High Hardness Nanocrystalline Invar Alloys Prepared from Fe-Ni Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
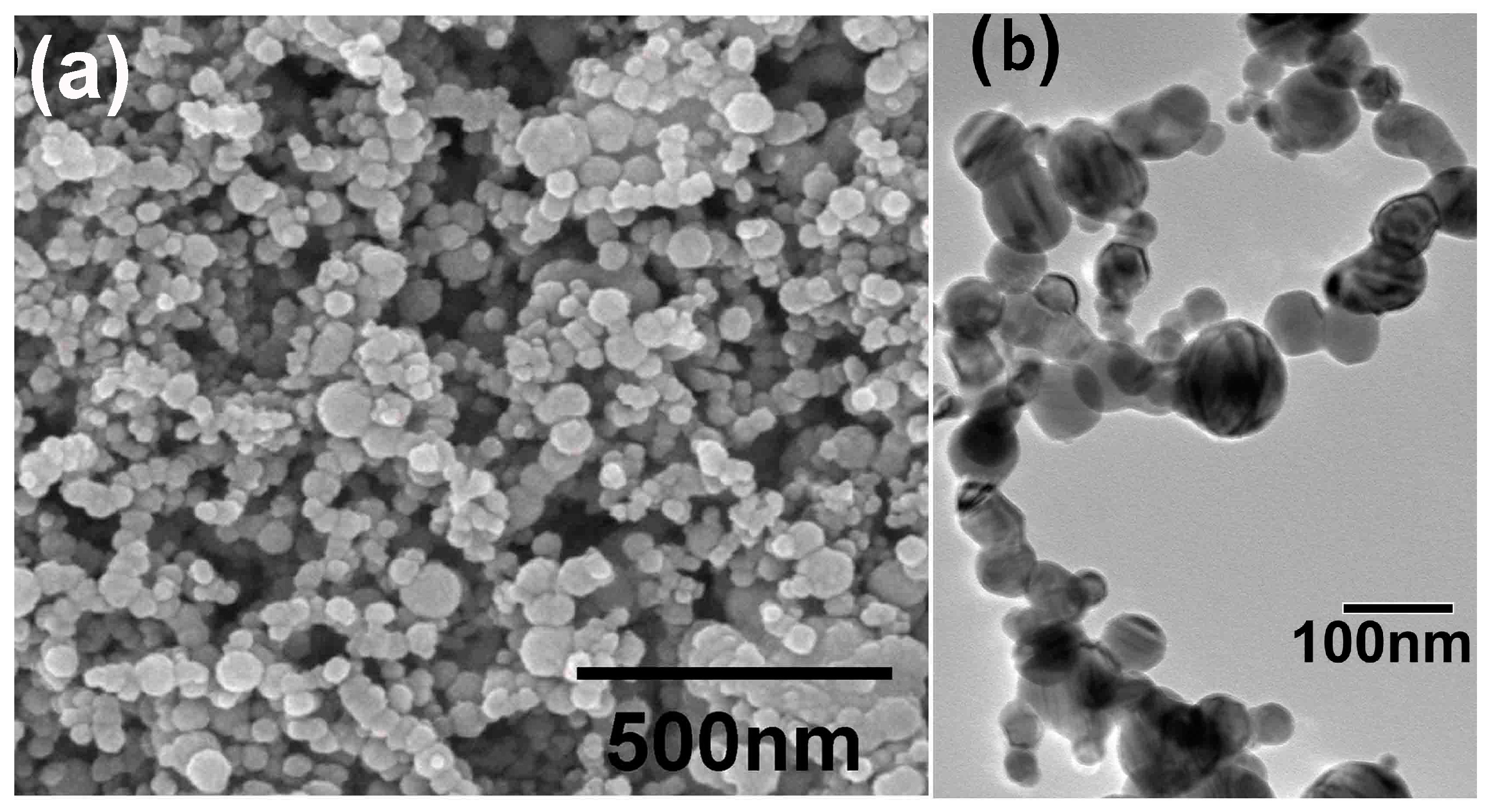
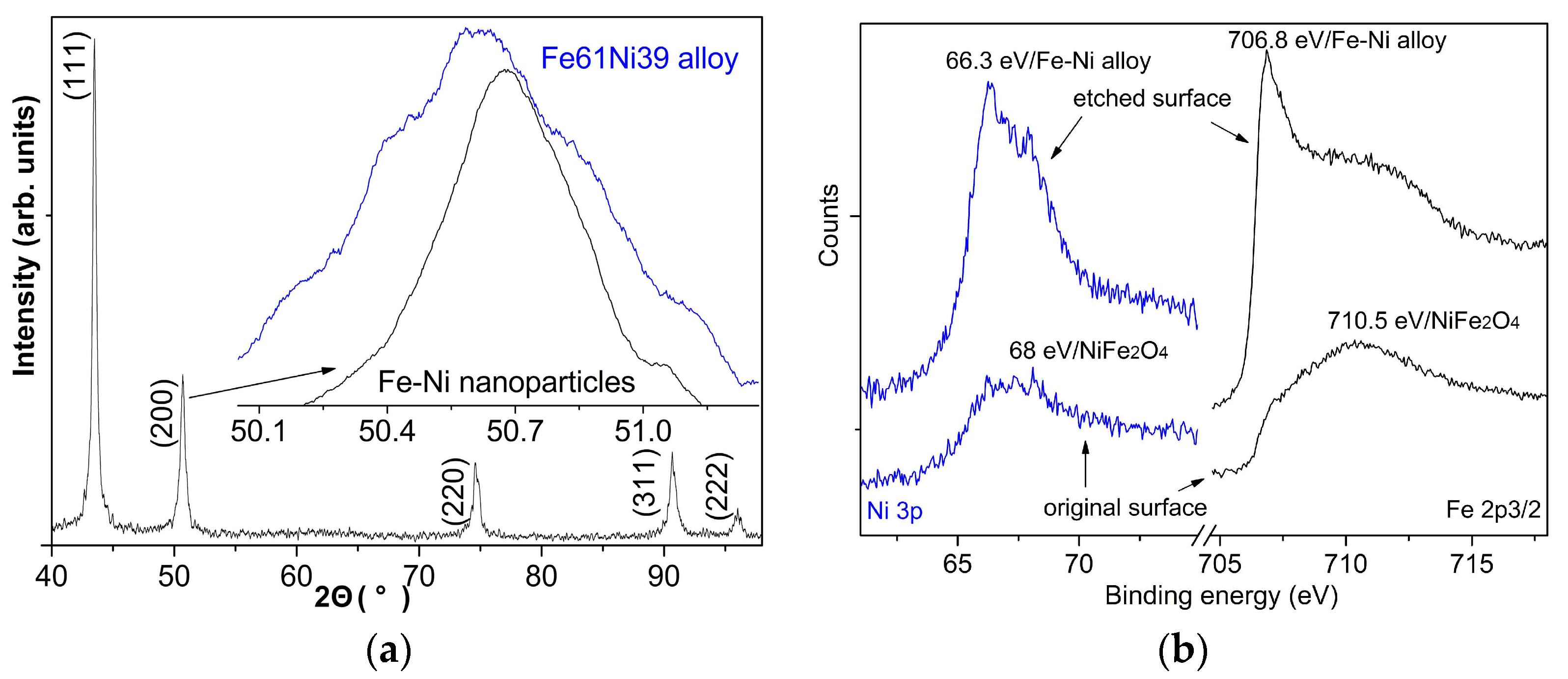
3.1. Charaterization of the Precusor Fe-Ni Nanoparticles
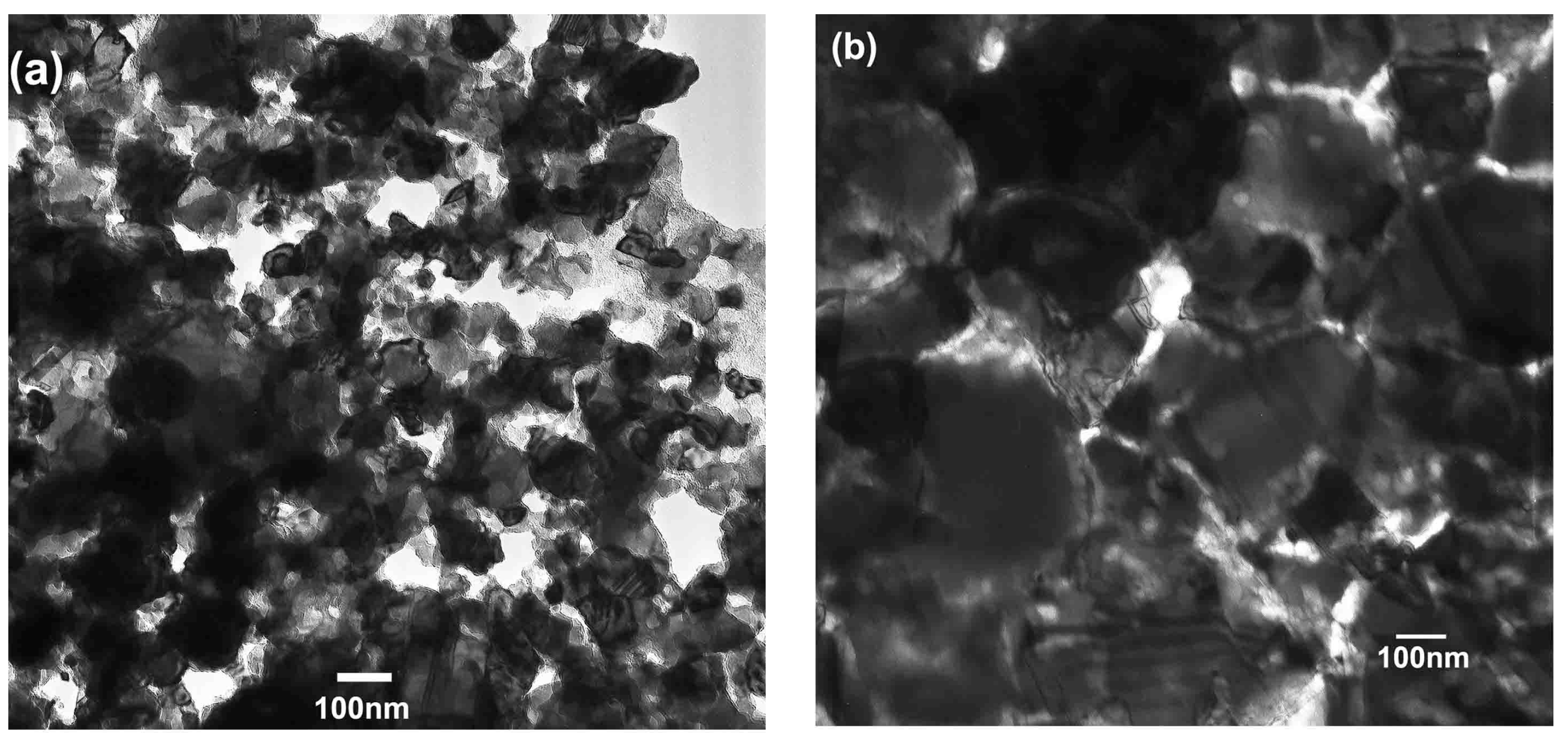
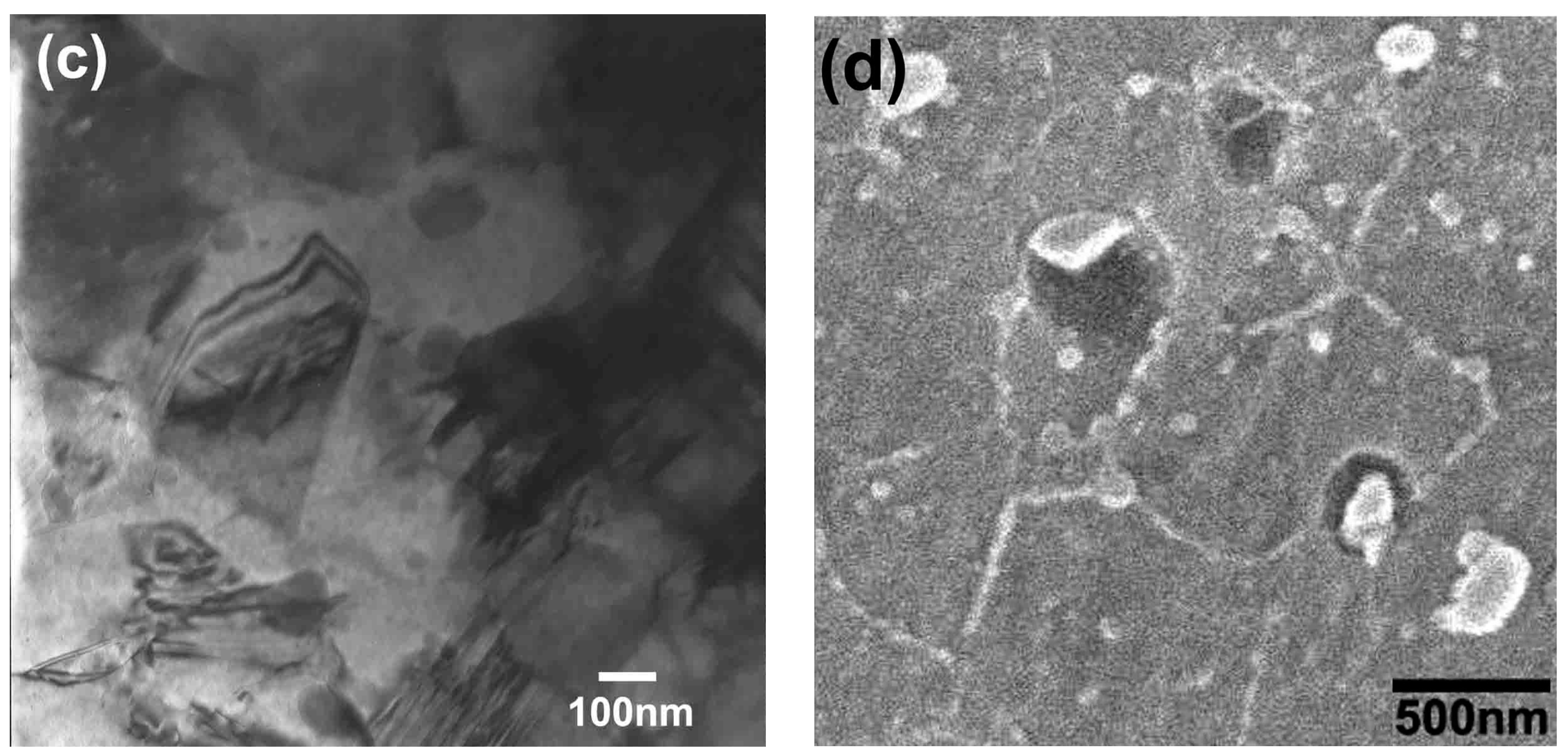
3.2. Characterization of the Nanocrystalline Invar Alloys
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guillaume, C.E. Recherches sur les aciers au nickel. Dilatations aux temperatures elevees; resistance electrique. CR Acad. Sci. 1897, 125, 235–238. [Google Scholar]
- Qiu, C.; Nicholas, J.E.A.; Attallah, M.M. Selective laser melting of Invar 36: Microstructure and properties. Acta Mater. 2016, 103, 382–395. [Google Scholar] [CrossRef]
- Schilfgaarde, M.; Abrikosov, I.A.; Johansson, B. Origin of the Invar effect in iron-nickle alloys. Nature 1999, 400, 46–49. [Google Scholar] [CrossRef]
- Bobbio, L.D.; Otis, R.A.; Borgonia, J.P.; Dillon, R.P.; Shapiro, A.A.; Liu, Z.K.; Beese, A.M. Additive manufacturing of a functionally graded material from Ti-6Al-4V to Invar: Experimental characterization and thermodynamic calculations. Acta Mater. 2017, 127, 133–142. [Google Scholar] [CrossRef]
