Effect of CaF2/CaO Composite Additive on Roasting of Vanadium-Bearing Stone Coal and Acid Leaching Kinetics
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Experimental Procedure
3. Results and Discussion
3.1. Roasting Process
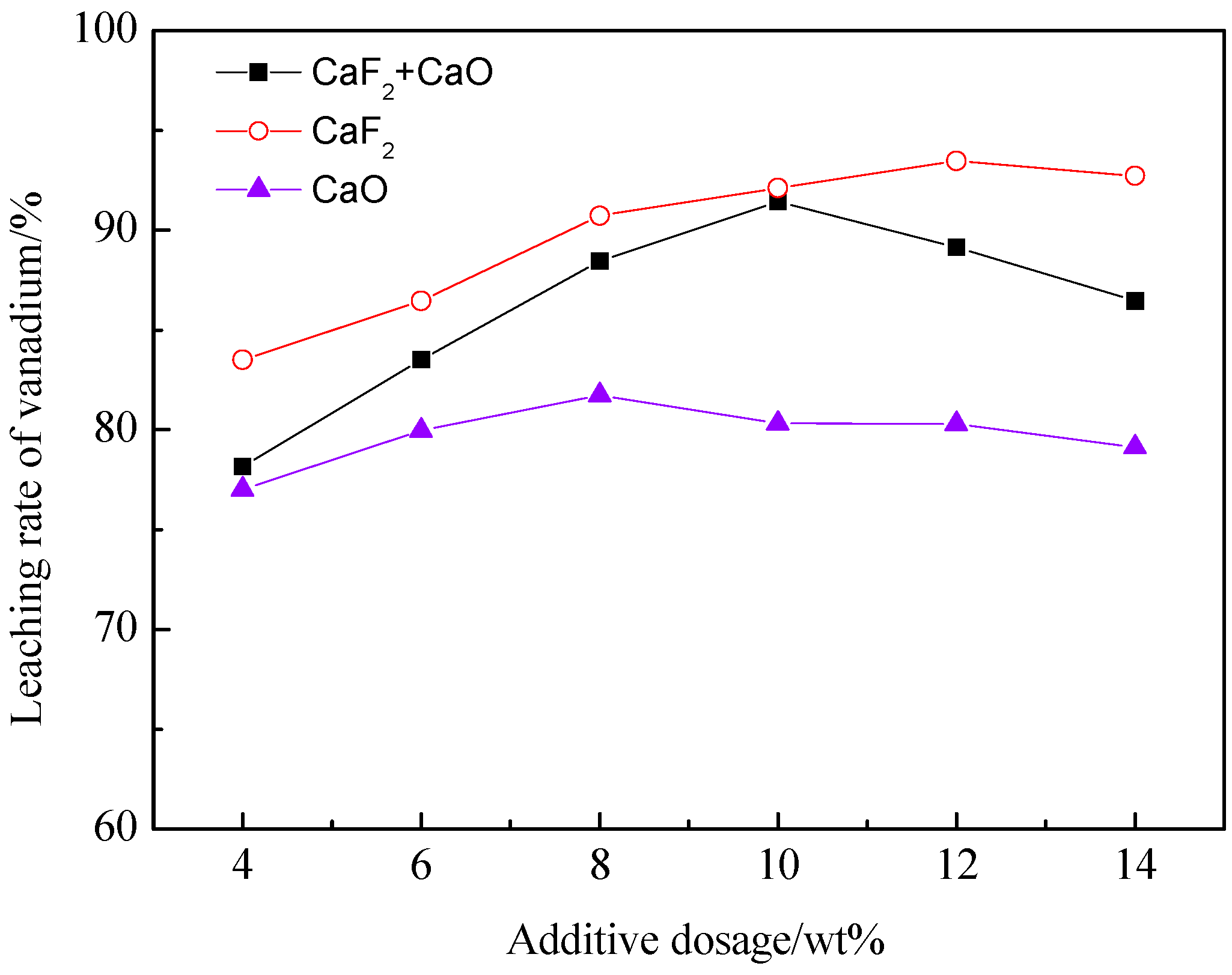
3.1.1. The Effect of the Proportion of Additives on the Leaching Rate of Vanadium
3.1.2. The Effect of the Total Amount of Additives on the Leaching Rate of Vanadium
3.1.3. The Effect of Temperature and Time on Leaching Rate of Vanadium
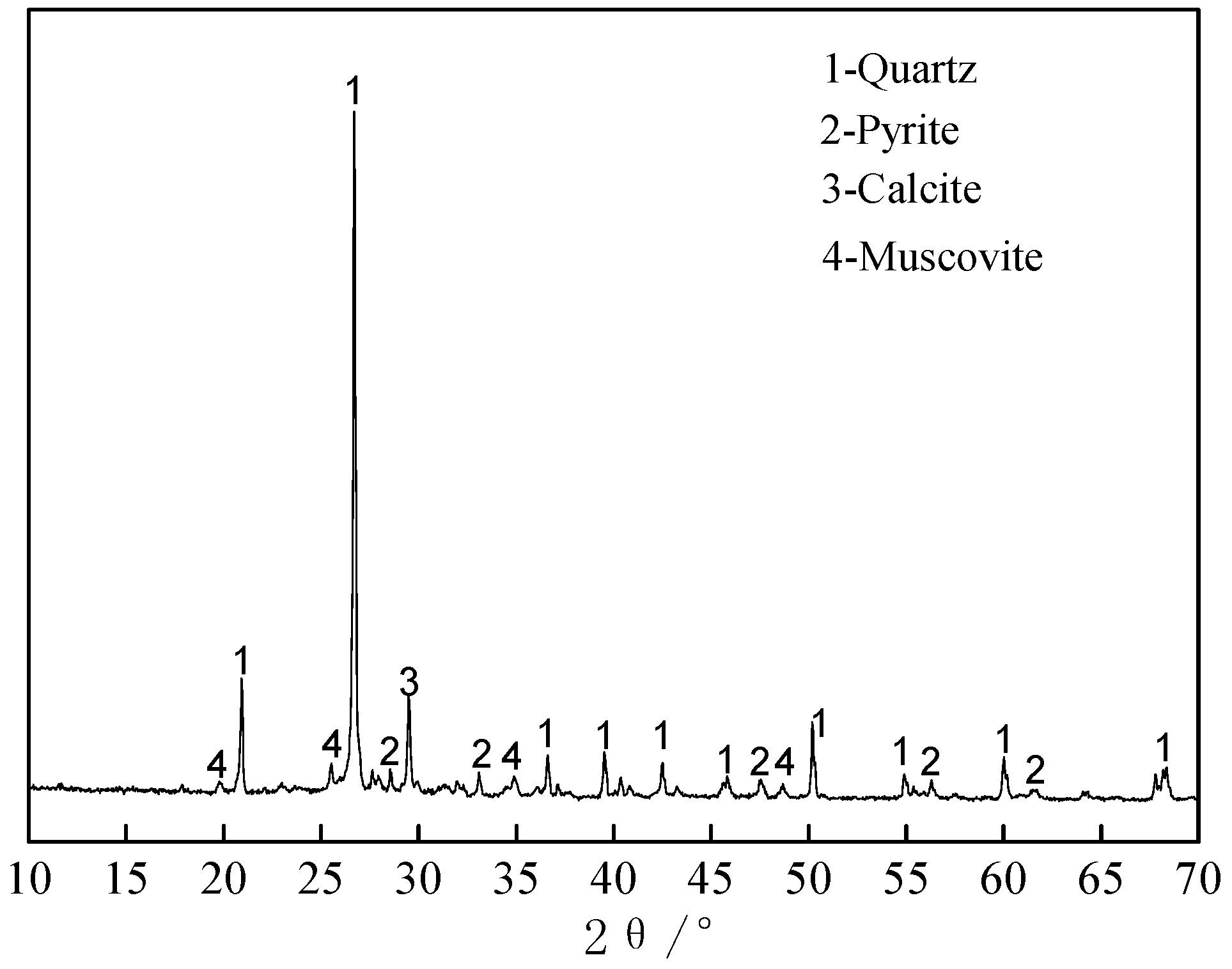
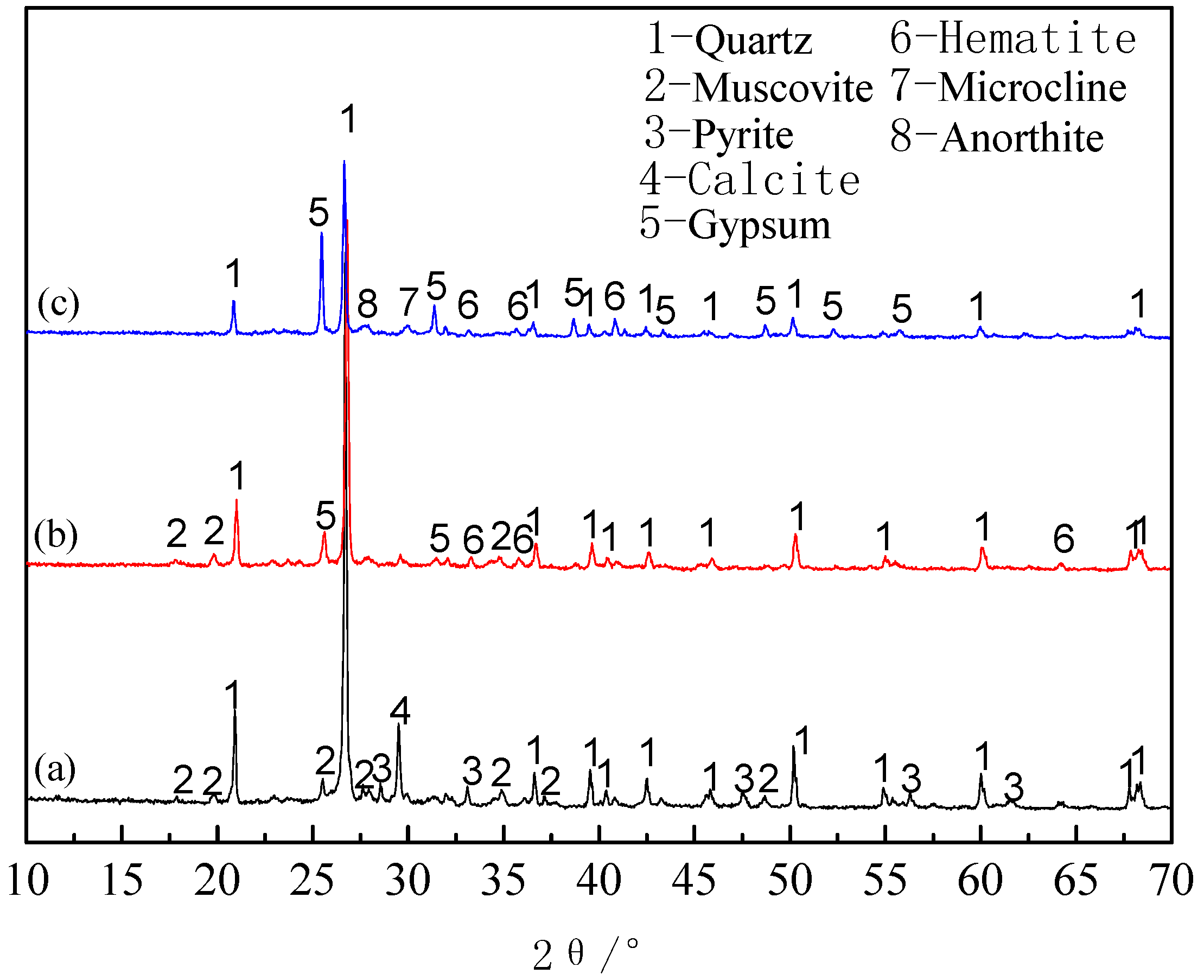
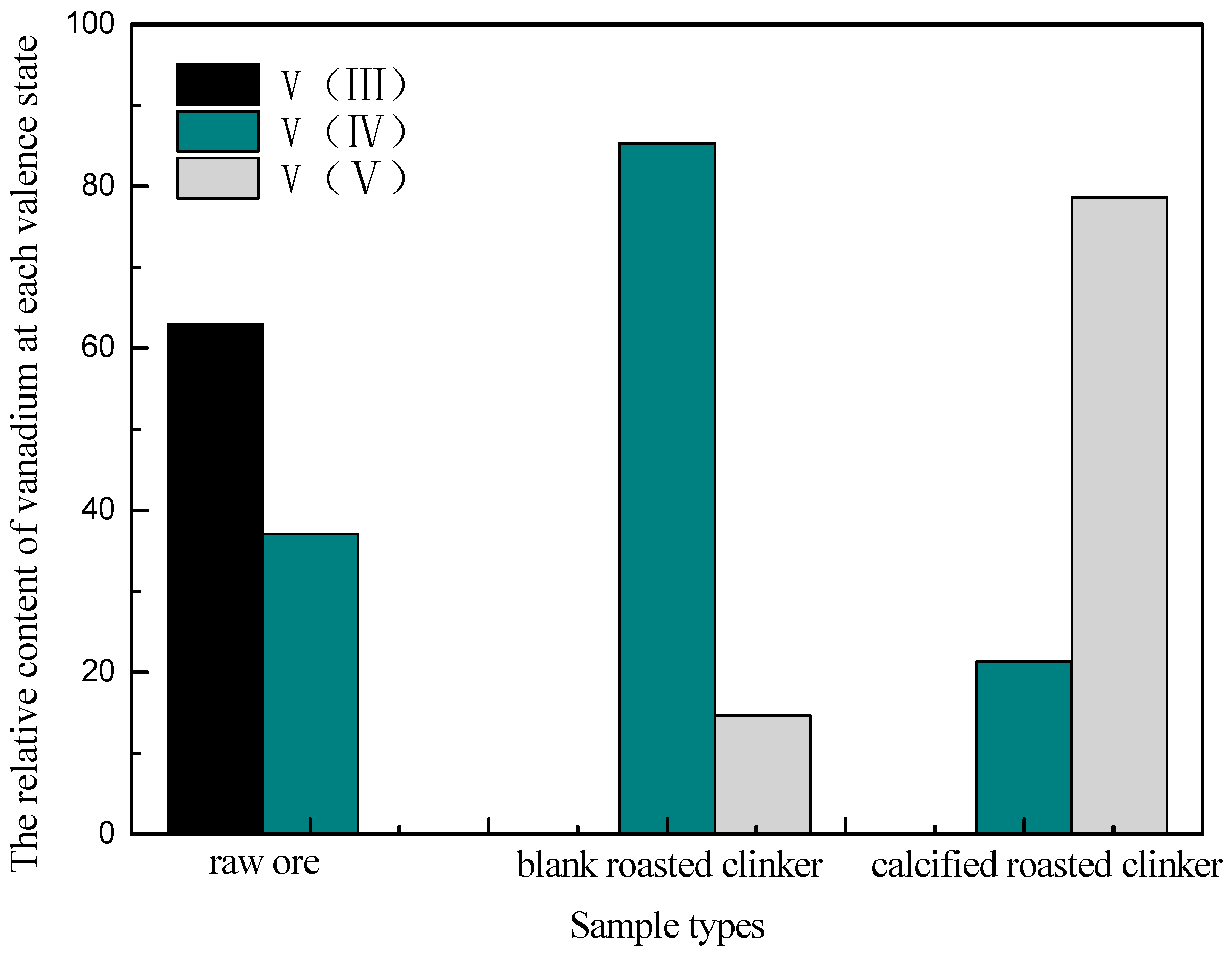
3.1.4. Phase Analysis of Roasted Products
3.2. Leaching Process
3.2.1. Effect of Sulfuric Acid Concentration on Vanadium Leaching Efficiency
3.2.2. Effect of Leaching Temperature on Vanadium Leaching Efficiency
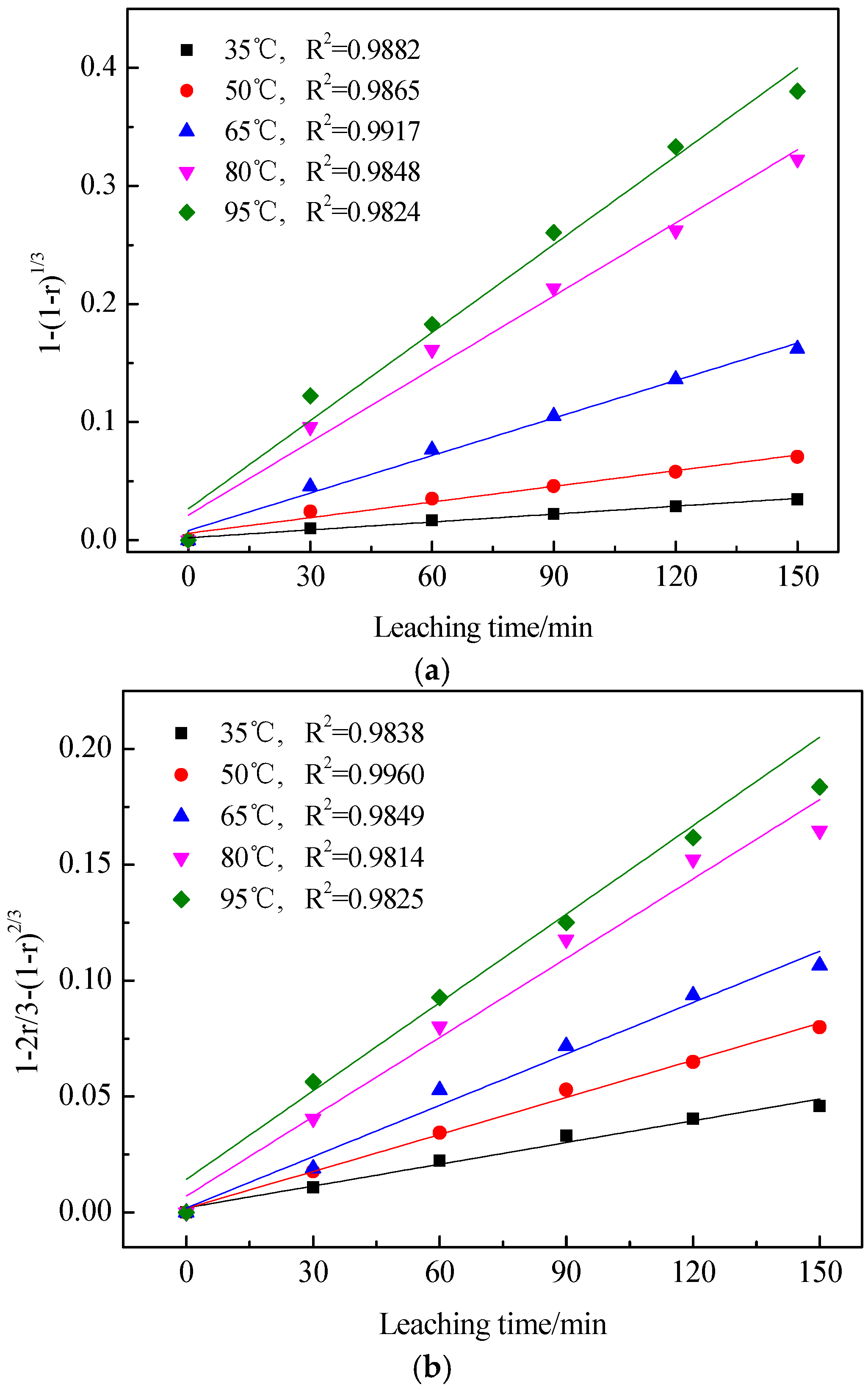
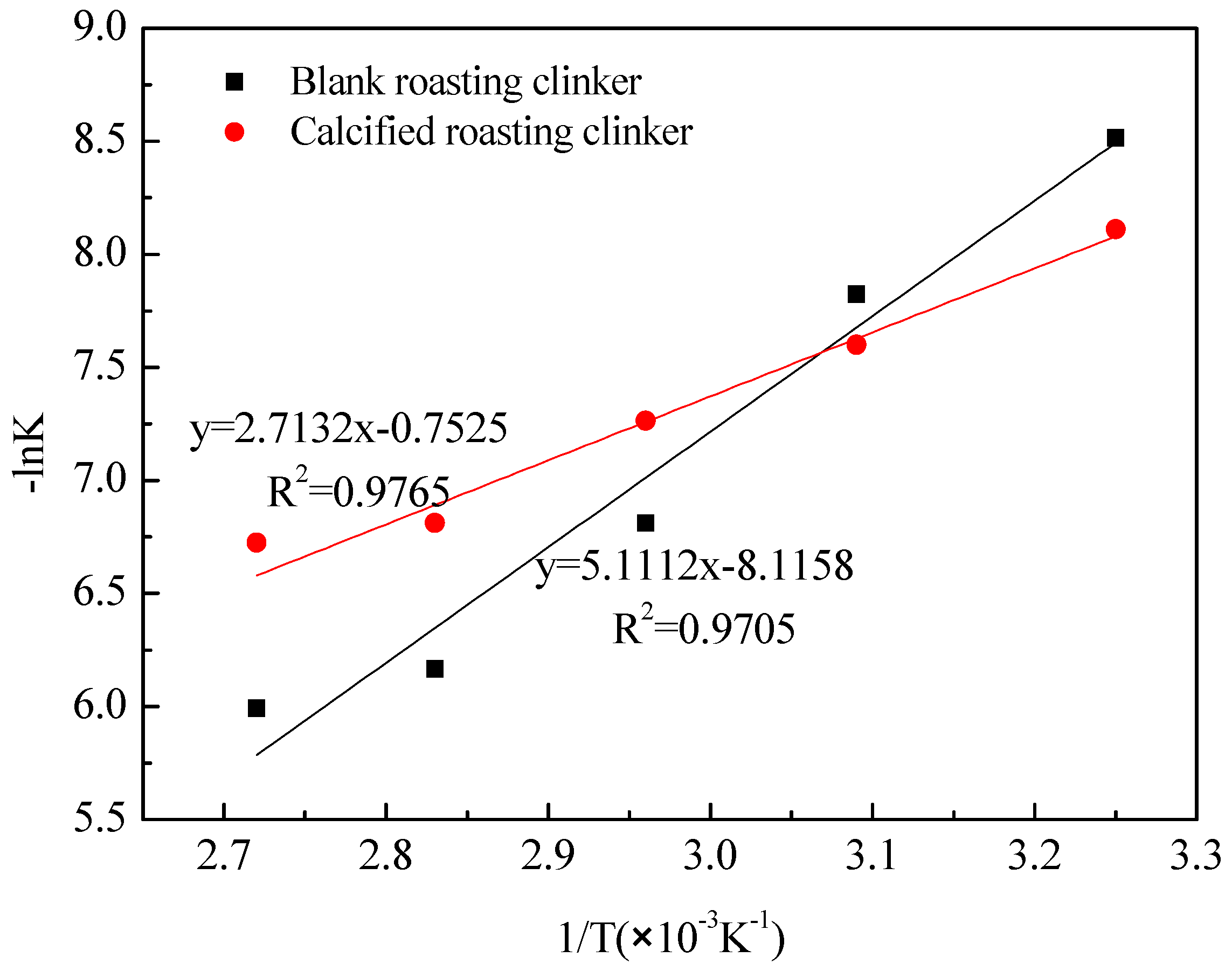
