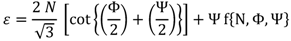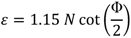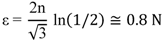1. Introduction
Since the last few years the effects of mechanical deformation on the hydrogen storage behavior of metal hydrides have been intensively studied. Mechanochemistry, mainly performed using Ball Milling (BM), has demonstrated the possibility to conduct various reactions, such as synthesis of nanocrystalline and amorphous materials at low temperatures without dissolution or fusion of the reactants. Forty years after the pioneering works of Benjamin [
1] the mechanochemical treatment of substances in producing new thermodynamically stable and metastable materials, often non-achievable by traditional methods, is well established and received industrial applications. While the method is progressing at the industrial level, knowledge about the physical mechanisms operating during the mechanochemical treatment (e.g., ball milling) is still very limited due to serious difficulties in quantifying this complex process [
2].
The mechanochemical techniques of ball-milling (BM) and severe plastic deformation (SPD) methods are widely used in research laboratories as well as in the industry. In the field of metal hydrides BM is intensively used for preparation of new compounds and to obtain nanocrystalline structure. Mechanical milling effects could be enhanced by changing parameters such as milling atmosphere (reactive milling), temperature, addition of anti-sticking agents, etc. This level of sophistication led to impressive results in enhancement of hydrogen storage properties of metal hydrides by the use of mechanical milling. The next challenge is to scale-up these types of technologies to industrial levels. Here the main hurdle may be the high energy milling (power and time) which is most usually needed for improving metal hydride properties. This imposes additional requirements in terms of specific energy of the milling machine and size of batches that could be processed. These problems are compounded if the milling has to be performed under hydrogen pressure and at high temperature. One also has to take into account the safety aspect of handling metal hydrides. The engineering aspects of these problems could surely be solved. The main challenge will be to keep the capital and operation cost low in order to make the technology competitive. However, the use of this technique poses the problem of scaling-up a high-energy density method.
In the case of SPD it has just recently been used for metal hydrides and the results are relatively scarce. In an early investigation, Ueda
et al. have prepared a Mg–Ni laminated composite by cold rolling stacked Mg and Ni sheets [
3]. However, to obtain the intermetallic Mg
2Ni the samples had to be heat treated at 673 K. Similar results were later found by Pedneault
et al. [
4,
5]. They showed that cold rolling combined with heat treatment resulted in nanostructured metal hydrides with good electrochemical properties. In the case of Ti-based alloys, Zhang
et al. [
5,
6] studied the effect of cold rolling on the hydrogen sorption properties of Ti–22Al–27Nb alloy and found better hydrogenation kinetics for cold rolled samples but only for the first hydrogenation cycle. Magnesium alloys have been studied by a few groups using different SPD techniques or combination of them [
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23]. These studies will be discussed in more details in the next sections but the general conclusion is that SPD techniques could improve the hydrogen sorption properties of magnesium alloys.
