The Effect of Ultrafast Heating on Cold-Rolled Low Carbon Steel: Formation and Decomposition of Austenite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Steel and Heat Treatment
2.2. Characterization
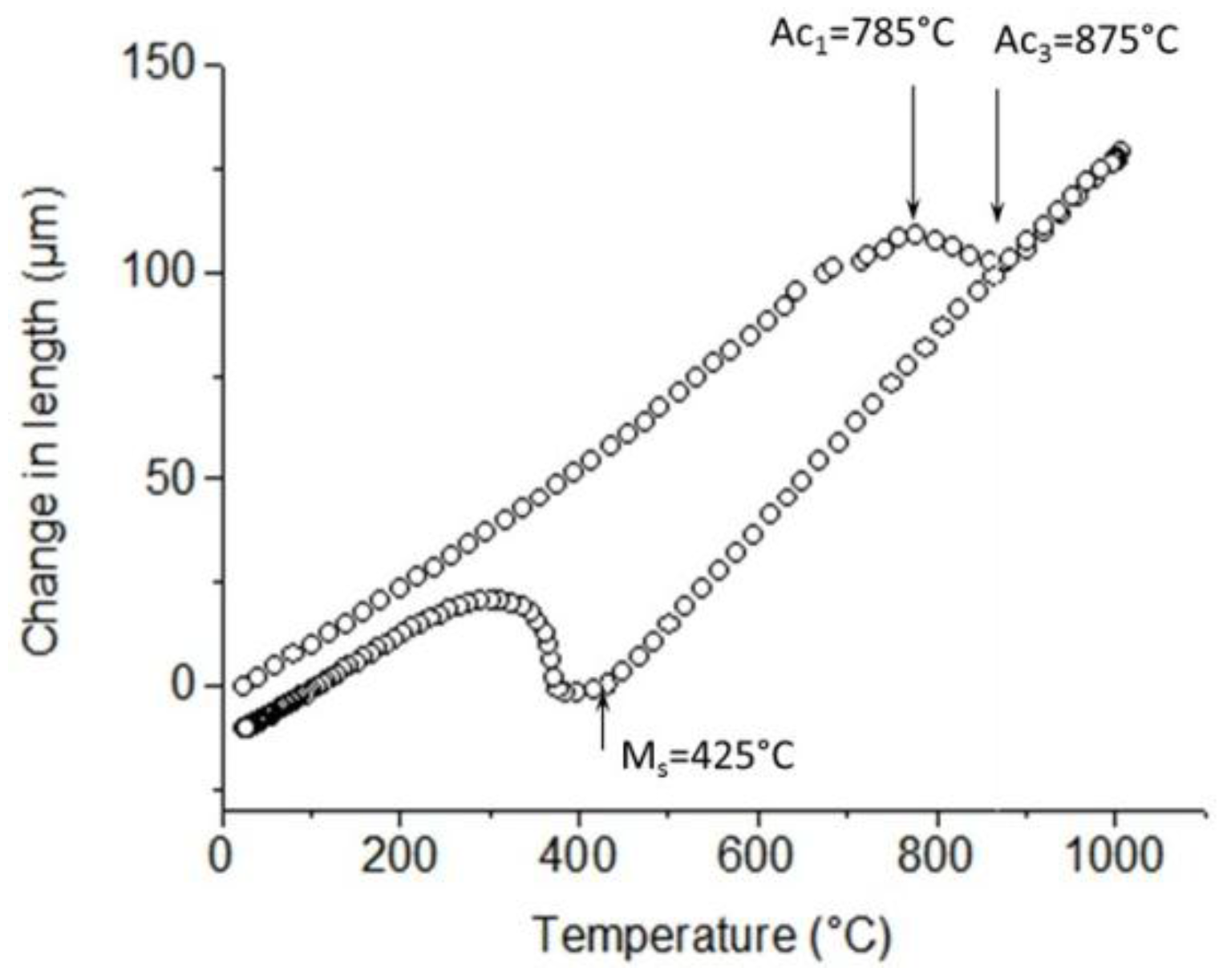
2.3. Data Post-Processing and Analysis
3. Results
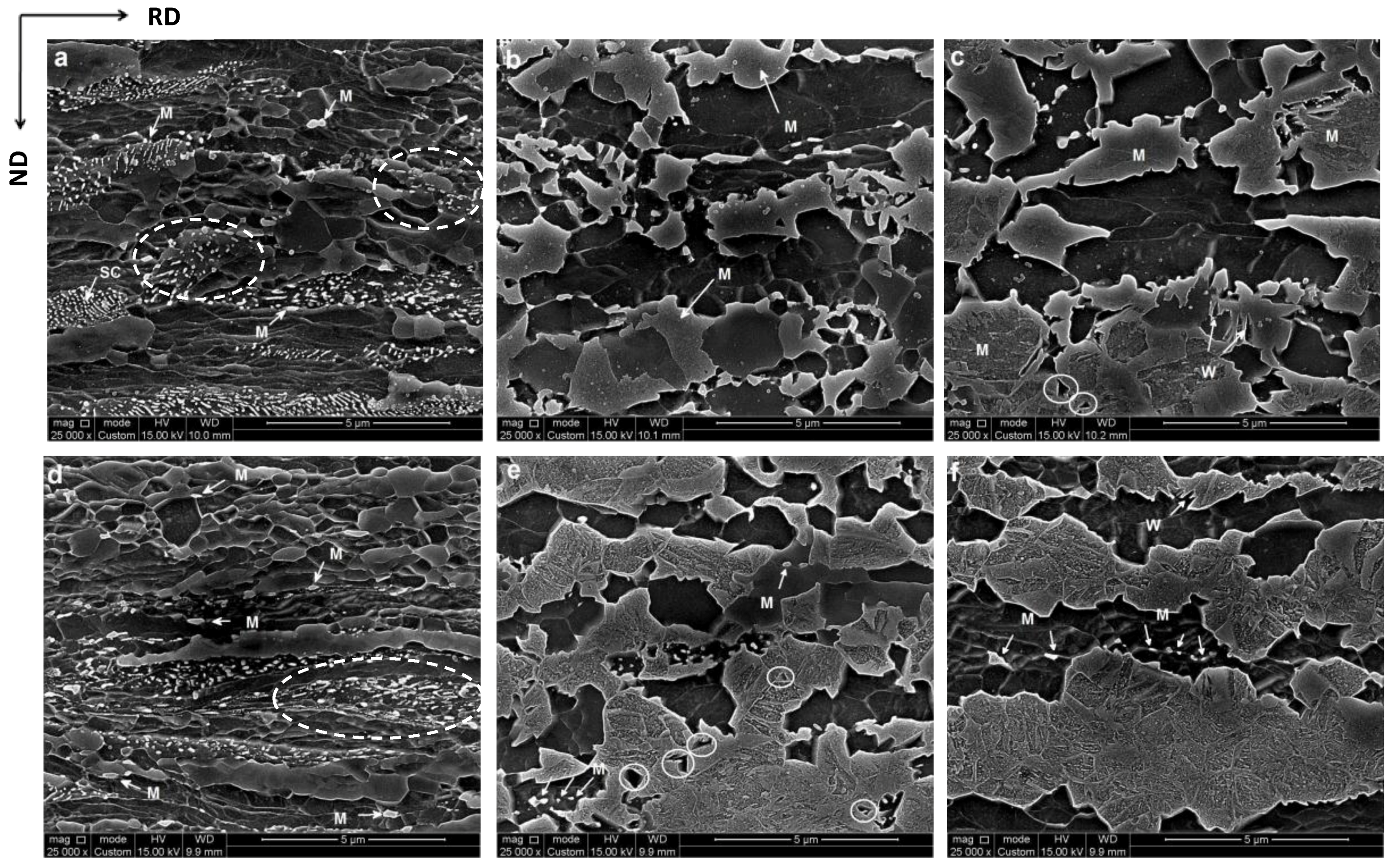
3.1. Microstructure after Cold-Rolling and Ultrafast Heating
3.2. Phase Quantification
4. Discussion
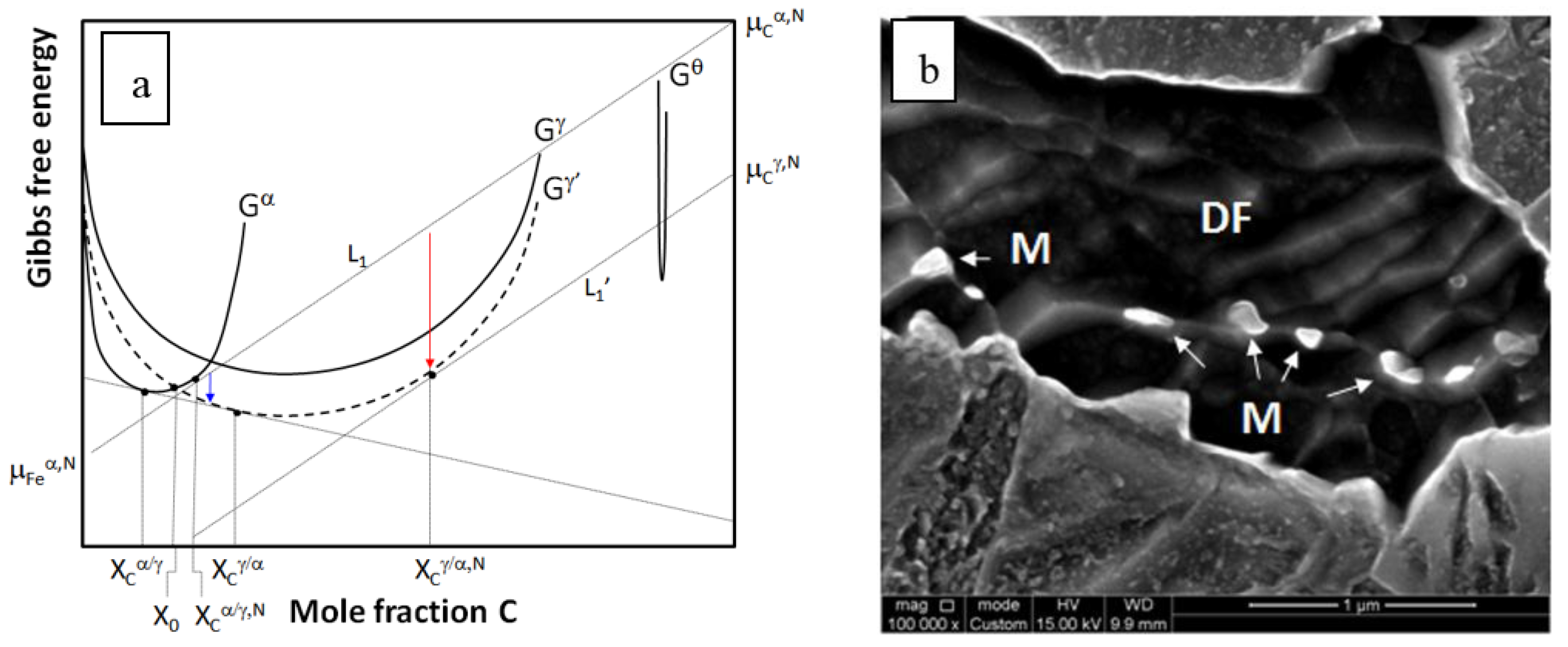
4.1. Formation of Austenite
4.1.1. Nucleation
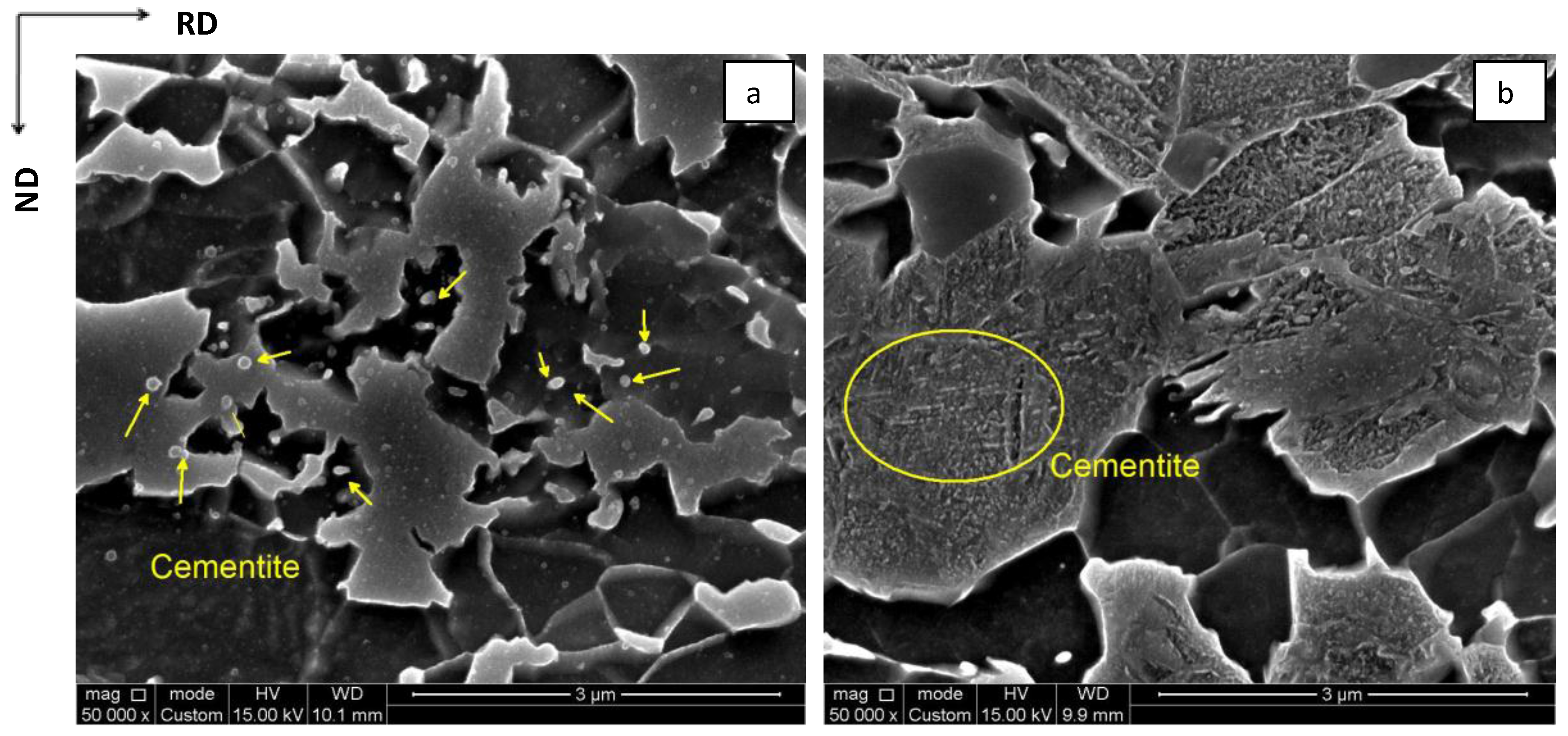
4.1.2. Austenite Growth into Pearlite
4.1.3. Growth into Proeutectoid Ferrite
4.1.4. Massive Formation of Austenite
4.2. Transformation of Proeutectoid Ferrite during Cooling
4.3. Texture Inheritance Effect in Martensite
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lolla, T.; Cola, G.; Narayanan, B.; Alexandrov, B.; Babu, S.S. Development of rapid heating and cooling (flash processing) process to produce advanced high strength steel microstructures. Mater. Sci. Technol. 2011, 27, 863–875. [Google Scholar] [CrossRef]
- Meng, Q.; Li, J.; Zheng, H. High-efficiency fast-heating annealing of a cold-rolled dual-phase steel. Mater. Des. 2014, 58, 194–197. [Google Scholar] [CrossRef]
