Raman Investigations to Identify Corallium rubrum in Iron Age Jewelry and Ornaments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Microscopy
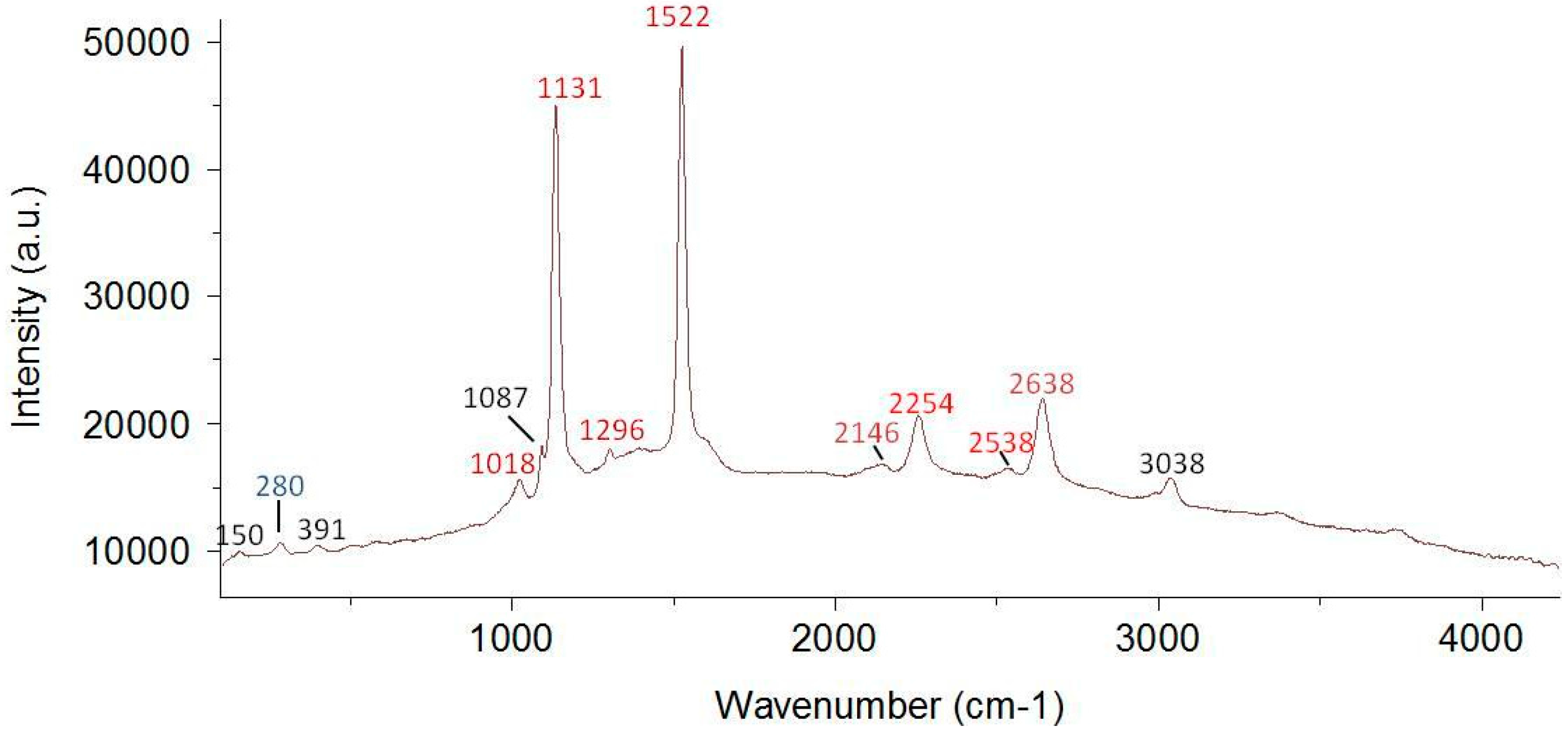
2.2.2. Raman Spectroscopy
3. Results
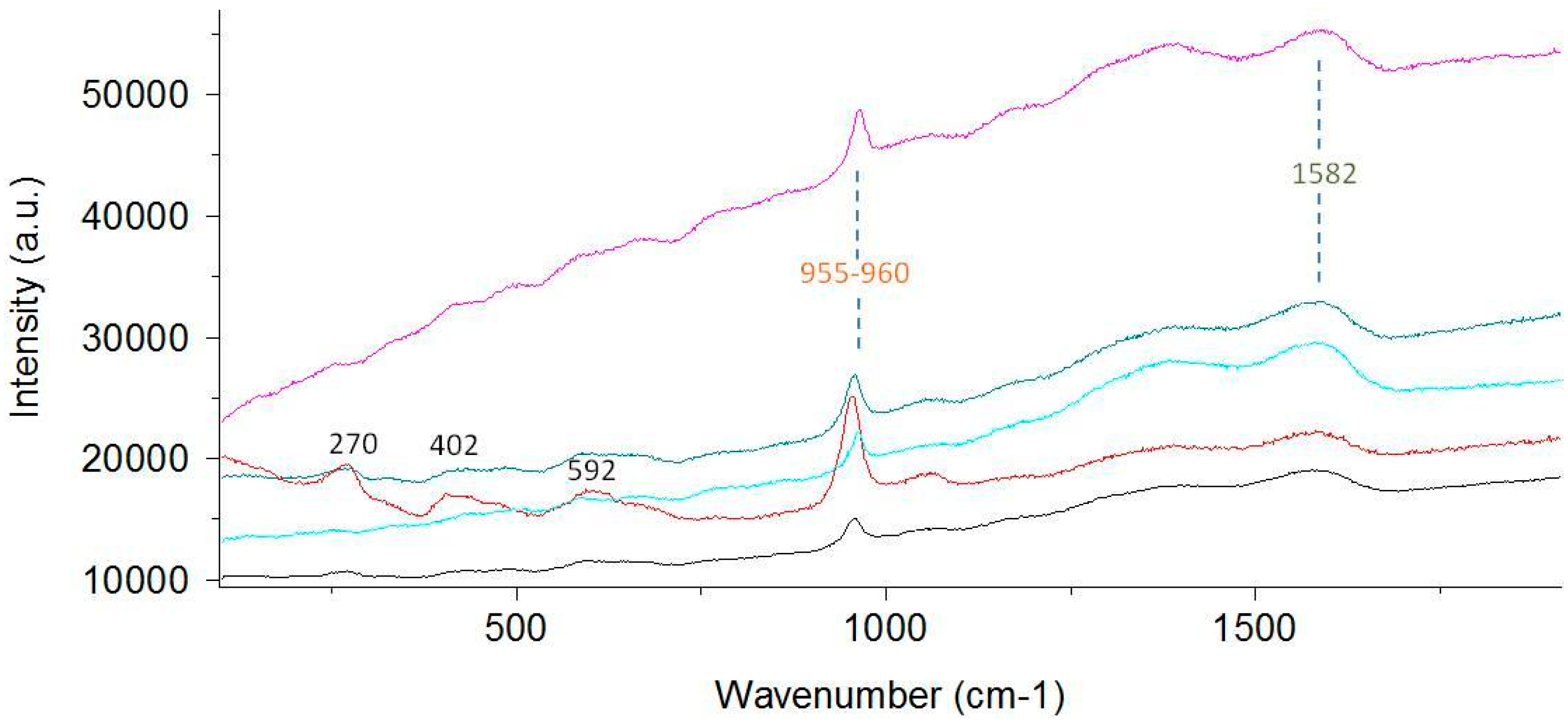
3.1. Optical Differences between the Surface Structures of Ancient and Recent Precious Corals
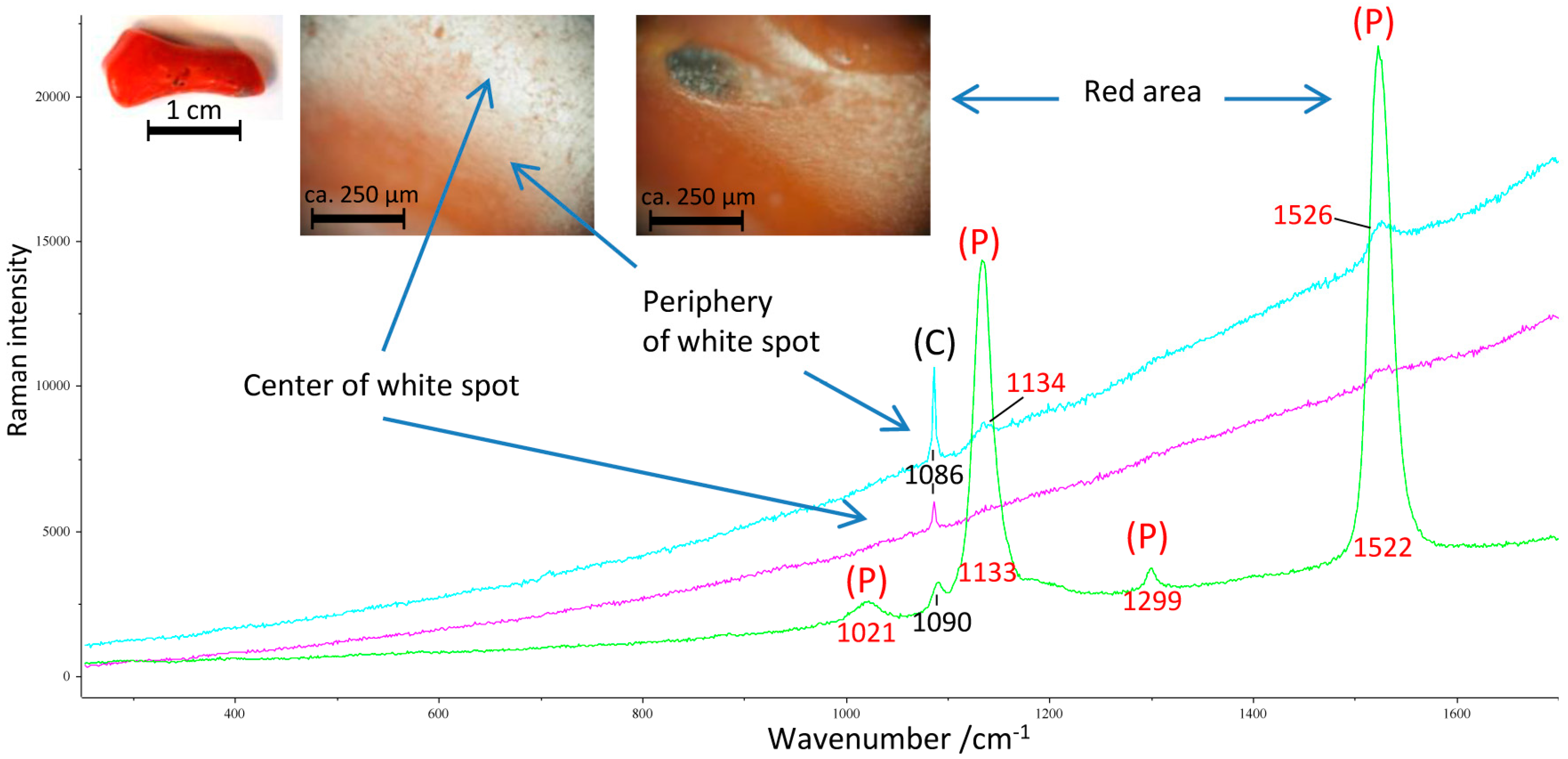
3.2. Remains of the Former Coloring
3.3. Identification of Ancient Corals: Case Studies
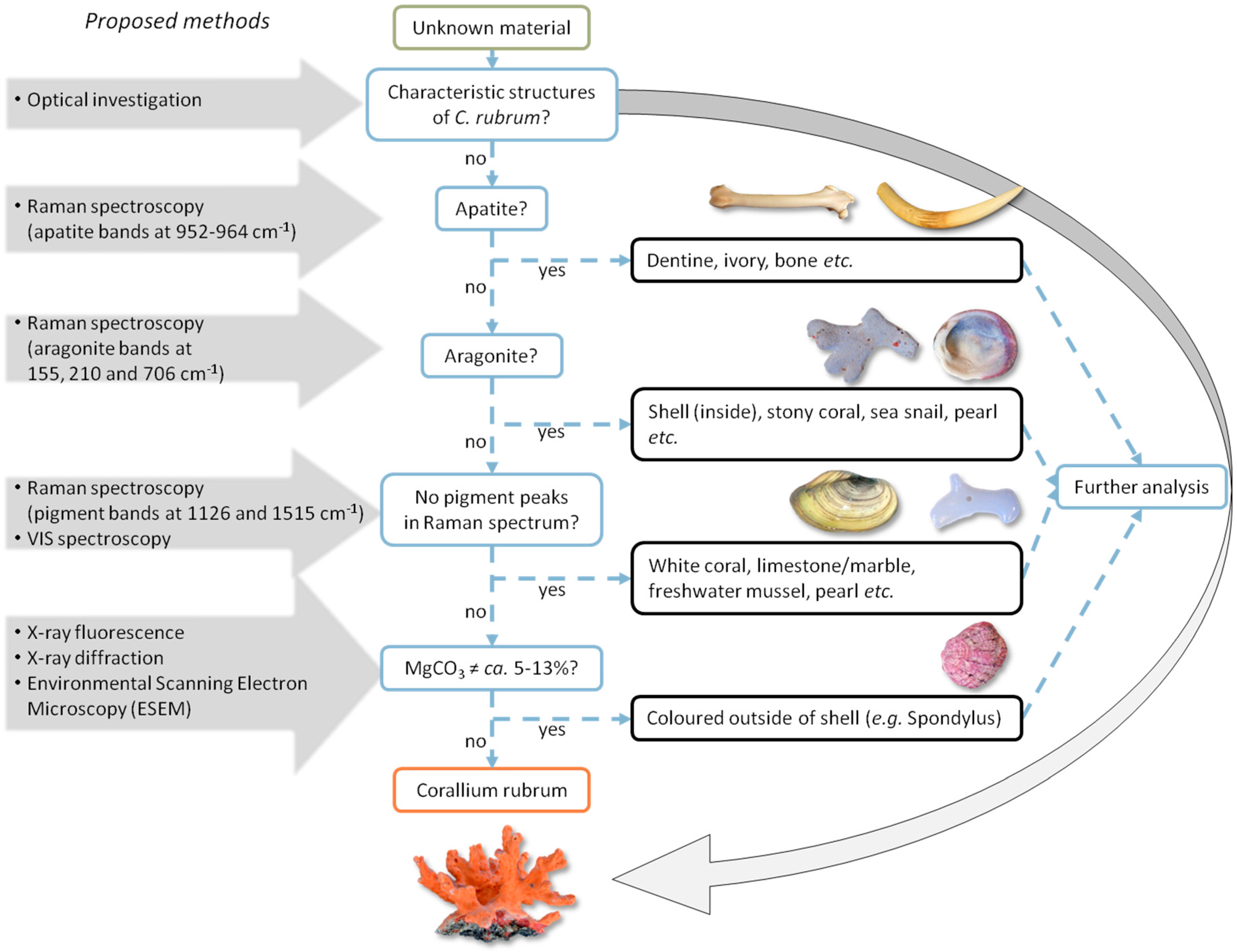
3.4. Identification Methodology
3.5. Summarized Results
4. Discussion
4.1. Challenges of Optical Identification
4.2. Possibilities and Limitations of Raman Spectroscopy for the Identification of C. rubrum
4.3. Hypotheses for the Loss of Color of Archaeological Corals
4.4. Archaeological Implications
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liverino, B. Red Coral; Jewel of the Sea: Bologna, Italy, 1989. [Google Scholar]
- Skeates, R. Mediterranean coral: Its use and exchange in and around the Alpine region during the later neolithic and copper age. Oxf. J. Archaeol. 1993, 108, 281–292. [Google Scholar] [CrossRef]