One important advantage of SPD techniques over high energy milling is that they are potentially easier to scale up to industrial level. Therefore, the study of SPD applied to metal hydrides is interesting from a fundamental point of view because of the different action of SPD on the processed material compared to high energy milling and also from a practical point of view because of the possibility of reducing cost of hydrides processing. In this paper we will review the use of two SPD techniques, Equal Channel Angular Pressing (ECAP) and Cold Rolling (CR) for the preparation of magnesium for hydrogen storage. After a short description of each technique we will present recent studies of the use of them in the case of magnesium. More specific techniques such as High Pressure Torsion (HPT) and Fast Forging (FF) are not considered here because at this moment very few investigations have been realized.
2. Equal Channel Angular Pressing
2.1. Description of the Technique
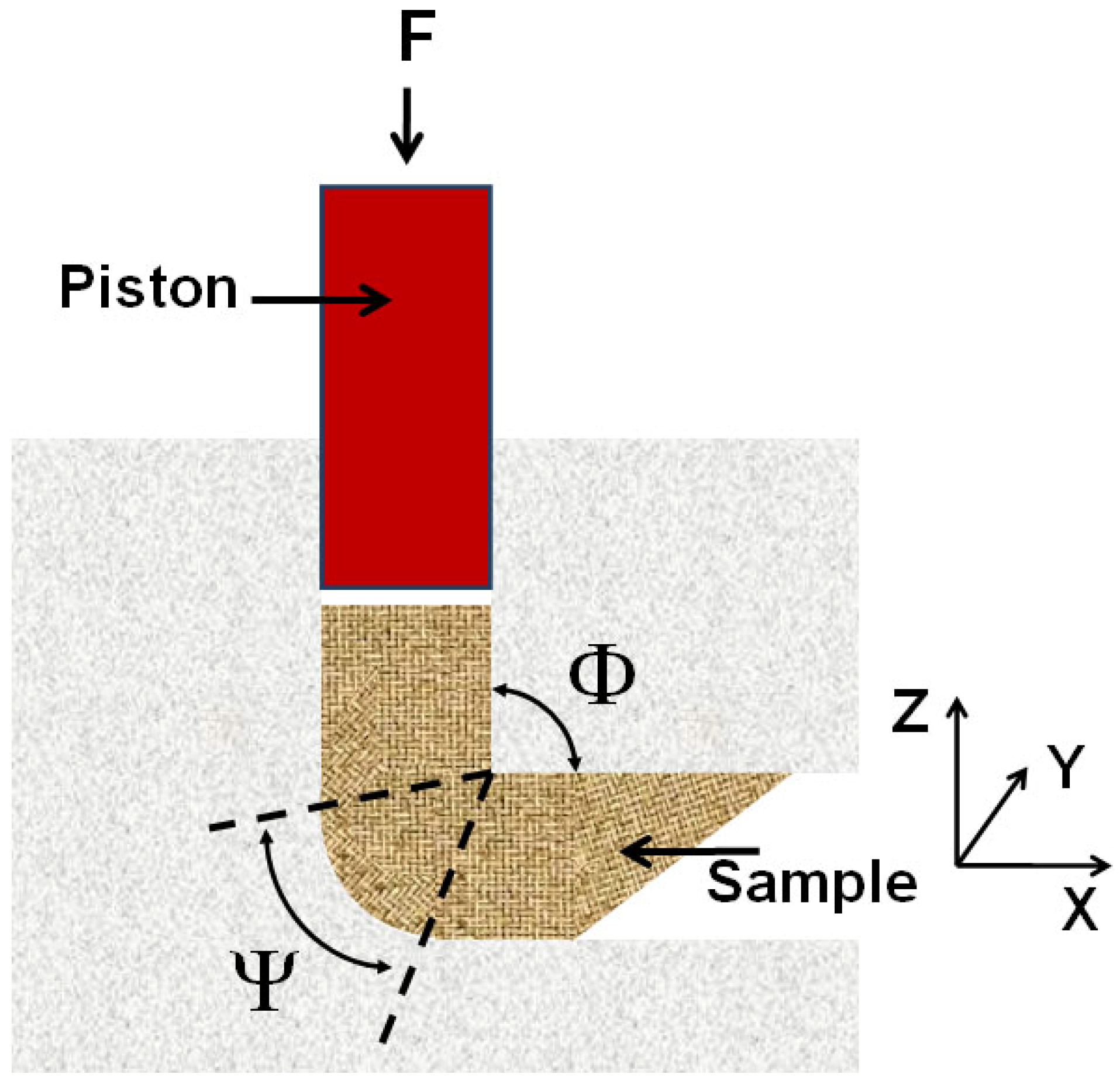
In the Equal Channel Angular Pressing (ECAP) severe plastic deformations are introduced into a material by forcing a sample (billet) with a piston through a die consisting of two channels of equal cross-section, which intersect at an angle (Φ) between 90° and 120° [
24] (see
Figure 1). The outer arc of curvature where the two channels intersect is labeled Y. Since the billet assumes the form and the cross-section of the die, it can be repeatedly processed to increase the microstrain density and simultaneously reduce the size of crystallites to meso- or nanometer down to 40 to 100 nm range. ECAP is quite efficient in processing metals and allows to produce porosity-free materials in substantial quantities with lower concentration of impurities and at a lower cost than ball-milling [
25]. Through the crystallite size refinement process, the proportion of high angle grain boundary (HAGB) increases due to dislocations recovery [
26]. High texturation effects could also be introduced by this process, thus organizing specific grain boundaries distributions, indeed depending on the crystal symmetry of the processed system (e.g., cubic
versus hexagonal).
Figure 1.
Schematic illustration of Equal Channel Angular Pressing (ECAP).
2.2. Strain
The equivalent strain (ε) introduced in the material by the ECAP process is determined by an equation incorporating the angle between the two parts of the channel, Φ, and the angle representing the outer arc of curvature where the two parts of the channel intersect, Ψ. The relationship given by:
where
N is the number of passes through the die [
27], and accounting the weakness of the f term contribution an expression that is often simplified [
7], to:
However, if the number of passes seems to be a dominant parameter, the most important refinement of the microstructure takes place during the first pressing passes, since the development of an ultrafine grain size leads to an increase of the yield stress.
2.3. Effect of Billet Rotation
From
Figure 1 it could be seen that after a single pressing a cubic element is sheared into a rhombohedral shape. If the same billet is pressed a second time then the operator has the choice to insert the billet with or without rotation. It has been recognized that, for repetitive pressing, the overall shearing characteristics within the crystalline sample may be changed by a rotation of the sample between the individual pressings [
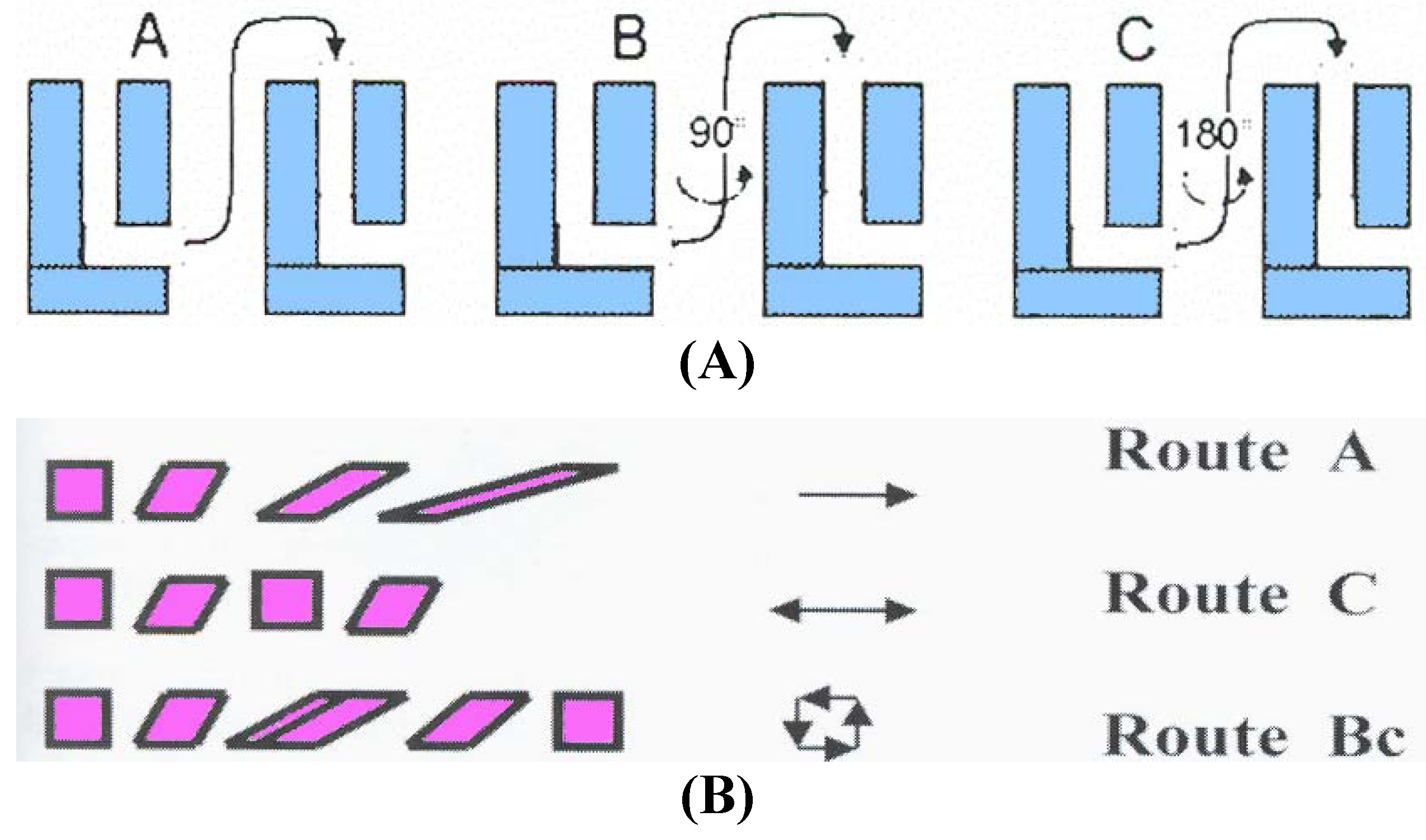
28]. When samples are pressed repetitively, different slip systems may be introduced by rotating the samples about the X-axis between consecutive passes through the die. Several processing routes have been identified for use in ECAP: route A in which the sample is pressed repetitively without rotation, route B
C in which the sample is rotated by 90° in the same sense between each pass and route C where the sample is rotated by 180° between passes [
29]. More complex combination of these routes could also be performed.