- De Knijf, D.; Puype, A.; Föjer, C.; Petrov, R. The influence of ultra-fast annealing prior to quenching and partitioning on the microstructure and mechanical properties. Mater. Sci. Eng. A 2015, 627, 182–190. [Google Scholar] [CrossRef]
- Puype, A. Developing of Advanced High Strength Steel via Ultrafast Annealing. Master’s Thesis, Ghent University, Ghent, Belgium, 2013. [Google Scholar]
- Cerda, F.M.C.; Goulas, C.; Sabirov, I.; Papaefthymiou, S.; Monsalve, A.; Petrov, R.H.; Sabirov, I.; Schulz, B. Microstructure, texture and mechanical properties in a low carbon steel after ultrafast heating. Mater. Sci. Eng. A 2016, 672, 17–20. [Google Scholar] [CrossRef]
- Meshkov, Y.Y.; Pereloma, E. V 17—The effect of heating rate on reverse transformations in steels and Fe-Ni-based alloys. In Phase Transformations in Steels; Pereloma, E., Edmonds, D.V., Eds.; Woodhead Publishing Series in Metals and Surface Engineering; Woodhead Publishing: Cambridge, UK, 2012; Volume 1, pp. 581–618. [Google Scholar]
- Christian, J.W. The Theory of Transformations in Metals and Alloys, 3rd ed.; Elsevier Science Ltd.: Oxford, UK, 2002. [Google Scholar]
- Kaluba, W.J.; Taillard, R.; Foct, J. The bainitic mechanism of austenite formation during rapid heating. Acta Mater. 1998, 46, 5917–5927. [Google Scholar] [CrossRef]
- Aaronson, H.I.; Nie, J.F. Discussion to “The bainitic mechanism of austenite formation during rapid heating”. Scr. Mater. 2000, 42, 505–509. [Google Scholar] [CrossRef]