- Li, H.; Ebrahimi, F. Ductile-to-brittle transition in nanocrystalline metals. Adv. Mater. 2005, 17, 1969–1972. [Google Scholar] [CrossRef]
- Nadutov, V.M.; Ustinov, A.I.; Demchenkov, S.A.; Svystunov, Y.O.; Skorodzievski, V.S. Structure and properties of nanostructured vacuum-deposited foils of Invar Fe-(35–38 wt %)Ni alloys. J. Mater. Sci. Technol. 2015, 31, 1079–1086. [Google Scholar] [CrossRef]
- Park, H.K.; Hwang, N.M.; Park, Y.B. Abnormal grain growth in the nanostructured Invar alloy fabricated by electrodeposition. Philos. Mag. Lett. 2012, 92, 589–596. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Shen, B.; Hu, W. A study of thermal stability in electrodeposited nanocrystalline Fe-Ni Invar alloy. Mater. Sci. Eng. A 2011, 18, 5701–5705. [Google Scholar] [CrossRef]
- Tjong, S.C.; Chen, H. Nanocrystalline materials and coatings. Mater. Sci. Eng. R Rep. 2004, 45, 1–88. [Google Scholar] [CrossRef]
- Wu, X.J.; Du, L.G.; Zhang, H.F.; Liu, J.F.; Zhou, Y.S.; Li, Z.Q.; Xiong, L.Y.; Bai, Y.L. Synthesis and tensile property of nanocrystalline metal copper. Nanostruct. Mater. 1999, 12, 221–224. [Google Scholar] [CrossRef]