Inspection of
Figure 2 shows that route A markedly increase the distortion of the rhombohedron, route B increases the distortion in the
X and
Z planes and route C restores the cubic element so that strain has been introduced but with no ultimate distortion of the bulk of the sample [
30]. We are looking for a route that will introduce maximum strain in all directions while recovering a cubic shape after
N passes. Immediately, we see that route A is not optimal because the cubic structure is not recovered. In route C the cubic structure is recovered after each 2
N pressings but no deformation is induced in the
Z plane. Therefore, the only optimal route is B
C since introducing strains in each plane while recovering a cubic structure after 4
N passes.
Figure 2.
Characteristics of most important processing routes: (A) The modes of successive passes in the ECAP die; (B) Effective deformation of the shape of a billet.
It has been shown experimentally that, when using an ECAP die with an angle of 90° between the two parts of the channel, optimum processing is achieved using route B
C because this leads most rapidly both to an array of reasonably equiaxed grains and to a high fraction of grain boundaries having high angles of misorientation [
31]. The influence of various ECAP parameters such as value of angles Ψ and Φ, pressing speed, temperature, and back pressure are discussed in a recent review [
24].
2.4. ECAP Effect on Magnesium
The effect of ECAP on the hydrogen storage behavior of Mg-based alloys has been investigated by Skripnyuk
et al. [
6,
7,
8,
9]. The structural alloy ZK60 of composition Mg–4.95 wt % Zn–0.71 wt % Zr were processed by high energy ball milling (HEBM), ECAP, and a combination of ECAP and HEBM. The ECAP processing was made through route A with eight passes at 250–300 °C and one additional pass at room temperature [
6]. The results show that after ECAP/HEBM treatment the hysteresis in the pressure-composition isotherm completely disappears. However, the most important effect of processing was on the hydrogen desorption kinetics. As shown in
Figure 3, ECAP processed samples shows much improved kinetics compare to as-cast and annealed samples. The fact that ZK60 desorbed hydrogen faster than HEBM-processed Mg was supposed being due to the catalytic effect of Zn and Zr. In turn, ECAP, HEBM, and ECAP/HEBM-processed alloys desorbed hydrogen at approximately the same rate, which was much higher than the desorption rate for annealed ZK60. Also combined ECAP/HEBM treatment did not improved the kinetics of desorption as compared to the effect of ECAP alone.
Figure 3.
Kinetics of hydrogen desorption at 300 °C from differently processed samples of ZK60 alloy and from high energy ball milling (HEBM)-processed pure Mg. [
6]
In the case of as-cast eutectic Mg-Ni alloy, after 10 ECAP passes (route B
C) sub-micrometer size of Mg and Mg
2Ni grains was achieved [
7]. The Mg grains showed supersaturation in Ni, which was non-homogeneously distributed across the grains. The Ni concentrations in the Mg grains with high dislocation density were consistently higher than in the grains with fewer dislocations. Gravimetric hydrogen storage capacity of about 6 wt % was measured. Pressure-composition isotherm measurements indicated that both Mg and Mg
2Ni phases are destabilized compare to their as-cast counterparts. The ECAP processed alloy also exhibited excellent hydrogen desorption kinetics. In fact, in terms of hydrogen desorption pressure the ECAP treated Mg-Ni alloy outperformed the alloys of similar composition nanostructured by alternative processing techniques [
7].
Recently, Skripnyuk
et al. investigated the hydrogenation properties of magnesium doped with carbon nano-tubes (CNT). When HEBM is used to synthesize Mg-CNT mixtures destruction of CNT is happening after a relatively short time of milling. Their idea was to use ECAP to get a good contact between Mg and CNT [
9]. They found that ECAP led to a slower absorption/desorption kinetics at the initial stages of the processes, and to their acceleration at the later stages. Two competing factors are proposed to explain the complex effect of ECAP on the hydrogenation kinetics of the composite. Namely, a decrease in hydrogen diffusivity along the CNTs due to their rupture and kinking, and concurrent enhancement of the hydrogen diffusion kinetics in the Mg matrix [
9].
In another investigation, Løcken
et al. used ECAP and HEBM to process the ternary eutectic Mg–Mg
2Ni–MmMg
12 (72 wt % Mg–20 wt % Ni–8 wt % Mm, Mm = mischmetal) [
32]. After eight ECAP passes at 400 °C (route B
C) an improvement in the hydrogen absorption and desorption rates was noticed. However, HEBM gave an even larger improvement and reduced the absorption and desorption times to one third of those of the as-cast alloy.
2.5. Effect of Extrusion Temperature
In more recent works, the processing temperature as was studied a key parameter for grain size control [
11,
33,
34].