- Henn, U. Korallen im Edelstein-und Schmuckhandel. Zeitschr. Dt. Gemmol. Ges. 2006, 55, 77–104. (In German) [Google Scholar]
- Tescione, G. Il Corallo Nella Storia e Nell’ Arte; Montanino: Neapel, Italy, 1965. (In Italian) [Google Scholar]
- Galasso, M. Korallenfischerei in Sardinien. Archäologische Zeugnisse und Dokumente von der Vorgeschichte bis heute. Skyllis 2000, 3, 80–113. (In German) [Google Scholar]
- Tsounis, G.; Rossi, S.; Gili, J.-M.; Arntz, W.E. Red coral fishery at the Costa Brava (NW Mediterranean): Case study of an overharvested precious Coral. Ecosystems 2007, 10, 975–986. [Google Scholar] [CrossRef]
- Proceedings of the International Workshop on Red Coral Science, Management, and Trade: Lessons from the Mediterranean; NOAA Technical Memorandum CRCP-13; Bussoletti, E.; Cottingham, D.; Bruckner, A.W.; Roberts, G.G.; Sandulli, R. (Eds.) National Oceanic and Atmospheric Administration: Silver Spring, MD, USA, 2010.
- Rolandi, V. Gems from the animal kingdom: A gemmological study of materials from cnidaria. Rendiconti 1981, 37, 703–718. [Google Scholar]