- Haghighi, S.E.; Janghorban, K.; Izadi, S. Structural evolution of Fe-50 at. % Al powders during mechanical alloying and subsequent annealing processes. J. Alloys Compd. 2010, 495, 260–264. [Google Scholar] [CrossRef]
- Haghighi, S.E.; Janghorban, K.; Izadi, S. Order-sintering of mechanically alloyed FeAl nanostructrues. J. Alloys Compd. 2010, 503, 375–379. [Google Scholar] [CrossRef]
- Peng, H.R.; Gong, M.M.; Chen, Y.Z.; Liu, F. Thermal stability of nanocrystalline materials: Thermal dynamics and kinetics. Int. Mater. Rev. 2017, 62, 303–333. [Google Scholar] [CrossRef]
- Si, P.Z.; Jiang, W.; Wang, H.X.; Li, Z.F.; Liu, J.J.; Lee, J.G.; Choi, C.J. Large scale synthesis of nitrogen doped TiO2 nanoparticles by reactive plasma. Mater. Lett. 2012, 68, 161–163. [Google Scholar] [CrossRef]
- Si, P.Z.; Skorvanek, I.; Kovac, J.; Geng, D.Y.; Zhao, X.G.; Zhang, Z.D. Structural and magnetic properties of Gd nanoparticles and carbon coated Gd/GdC2 nanocapsules. J. Appl. Phys. 2003, 94, 6779–6784. [Google Scholar] [CrossRef]