- Hillert, M. Comments on “The bainite mechanism of austenite formation during rapid heating”. Scr. Mater. 2000, 43, 1045–1046. [Google Scholar] [CrossRef]
- Judd, R.R.; Paxton, H.W. Kinetics of austenite formation from a spheroidized ferrite-carbide aggregate. Trans. AIME 1968, 242, 206–215. [Google Scholar]
- Speich, G.R.; Szirmae, A. Formation of austenite from ferrite and ferrite-carbide aggregates. Trans. AIME 1969, 245, 1063–1073. [Google Scholar]
- Hillert, M.; Nilsson, K.; Torndahl, L.-E. Effect of alloying elements on the formation of austenite and dissolution of cementite. J. Iron Steel Inst. 1971, 209, 49–66. [Google Scholar]
- Schmidt, E.; Wang, Y.; Sridhar, S. A study of nonisothermal austenite formation and decomposition in Fe-C-Mn alloys. Metall. Mater. Trans. A 2006, 37, 1799–1810. [Google Scholar] [CrossRef]
- Schmidt, E.D.; Damm, E.B.; Sridhar, S. A study of diffusion- and interface-controlled migration of the Austenite/Ferrite front during Austenitization of a case-hardenable alloy steel. Metall. Mater. Trans. A 2007, 38, 698–715. [Google Scholar] [CrossRef]
- Dykhuizen, R.C.; Robino, C.V.; Knorovsky, G.A. A method for extracting phase change kinetics from dilatation for multistep transformations: Austenitization of a low carbon steel. Metall. Mater. Trans. B 1999, 30, 107–117. [Google Scholar] [CrossRef]