- Tsounis, G.; Rossi, S.; Grigg, R.; Santangelo, G.; Bramanti, L.; Gili, J.-M. The exploitation and conservation of precious corals. Oceanogr. Mar. Biol. 2010, 48, 161–212. [Google Scholar]
- Lacaze-Duthiers, H. Histoire Naturelle du Corail: Organisation, Reproduction, Pèche en Algerie, Industrie et Commerce; J.B. Ballière et Fils Publisher: Paris, France, 1864. (In French) [Google Scholar]
- Smith, C.P.; McClure, S.F.; Eaton-Magaña, S.; Kondo, D.M. Pink-to-red coral: A guide to determining origin of color. Gems Gemol. 2007, 43, 4–15. [Google Scholar] [CrossRef]
- Dauphin, Y. Mineralizing matrices in the skeletal axes of two Corallium species (Alcyonacea). Comp. Biochem. Physiol. A 2006, 145, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Vielzeuf, D.; Garrabou, J.; Barronet, A.; Grauby, O.; Marschal, C. Nano to macroscale biomineral architecture of red coral (Corallium rubrum). Am. Miner. 2008, 93, 1799–1815. [Google Scholar] [CrossRef]
- Harmelin, J.-G. Le corail rouge de Méditerranée: Quelques aspects de sa biologie et de son écologie. In Corallo di Ieri, Corallo di Oggi: Atti del Convegno, Ravello, Villa Rufolo, 13–15 Dicembre 1996; Morel, J.-P., Rondi-Costanzo, C., Ugolini, D., Eds.; Edipuglia: Bari, Italy, 2000; pp. 11–20. (In French) [Google Scholar]