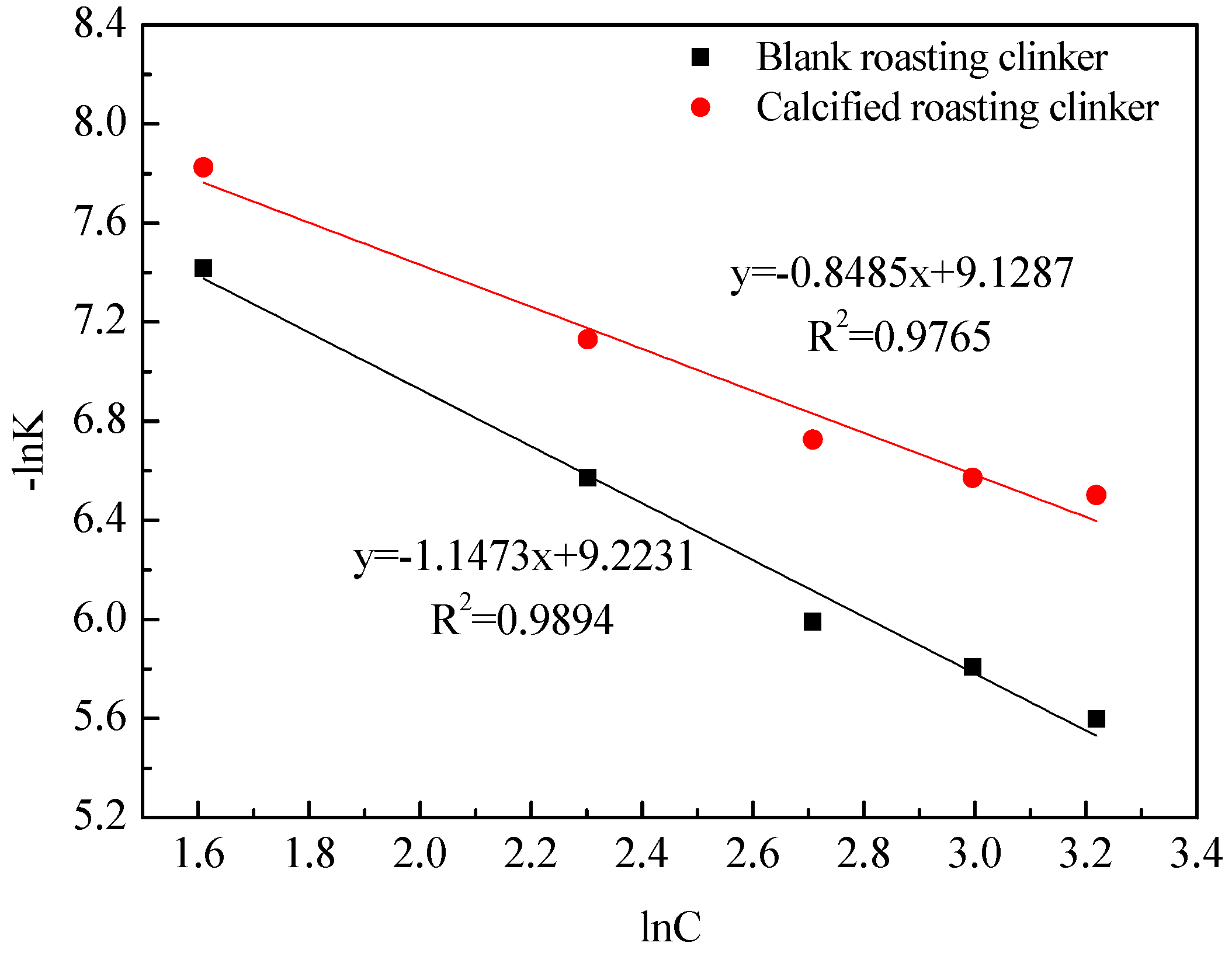
3.3. Kinetics of Vanadic Acid Leaching Process
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, F.; Zhang, Y.M.; Huang, J.; Liu, T.; Xue, N.N. Mechanism of enhancing extraction of vanadium from stone coal by roasting with MgO. Minerals 2017, 7, 33. [Google Scholar] [CrossRef]
- Aureliano, M. Decavanadate Toxicology and Pharmacological Activities: V10 or V1, Both or None? Oxid. Med. Cell. Longev. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Skyllaskazacos, M.; Cao, L.Y.; Kazacos, M.; Kausar, N.; Mousa, A. Vanadium electrolyte studies for the vanadium redox battery–A review. Cheminform 2016, 47, 1521–1543. [Google Scholar] [CrossRef]
- Zheng, Q.S.; Zhang, Y.M.; Huang, J.; Liu, T.; Xue, N.N.; Shi, Q.H. Optimal location of vanadium in muscovite and its geometrical and electronic properties by DFT calculation. Minerals 2017, 7, 32. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Keller, D.E. Chemistry, spectroscopy and the role of supported vanadium oxides in heterogeneous catalysis. Catal. Today 2003, 78, 25–46. [Google Scholar] [CrossRef]
- Hu, P.C.; Zhang, Y.M.; Liu, T.; Huang, J.; Yuan, Y.Z. Highly selective separation of vanadium over iron from stone coal by oxalic acid leaching. Ind. Eng. Chem. 2017, 45, 241–247. [Google Scholar] [CrossRef]
- Yao, Z.L.; Jin, L. Review of Vanadium Processing in China. Eng. Sci. 2005, 3, 58–62. [Google Scholar]
- Wang, M.Y.; Wang, X.W. Research status and prospect of vanadium leaching processes from stone coal. Chin. J. Rare Met. 2010, 34, 90–97. [Google Scholar]
- Xue, N.N.; Zhang, Y.M.; Liu, T.; Huang, J. Study of the dissolution behavior of muscovite in stone coal by oxygen pressure acid leaching. Metall. Mater. Trans. B 2016, 47, 1–8. [Google Scholar] [CrossRef]
- Zhu, X.B.; Zhang, Y.M.; Huang, J.; Liu, T.; Wang, Y. A kinetics study of multi-stage counter-current circulation acid leaching of vanadium from stone coal. Int. J. Miner. Process. 2012, 114, 1–6. [Google Scholar] [CrossRef]
- Zhu, J.; Guo, J.K. Research on the technology and recovery of vanadium extraction from stone coal. Mod. Min. 2011, 3, 24–27. [Google Scholar]
- Li, H.R.; Feng, Y.L.; Liang, J.L.; Luo, X.B.; Du, Z.W. Vanadium recovery from clay vanadium mineral using an acid leaching method. Rare Met. 2008, 27, 116–120. [Google Scholar] [CrossRef]
- Hu, K.L.; Liu, X.H. Review of Roasting Process of Vanadium-bearing Carbonaceous Shale. Rare Met. Cem. Carbides 2015, 1, 1. [Google Scholar]
- Bei, S.; Wang, Z.J.; Li, Q.H.; Zhang, Y.G. Review of vanadium extraction from stone coal by roasting technique with sodium chloride and calcium oxide. Chin. J. Rare Met. 2010, 2, 24. [Google Scholar]