Figure 4 shows the effect of ECAP temperature processing for AZ31 alloy. This figure shows the effect of two ECAP passes (mode B
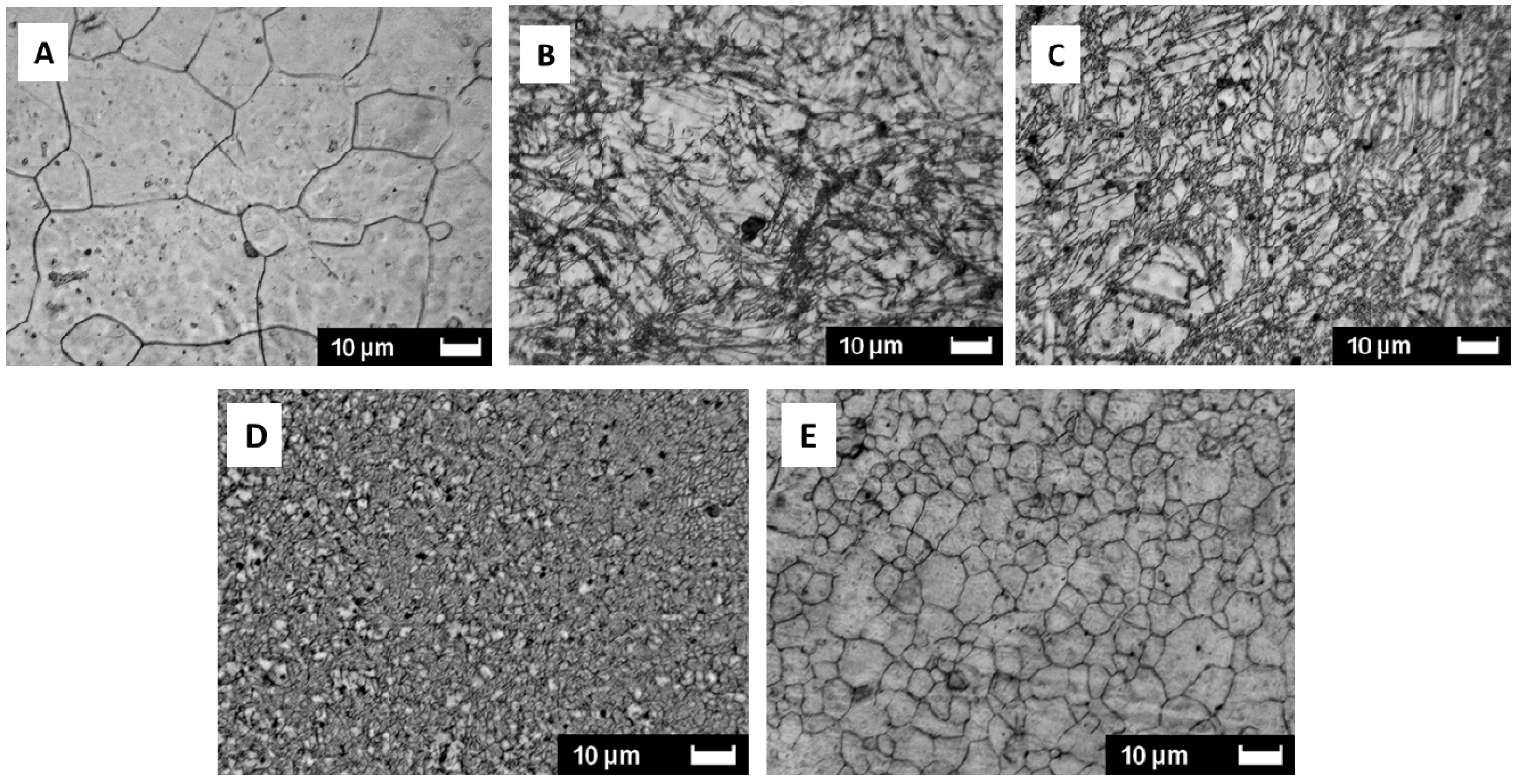
C) performed at different temperatures. Each experiment was performed by starting from the as-cast material. With increasing the number of passes the elongations of fractures is increasing, mainly for temperatures lower than 200 °C. For higher temperature (e.g., 250 °C) the yield stress (or the maximum stress) shows only small changes. The as-cast structure, shown in
Figure 4A, contains large well crystallized grains (from 10 to 50 µm).
Figure 4B shows that after two ECAP passes at 150 °C, the initial structure is severely crushed by the deformation process. Grains are elongated and have a general orientation. A similar structure is still seen after two passes at 200 °C as presented in
Figure 4C. A completely different structure is obtained by ECAP at 250 °C as displayed in
Figure 4D. Here, there are no signs of deformation. This indicates that recrystallization took place during ECAP deformation. If the processing temperature is increased to 300 °C, after two ECAP passes the sample is fully recrystallized. There are no remains of plastic deformation effect but during ECAP there was a grain size growth. These results clearly show that dynamic re-crystallization could occur during ECAP and that the operation temperature can markedly influence the microstructure of the sample, thus the hydrogenation/dehydrogenation reactions. Practically, during the SPD process a few ten degrees increase of temperature may occurs for some systems.
Comparative experiments performed more recently on ECAP processed AZ31 samples reveal that the most effective reaction in terms of hydrogen absorption/desorption kinetics correspond to samples treated in the range of brittle to ductile mechanical characteristics (150–210 °C—see
Figure 4B,C).
Figure 4.
Optical micrographs of AZ31 magnesium alloy. (A) as-cast; (B) two passes at 150 °C; (C) two passes at 200 °C; (D) two passes at 250 °C; (E) two passes at 300 °C.