- Allemand, D.; Bénazet-Tambutté, S. Dynamics of calcification in the Mediterranean red coral, Corallium rubrum (Linnaeus) (Cnidaria, Octocorallia). J. Exp. Zool. 1996, 276, 270–278. [Google Scholar] [CrossRef]
- Vielzeuf, D.; Garrabou, J.; Gagnon, A.; Ricolleau, A.; Adkins, J.; Günther, D.; Hametner, K.; Devidal, J.-L.; Reusser, E.; Perrin, J.; et al. Distribution of sulphur and magnesium in the red coral. Chem. Geol. 2013, 355, 13–27. [Google Scholar] [CrossRef]
- Perrin, J.; Vielzeuf, D.; Ricolleau, A.; Dallaporta, H.; Valton, S.; Floquet, N. Block-by-block and layer-by-layer growth modes in coral skeletons. Am. Miner. 2015, 100, 681–695. [Google Scholar] [CrossRef]
- Urmos, J.; Sharma, S.K.; Mackenzie, F.T. Characterization of some biogenic carbonates with Raman spectroscopy. Am. Miner. 1991, 76, 641–646. [Google Scholar]
- Parker, J.E.; Thompson, S.P.; Lennie, A.R.; Potter, J.; Tang, C.C. A study of the aragonite-calcite transformation using Raman spectroscopy, synchrotron powder diffraction and scanning electron microscopy. CrystEngComm 2010, 12, 1590–1599. [Google Scholar] [CrossRef]
- Merlin, J.C.; Dele, M.L. Étude par spectroscopie Raman de résonance de la pigmentation des squelettes calcaires de certains coraux. Bull. Soc. Zool. Fr. 1983, 108, 289–301. (In French) [Google Scholar]
- Merlin, J.C.; Delé-Dubois, M.L. Resonance Raman characterization of polyacetylenic pigments found in the calcareous skeleton. Comp. Biochem. Physiol. B 1986, 84, 97–103. [Google Scholar]
- Cvejic, J.; Tambutté, S.; Lotto, S.; Mikov, M.; Slacanin, I.; Allemand, D. Determination of canthaxanthin in the red coral (Corallium rubrum) from Marseille by HPLC combined with UV and MS detection. Mar. Biol. 2007, 152, 855–862. [Google Scholar] [CrossRef]
- Karampelas, S. Determination by Raman scattering of the nature of pigments in cultured freshwater pearls from the mollusk Hyriopsis cumingi. J. Raman Spectrosc. 2007, 38, 217–230. [Google Scholar] [CrossRef]
- Barnard, W.; de Waal, D. Raman investigation of pigmentary molecules in the molluscan biogenic matrix. J. Raman Spectrosc. 2006, 37, 342–352. [Google Scholar] [CrossRef]