- Jia, X.M.; Li, P.Y.; Huang, Y.; Chen, T.B.; Liu, Z.C.; Liu, H.W.; Wen, Z.Q. Calcified roast-acid leaching of vanadium from vanadium ore in Shaanxi. Hydrometall. China 2015, 34, 182–185. [Google Scholar]
- Li, C.L.; Zhou, X.Y.; Wang, H.; Zhang, T.K.; Li, J.; Ou, X.; Jiang, X.D. Effect of oxidation on vanadium extraction from stone coal with calcified roasting. J. Cent. South Univ. 2011, 41, 7–10. [Google Scholar]
- Gao, Y.M. Determining vanadium content in vanadium-bearing slag with ferrous iron volumetric method. Ferro-Alloys 2003, 3, 44–46. [Google Scholar]
- Wang, T.Y.; Xu, L.J.; Liu, C.L.; Zhang, Z.D. Calcified roasting-acid leaching process of vanadium from low-grade vanadium-containing stone coal. Chin. J. Geochem. 2014, 33, 163–167. [Google Scholar] [CrossRef]
- Sadykhov, G.B.; Goncharov, K.V.; Goncharenko, T.V.; Olyunina, T.V. Phase transformations during the oxidation of calcium-containing titanium-vanadium slags and their influence on the formation of calcium vanadates. Russ. Metall. 2013, 3, 161–168. [Google Scholar] [CrossRef]