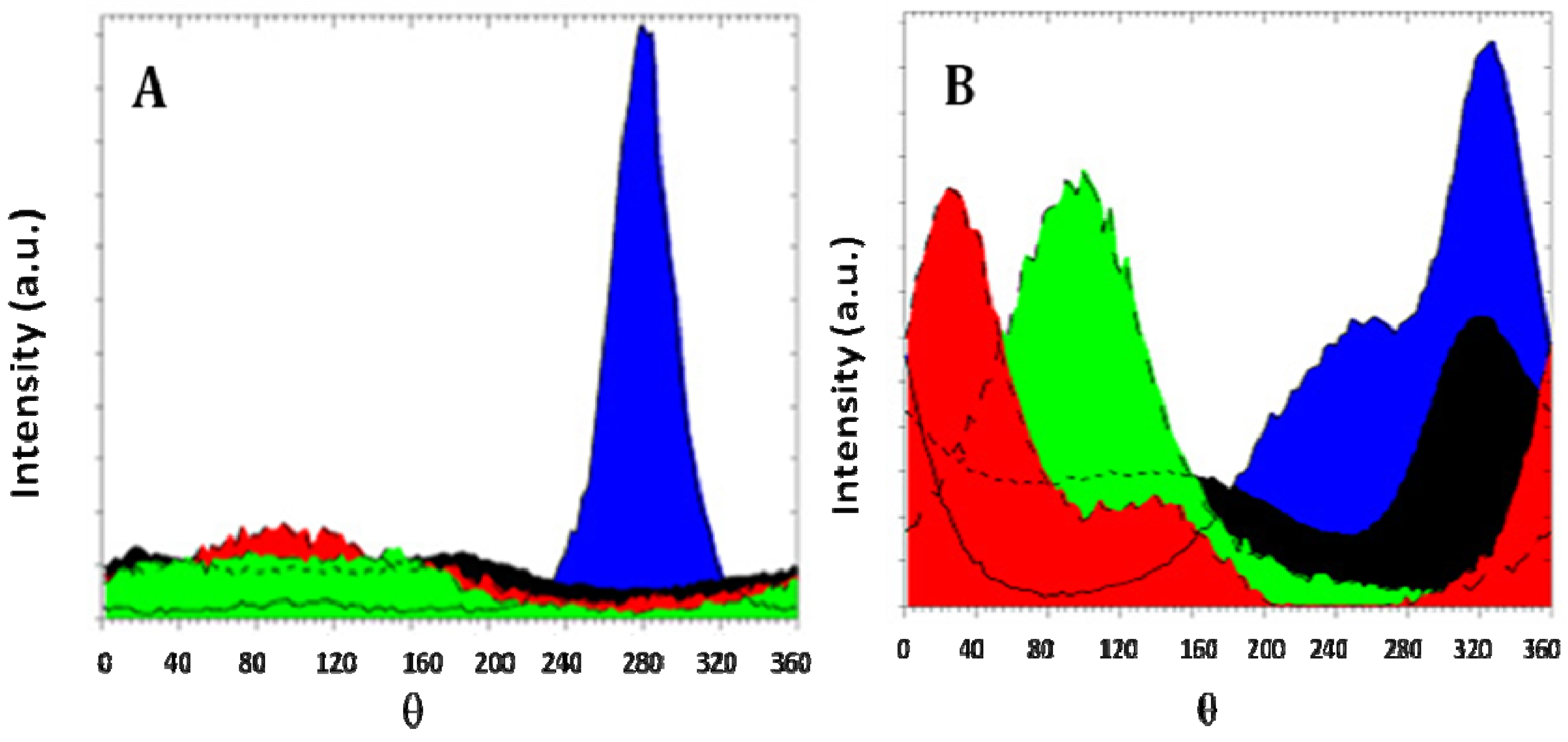
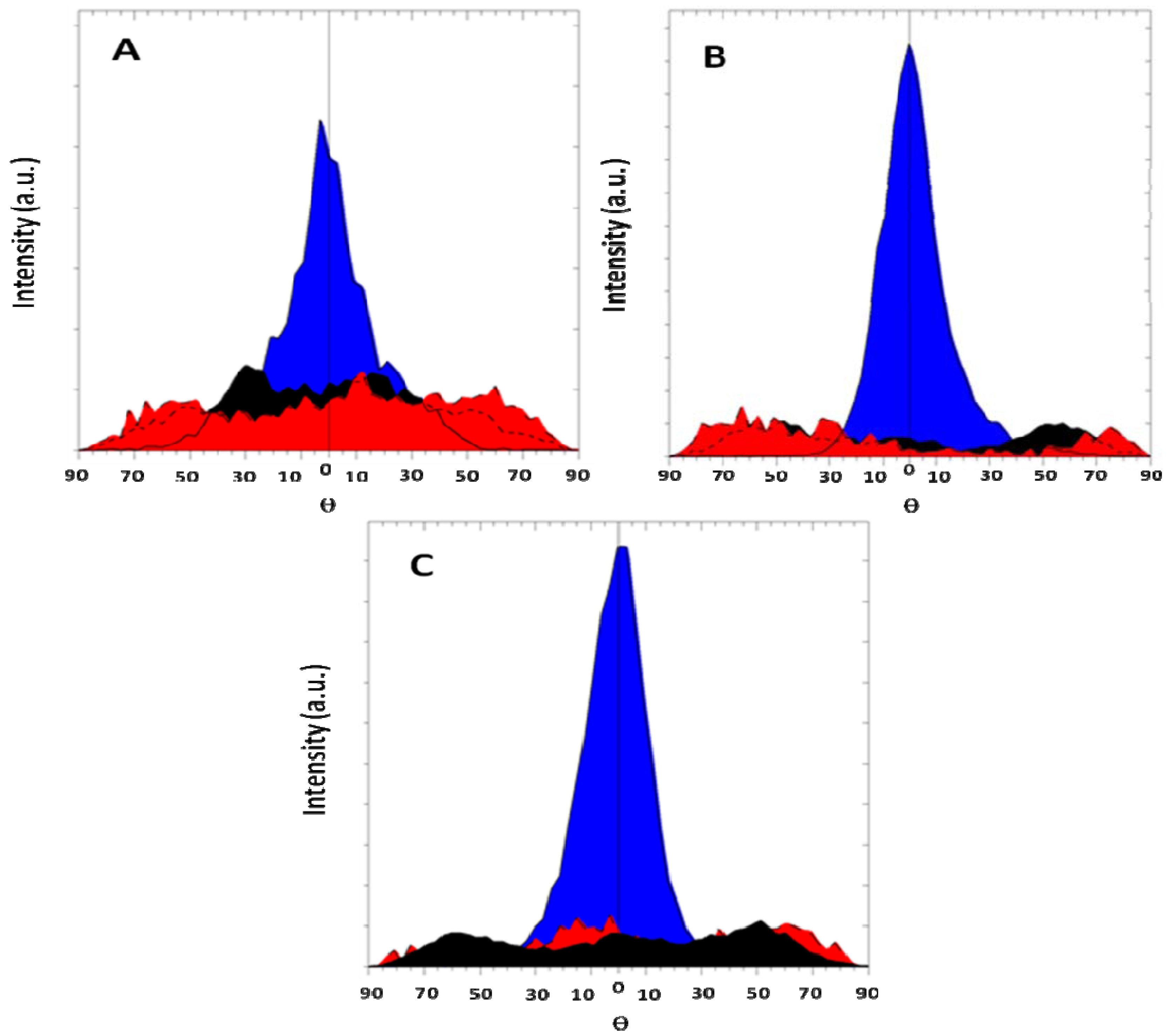
Figure 5 shows the evolution of texture for AZ31 magnesium alloy for different ECAP routes. It is seen that a very marked texture is delivered by route A processing, contrarily to mode B
C that distributes almost equally the grains along all crystalline directions. Also, the profiles of lines are much broadened for the route B
C than for route A, meaning that more defects are developed during the multi-orientation process B
C. So, as said above, both effects impact quite differently the hydrogen sorption properties.
Figure 5.
