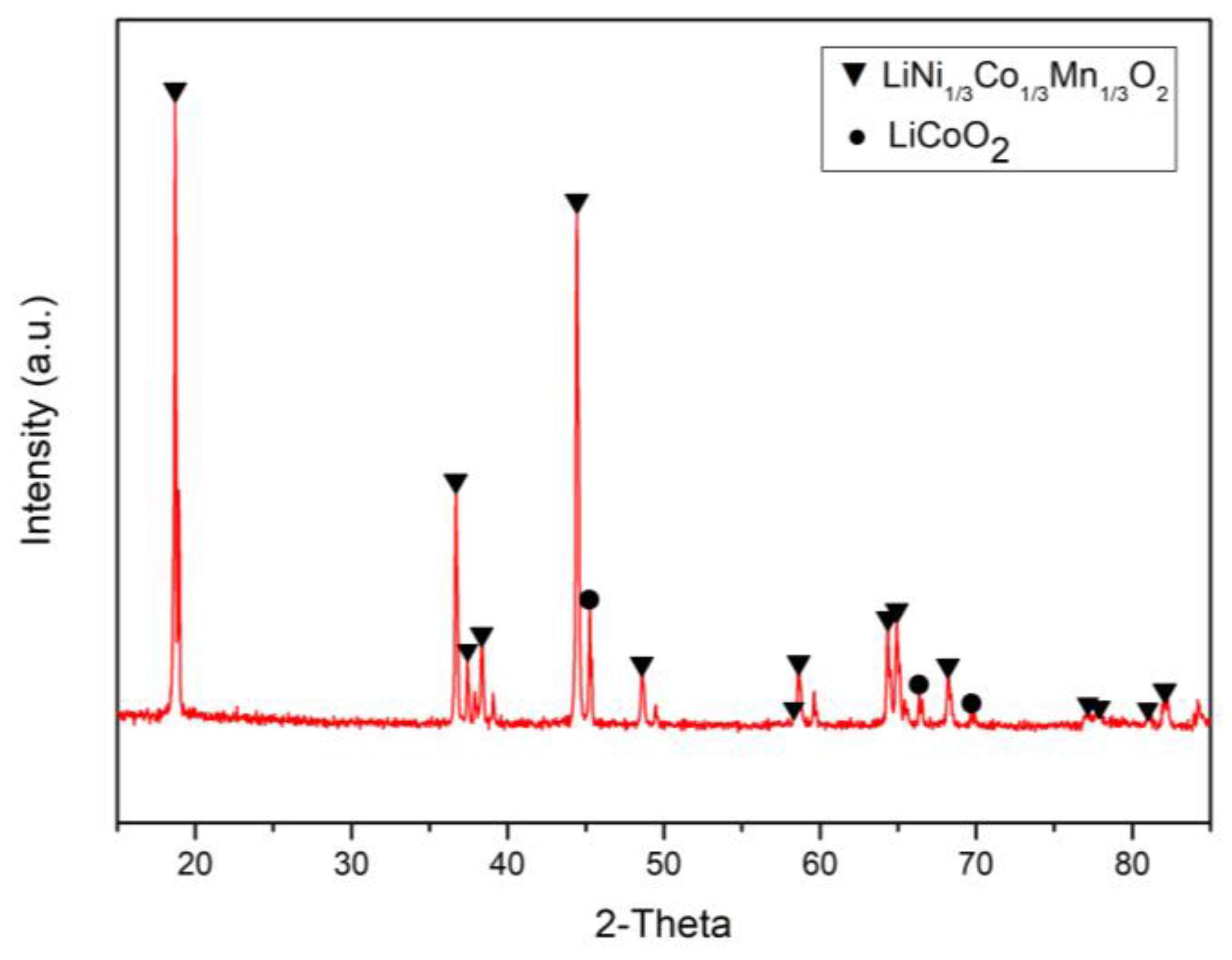









| [H2SO4] | [H2O2] | Liquid-Solid Ratio | Temperature | Leaching Time | |
|---|---|---|---|---|---|
| NMC battery cathode material | 2.0 mol/L | 10.0% | 30 mL/g | 70 °C | 90 min |
| Equilibrium pH Value | Concentration (M) | Organic-Aqueous Ratio | Extraction Time (min) | |
|---|---|---|---|---|
| Na-Cyanex 272 | 6.0 | 0.1 | 1.5 | 15 |
| Na-D2EHPA | 2.95 | 0.2 | 1.0 | 5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-S.; Ho, H.-J. Recovery of Valuable Metals from Lithium-Ion Batteries NMC Cathode Waste Materials by Hydrometallurgical Methods. Metals 2018, 8, 321. https://doi.org/10.3390/met8050321
Chen W-S, Ho H-J. Recovery of Valuable Metals from Lithium-Ion Batteries NMC Cathode Waste Materials by Hydrometallurgical Methods. Metals. 2018; 8(5):321. https://doi.org/10.3390/met8050321
Chicago/Turabian StyleChen, Wei-Sheng, and Hsing-Jung Ho. 2018. "Recovery of Valuable Metals from Lithium-Ion Batteries NMC Cathode Waste Materials by Hydrometallurgical Methods" Metals 8, no. 5: 321. https://doi.org/10.3390/met8050321