- Savran, V.I.; Offerman, S.E.; Sietsma, J. Austenite nucleation and growth observed on the level of individual grains by three-dimensional X-ray diffraction microscopy. Metall. Mater. Trans. A 2010, 41, 583–591. [Google Scholar] [CrossRef]
- Petrov, R.H.; Kestens, L.A.I. Advanced High-Strength Steels: Electron Backscatter Diffraction (EBSD). In Encyclopedia of Iron, Steel, and Their Alloys; Colás, R., Totten, G.E., Eds.; CRC Press: Boca Ratón, FL, USA, 2016; pp. 46–69. [Google Scholar]
- Pinard, P.T.; Schwedt, A.; Ramazani, A.; Prahl, U.; Richter, S. Characterization of dual-phase steel microstructure by combined submicrometer EBSD and EPMA carbon measurements. Microsc. Microanal. 2013, 19, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Oddy, A.; Mcdill, J.; Karlsson, L. Microstructural predictions including arbitrary thermal histories, reaustenization and carbon segregation effects. Can. Metall. Q. 1996, 35, 275–283. [Google Scholar] [CrossRef]
- Onink, M.; Tichelaar, F.D.; Brakman, C.M.; Mittemeijer, E.J.; van der Zwaag, S. An in situ hot stage transmission electron microscopy study of the decomposition of Fe-C austenites. J. Mater. Sci. 1995, 30, 6223–6234. [Google Scholar] [CrossRef]