Texture of AZ31 magnesium alloy. (A) One pass at RT, mode A; (B) Six passes at RT, mode BC. Orientations are color coded. (002)-blue; (100)-red; (101)-black; (110)-green.
Samples processed N-times following route A (2 < N < 8) develop an initial incubation time before a continuous hydrogen absorption rate, while absorption started readily in samples processed by route B
C. It may be concluded that the shape, the size and the texture (as shown on
Figure 5) of the deformed grains are pertinent parameters to be consider in the hydrogen activation and diffusion processes [
18]. For these samples, an hydrogen uptake up to ~7.6% was measured.
3. Cold Rolling
3.1. Description of the Technique
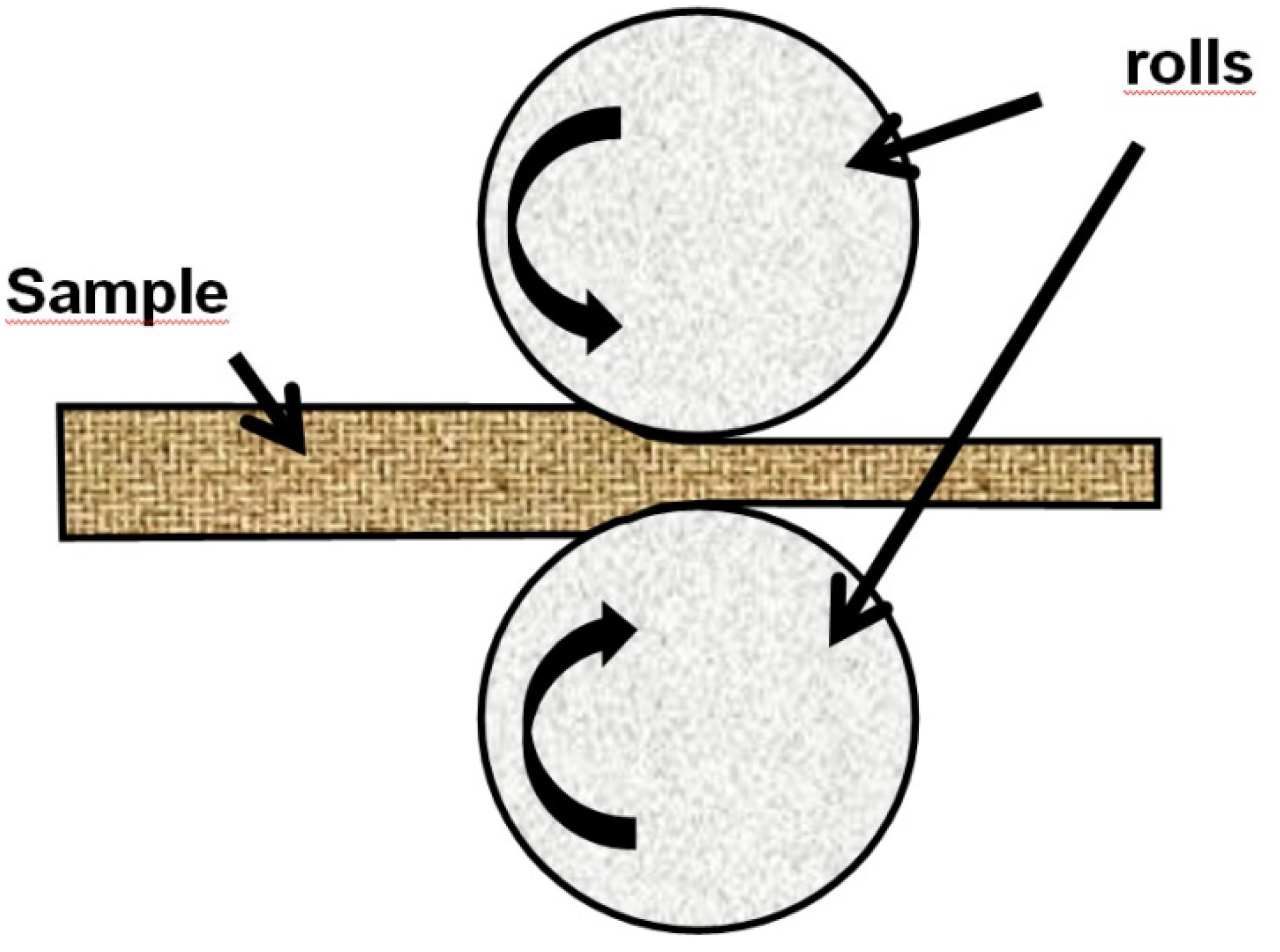
In cold rolling technique (CR), a sheet of metal is introduced between rollers where it is compressed and squeezed (
Figure 6). The hardness and other properties of the finished product depends on the amount of strain induced in each Cr pass.
Figure 6.
Schematic illustration of Cold Rolling (CR).
Usually, rolling is classified according to the processing temperature as compared with the metal re-crystallization temperature. In Hot Rolling (HR) the process is carried out at a temperature exceeding the re-crystallization temperature of the rolled material while in Cold Rolling (CR) rolling temperature is below re-crystallization temperature. In this review only CR will be discussed.
3.2. Strain
If we represent the rolling direction (RD), transverse direction (TD) and normal direction (ND) by directions by subscript 1, 2, and 3 respectively then, to a very rough approximation for macroscopic sheets, rolling deformation is plane strain compression for which ε
11 = −ε
33 and all other strains ε
22 = ε
12 = ε
13 = ε
23 = 0. Under these idealized assumptions the effective strain equals the von Mises equivalent strain [
35].