- Duhamel, C.; Champion, Y.; Tence, M.; Walls, M. Synthesis of controlled-chemistry ultrafine FexNi1−x ferromagnetic powders. J. Alloys Compd. 2005, 393, 204–210. [Google Scholar] [CrossRef]
- Kurlyandskaya, G.V.; Bhagat, S.M.; Bagazeev, A.V.; Medvedev, A.I.; Ballesteros, A.; Beketov, I.V.; Safronov, A.P. Structure, magnetic and microwave properties of FeNi Invar nanoparticles obtained by electrical explosion of wire in different preparation conditions. J. Phys. Chem. Solids 2016, 98, 255–262. [Google Scholar] [CrossRef]
- Tadaki, T.; Murai, Y.; Koreeda, A.; Nakata, Y.; Hirotsu, Y. Structure and phase transformation of nano-scale particles of Fe-Ni alloys. Mater. Sci. Eng. A 1996, 217–218, 235–238. [Google Scholar] [CrossRef]
- Li, X.G.; Chiba, A.; Takahashi, S. Preparation and magnetic properties of ultrafine particles of Fe-Ni alloys. J. Magn. Magn. Mater. 2005, 170, 339–345. [Google Scholar] [CrossRef]
- Asaka, K.; Hirotsu, Y.; Tadaki, T. Martensitic transformation in nanometer-sized particles of Fe-Ni alloys. Mater. Sci. Eng. A 1999, 273–275, 262–265. [Google Scholar] [CrossRef]
- Mclntyre, N.S.; Zetaruk, D.G. X-ray photoelectron spectroscopic studies of iron oxides. Anal. Chem. 1977, 49, 1521–1529. [Google Scholar] [CrossRef]