- Kop, T.A. Dilatometric study of the Austenite/Ferrite Interface Mobility. Ph.D. Thesis, TU Delft, Delft, The Netherlands, 2000. [Google Scholar]
- Savran, V.I. Austenite Formation in C-Mn Steel. Ph.D. Thesis, TU Delft, Delft, The Netherlands, 2009. [Google Scholar]
- Hillert, M. The uses of free energy-composition diagrams. In Lectures on the Theory of Phase Transformations; Aaronson, H.I., Ed.; Metallurgical Society of AIME: New York, NY, USA, 1975; pp. 1–50. [Google Scholar]
- Mehl, R.F. The mechanism and rate formation of austenite from ferrite-cementite aggregates. Trans. ASM 1943, 31, 613–650. [Google Scholar]
- Zener, C. Kinetics of Decomposition of Austenite. Trans. AIME 1946, 167, 550–583. [Google Scholar]
- Sietsma, J.; van der Zwaag, S. A concise model for mixed-mode phase transformations in the solid state. Acta Mater. 2004, 52, 4143–4152. [Google Scholar] [CrossRef]
- Hutchinson, B.; Kestens, L.A.I. Origins of texture memory in steels. In Applications of Texture Analysis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 281–290. [Google Scholar]
- Dillamore, I.L.; Morris, P.L.; Smith, C.J.E.; Hutchinson, W.B. Transition bands and recrystallization in metals. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1972, 329, 405–420. [Google Scholar] [CrossRef]