In this idealized situation the strain is in normal direction contrary to HPT and ECAP processes which produce mainly shear deformations. However, in real situation deformation in the rolled sheet is strongly affected by frictional condition between the rolls and the metals. It is well-known that under high friction conditions the metals deform inhomogeneously through thickness because a large amount of redundant shear strain is introduced in the surface regions [
36].
3.3. Accumulative Roll Bonding
In accumulative roll bonding (ARB) [
37] the starting sample is made of a stack of two or more thin foils. The foils’ interfaces could be surface-treated in order to enhance bond strength. The foils could be made of the same element or of different elements in the desired stoichiometric composition. The stack is then rolled as in as in a conventional roll- bonding process. Then, the length of rolled material is sectioned into two halves. The sectioned foils are stacked one on top of the other and rolled again. The whole process is repeated for the desired number of times. In effect, ARB rolling is not only a deformation method but also a bonding process. The process can introduce ultra-high plastic strain without any geometrical change.
It should be noted, that in ARB process half of the surface regions comes to the center in the next cycle. This results in complicated distributions of the surface regions with large shear strain through thickness of the foil. With a 50% thickness reduction at each rolling pass and assuming no lateral spreading of the material (von Mises yield criterion and plane strain condition) the equivalent plastic strain after N cycles is [
37]:
From this expression we see that as the ARB procedure could be applied for a limitless number of time, large plastic strain could be reached for even a few numbers of rolls [
38].
3.4. Cold rolling of Magnesium
Magnesium has a limited number of slip planes and work hardening occurs rapidly. Thus, after only a few rolling passes, a magnesium foil will quickly break up in small pieces. Therefore, rolling many times (more than 10 rolling passes) could be difficult. However, for hydrogen storage applications and mechanical integrity is not so important. In fact, after a few hydrogenations many metal hydrides turn into powder because of the important decrepitation due to the significant volume change during hydrogenation. Consequently, work hardening is not a significant problem for hydrogen storage applications.
To our knowledge, Ueda
et al. were the first to try to synthesize a metal hydride (Mg
2Ni) using cold rolling of raw elements followed by heat treatment [
3]. When using the stoichiometry 2Mg + Ni, single phase Mg
2Ni was obtained, and the sample could be completely hydrogenated to Mg
2NiH
4. Interdiffusion between Mg and Ni during heat treatment was the mechanism for the formation of Mg
2Ni [
3]. Mg-Ni system for electrochemical applications was also studied by Pednault
et al. [
4,
5]. They investigated the structural and electrochemical evolution of 2Mg–Ni cold-rolled samples as a function of the number of rolling passes as well as heat treatment. They found that nanocrystalline Mg
2Ni alloy was obtained by an appropriate three step process involving rolling, heat treatment and rolling again. The best results were obtained by first rolling 90 times, followed by a heat treatment at 400 °C for 4 h and roll again 20 times. The treated material displayed an initial discharge capacity of 205 mAhg
−1, which is quite similar to that obtained with ball-milled Mg
2Ni alloy [
4].
For Mg-Pd system, Dufour and Huot have shown that cold rolled samples have a much better air contamination resistance than the same material prepared by ball milling [
23]. Takeichi
et al. [
39] as well as Dufour and Huot [
22] have also shown that the alloy Mg
6Pd could be synthesized by cold rolling followed by heat treatment. Other Mg-X systems such as Mg-Ti [
21,
40], Mg-Al [
41], Mg-Cu [
39,
42], Mg-Fe [
11,
13] as well as commercial Mg-Zr-Zn alloy [
43] have been investigated.
3.5. Texture Evolution of Cold Rolled Alloys
Figure 7 shows the evolution of texture in cold rolled magnesium. Rolling was performed at room temperature and there was a 50% thickness reduction at each pass. From
Figure 7A we see that after just one rolling pass the material is already highly textured. Further increase of the number of deformation passes leads to insignificant decrease in the intensity of the texture maximum ((002) direction). It should be noted that the width of maximum remains relatively broad in all cases. This means that the deformation is spread over the sample. In the case of ECAP the deformation is not so spread as for CR since the latter changes strongly the overall dimensions of the sample. In our opinion, it is due to the fact that in the course of rolling deformation of a sample is carried out both as a compression, and a shift of deformation. Therefore the structure comes out with larger dispersion than in the case of ECAP.
Figure 7.
Pole figure of cold rolled magnesium. Orientations are color coded. (002)-blue; (100)-red; (101)-black. (A): 1 rolling pass; (B): 10 rolling passes; (C): 100 rolling passes.
3.6. Hydrogen Sorption Properties of Cold Rolled Magnesium Alloys
Most of the studies of SPD on magnesium alloys have used ECAP techniques: to our knowledge the only systematic investigation of effect of cold rolling on commercial magnesium alloys have been reported by Amira and Huot on four different as-cast and die-cast alloys (AZ91, MRI153, AXJ530 and ZAEX10430) [
18]. Here we report the main features of AZ91D alloy compared to pure magnesium. Plates of AZ91D and pure magnesium were first rolled 50 times in air. A 50% reduction at each roll was obtained by folding the plates in two between two successive rolls. Final thickness was about 0.3 mm. In order to introduce nucleation points for the hydride phase, after the final roll the material was mixed with 5 wt % of MgH
2 and ball milled for 30 minutes. Hydrogenation was performed at 623 K under hydrogen pressure of 2 MPa for the absorption and 0.01 MPa for desorption.
Figure 8 compares the first hydrogenation (activation) of cold rolled AZ91D and magnesium.
Figure 8.
First hydrogenation (activation) of cold rolled AZ91D and magnesium.