- Mclntyre, N.S.; Cook, M.G. X-ray photoelectron studies on some oxides and hydroxides of cobalt, nickel, and copper. Anal. Chem. 1975, 47, 2208–2213. [Google Scholar] [CrossRef]

| Pressure\Temperature | 600 °C | 700 °C | 800 °C | 900 °C |
|---|---|---|---|---|
| 172 MPa | 90.36% (183 ± 3.1) | 98.11% (222.8 ± 2.9) | 99.19% (200 ± 3.5) | 97.71% (180.5 ± 2.8) |
| 682 MPa | 97.74% (214.9 ± 2.5) | 98.01% (227.7 ± 3.0) | 99.32% (214.7 ± 3.2) | 99.37% (194.4 ± 2.7) |
| 1194 MPa | 98.78% (242.2 ± 2.6) | 98.87% (225.2 ± 2.8) | 98.88% (207.7 ± 3.3) | 99.6% (190.8 ± 2.9) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, P.-Z.; Choi, C.-J. High Hardness Nanocrystalline Invar Alloys Prepared from Fe-Ni Nanoparticles. Metals 2018, 8, 28. https://doi.org/10.3390/met8010028
Si P-Z, Choi C-J. High Hardness Nanocrystalline Invar Alloys Prepared from Fe-Ni Nanoparticles. Metals. 2018; 8(1):28. https://doi.org/10.3390/met8010028
Chicago/Turabian StyleSi, Ping-Zhan, and Chul-Jin Choi. 2018. "High Hardness Nanocrystalline Invar Alloys Prepared from Fe-Ni Nanoparticles" Metals 8, no. 1: 28. https://doi.org/10.3390/met8010028