- Gobernado, P.; Petrov, R.H.; Kestens, L.A.I. Recrystallized {3 1 1}<1 3 6> orientation in ferrite steels. Scr. Mater. 2012, 66, 623–626. [Google Scholar] [CrossRef]
- Ray, R.K.; Jonas, J.J. Transformation textures in steels. Int. Mater. Rev. 1990, 35, 1–36. [Google Scholar] [CrossRef]



| C | Mn | Si | S | P | Fe |
|---|---|---|---|---|---|
| 0.14 | 2.05 | 1.20 | 0.001 | 0.012 | Rest |
| Heating Rate, °C/s | Peak Temperature, °C | ||||
|---|---|---|---|---|---|
| 150 | 704 | 741 | 782 | 838 | 1000 |
| 1500 | 749 | 842 | 862 | 1000 | 1050 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro Cerda, F.M.; Schulz, B.; Papaefthymiou, S.; Artigas, A.; Monsalve, A.; Petrov, R.H. The Effect of Ultrafast Heating on Cold-Rolled Low Carbon Steel: Formation and Decomposition of Austenite. Metals 2016, 6, 321. https://doi.org/10.3390/met6120321
Castro Cerda FM, Schulz B, Papaefthymiou S, Artigas A, Monsalve A, Petrov RH. The Effect of Ultrafast Heating on Cold-Rolled Low Carbon Steel: Formation and Decomposition of Austenite. Metals. 2016; 6(12):321. https://doi.org/10.3390/met6120321
Chicago/Turabian StyleCastro Cerda, Felipe Manuel, Bernd Schulz, Spyros Papaefthymiou, Alfredo Artigas, Alberto Monsalve, and Roumen H. Petrov. 2016. "The Effect of Ultrafast Heating on Cold-Rolled Low Carbon Steel: Formation and Decomposition of Austenite" Metals 6, no. 12: 321. https://doi.org/10.3390/met6120321