The first hydrogenations of unrolled materials are not shown because they are too slow, effectively taking a few days for partial activation. From
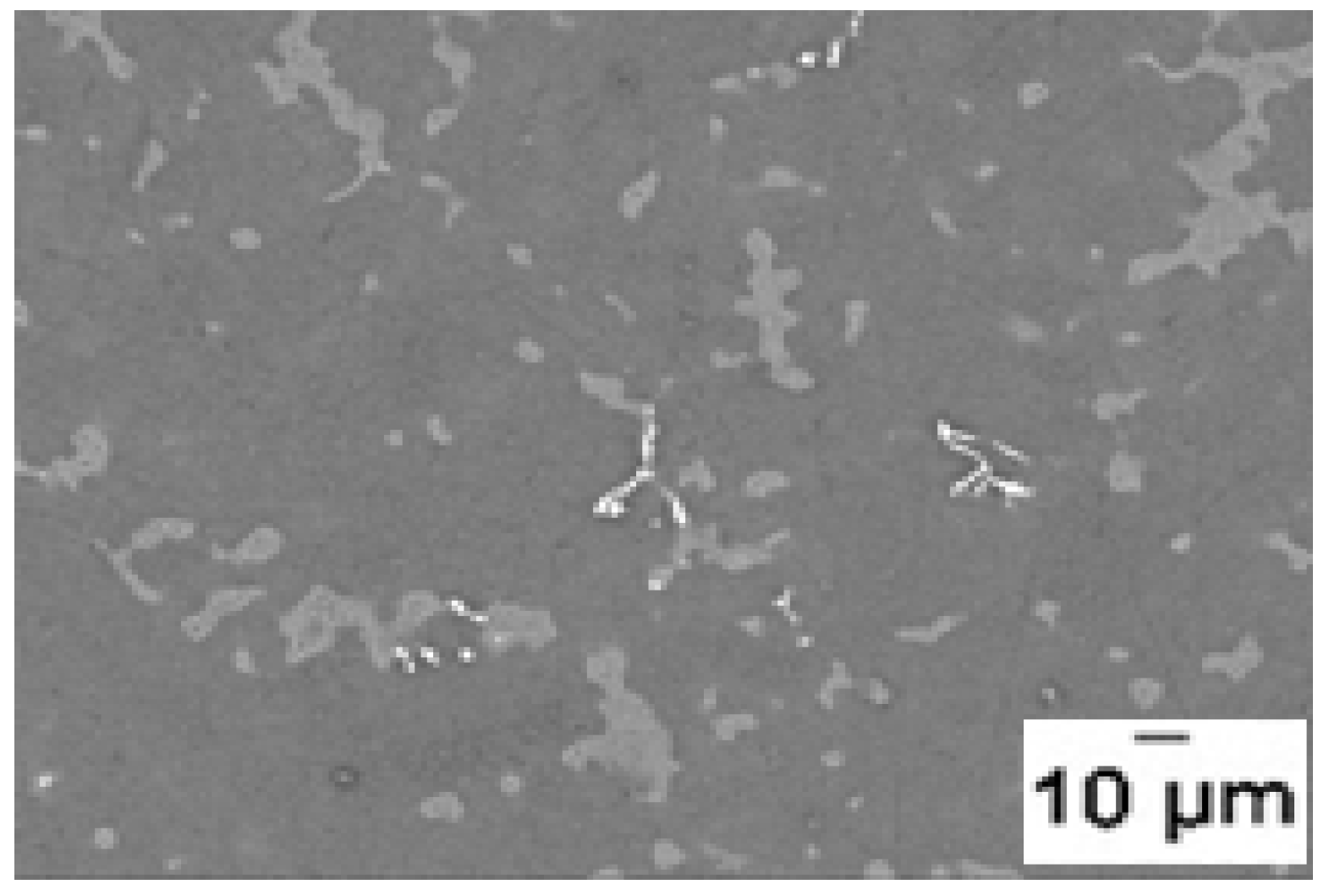
Figure 8 one can see that AZ91D activate faster than pure magnesium but the main difference is not so much the intrinsic kinetic but the fact that the alloy do not have the incubation time shown by pure magnesium. The absence of incubation time is not fully understood but it may be due to the presence of precipitates of Mg
17Al
12 and Al
8Mn
5 compounds seen in the micrograph presented on
Figure 9.
Figure 9.
Microstructure of as-cast AZ91D. Primary α-Mg with discontinuous and divorced Mg17Al12 eutectic. The white particles are Al8Mn5 compound (γ-brass).
Dehydrogenation of kinetics of cold rolled AZ91D and magnesium are shown in
Figure 10. The alloy has faster dehydrogenation kinetics and the incubation time is shorter than pure magnesium. In subsequent cycle, the AZ91D alloys kept its faster sorption kinetics compared to pure magnesium [
18].
Figure 10.
Dehydrogenation kinetics of cold rolled AZ91D and magnesium.
4. Conclusions
Mechanical milling and severe plastic deformation are valuable methods for the synthesis and preparation of metal hydrides, but the former is presently much more used than the later. Up to now, for SPD technique the level of understanding the impact of these techniques on the hydrogen storage properties of metal hydrides is still relatively small. Only a limited number of papers have been published and just a few research groups are investigating metal hydrides applications of SPD. However, first results are encouraging and show similarities with mechanical milling in the effectiveness of obtaining a nanocrystalline structure and enhancement of hydrogen storage properties. Some SPD techniques such as HPT could face the same and maybe bigger scaling-up problems but others, for instance, Cold Rolling, Fast Forging and even ECAP could be more easily adopted by the industry. The capital and operating cost of these techniques is potentially much less than mechanical milling. For example, a recent investigation has shown that at laboratory scale cold rolling could produce similar enhancement of hydrogen storage properties of magnesium hydride than ball milling but with energy requirement and processing time reduced by an order of magnitude [
20]. The same reduction is expected at industrial level.
Because of the multiple parameters of ECAP (angle of channel, type of routes, number of passes, rate of process, temperature of sample, etc.) this technique offers a large panel of control factors to design the microstructure, the texture as well as the defect density (from dislocations to twins) to apply to the wide mechanical strength properties of commercially available magnesium alloys. Cold rolling may not be as sophisticated as ECAP in making materials with a wide range of textures but its potential for industrial applications may be higher.
From a more fundamental point of view, both mechanical milling and SPD could produce materials with new characteristics such as nanocrystalline structure, metastable phases, high density of defects, texture, important microstrain, etc. Each of these features could have an impact on the hydrogen storage behavior but the exact multiple mechanisms are usually not fully understood. More studies on the basic mechanism of mechanical effect through milling and SPD within the scope of hydrogen storage are needed.