- Hu, Y.J.; Zhang, Y.M. Effect of calcium content on vanadium extraction from black shale by sodium roasting-water leaching. Nonferrous Met. 2014, 9, 34–38. [Google Scholar]
- Abdel-Aal, E.A.; Rashad, M.M. Kinetic study on the leaching of spent nickel oxide catalyst with sulfuric acid. Hydrometallurgy 2004, 74, 189–194. [Google Scholar] [CrossRef]
- Cai, Z.L.; Zhang, Y.M.; Liu, T.; Huang, J. Mechanisms of vanadium recovery from stone coal by novel BaCO3/CaO composite additive roasting and acid leaching technology. Minerals 2016, 6, 26–39. [Google Scholar] [CrossRef]
- Li, M.T.; Wei, C.; Qiu, S.; Zhou, X.J.; Li, C.X.; Deng, Z.G. Kinetics of vanadium dissolution from black shale in pressure acid leaching. Hydrometallurgy 2010, 104, 193–200. [Google Scholar] [CrossRef]
- Ju, Z.J.; Wang, C.Y.; Yin, F. Dissolution kinetics of vanadium from black shale by activated sulfuric acid leaching in atmosphere pressure. Int. J. Miner. Process. 2015, 138, 1–5. [Google Scholar] [CrossRef]
- Espiari, S.; Rashchi, F.; Sadrnezhaad, S.K. Hydrometallurgical treatment of tailings with high zinc content. Hydrometallurgy 2006, 82, 54–62. [Google Scholar] [CrossRef]







| Element | SiO2 | CaO | Al2O3 | V2O5 | Fe2O3 | K2O | Na2O | MgO | C | S |
|---|---|---|---|---|---|---|---|---|---|---|
| Content | 57.37 | 5.82 | 6.46 | 0.77 | 3.69 | 2.24 | 0.45 | 1.47 | 13.32 | 1.75 |
| Minerals | V2O5 | SiO2 | Al2O3 | FeO | MgO | CaO | K2O |
|---|---|---|---|---|---|---|---|
| Muscovite | 3.48 | 51.08 | 27.22 | 0.23 | 4.53 | 0.02 | 9.52 |
| Pyrite | 0 | 0.06 | 0 | 59.12 | 0 | 0 | 0 |
| Calcite | 0 | 0 | 0 | 0 | 1.10 | 65.94 | 0 |
| Quartz | 0 | 98.35 | 0 | 0 | 0 | 0 | 0 |
| Element | SiO2 | Al2O3 | Fe2O3 | CaO | V2O5 | S | K2O | Na2O |
|---|---|---|---|---|---|---|---|---|
| Raw ore | 57.37 | 6.46 | 3.69 | 5.82 | 0.77 | 1.75 | 2.24 | 0.45 |
| Blank roasted clinker | 68.92 | 7.78 | 4.37 | 7.17 | 0.9 | 0.94 | 2.20 | 0.40 |
| Calcified roasted clinker | 64.59 | 7.03 | 4.56 | 14.49 | 0.79 | 1.94 | 1.91 | 0.35 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Liu, T.; Zhang, Y.; Huang, J. Effect of CaF2/CaO Composite Additive on Roasting of Vanadium-Bearing Stone Coal and Acid Leaching Kinetics. Minerals 2017, 7, 43. https://doi.org/10.3390/min7030043
Wang B, Liu T, Zhang Y, Huang J. Effect of CaF2/CaO Composite Additive on Roasting of Vanadium-Bearing Stone Coal and Acid Leaching Kinetics. Minerals. 2017; 7(3):43. https://doi.org/10.3390/min7030043
Chicago/Turabian StyleWang, Bo, Tao Liu, Yimin Zhang, and Jing Huang. 2017. "Effect of CaF2/CaO Composite Additive on Roasting of Vanadium-Bearing Stone Coal and Acid Leaching Kinetics" Minerals 7, no. 3: 43. https://doi.org/10.3390/min7030043