- Fritsch, E.; Karampelas, S. Comment on: Determination of canthaxanthin in the red coral (Corallium rubrum) from Marseille by HPLC combined with UV and MS detection (Cvejic, et al. Mar. Biol. 2007, 152, 855–862). Mar. Biol. 2007, 152, 929–930. [Google Scholar] [CrossRef]
- Kupka, T.; Lin, H.M.; Stobiński, L.; Chen, C.-H.; Liou, W.-J.; Wrzalik, R.; Flisak, Z. Experimental and theoretical studies on corals I: Toward understandig the origin of color in precious red corals from Raman and IR spectroscopies and DFT calculations. J. Raman Spectrosc. 2010, 41, 651–658. [Google Scholar] [CrossRef]
- Karampelas, S.; Fritsch, E.; Rondeau, B.; Andouche, A.; Métivier, B. Identification of the endangered pink-to-red stylaster corals by Raman spectroscopy. Gems Gemol. 2009, 49, 48–52. [Google Scholar] [CrossRef]
- Bergamonti, L.; Bersani, D.; Mantovan, S.; Lottici, P.P. Micro-Raman investigation of pigments and carbonate phases in corals and molluscan shells. Eur. J. Mineral. 2013, 25, 845–853. [Google Scholar] [CrossRef]
- Soldati, A.L.; Jacob, D.E.; Wehrmeister, U.; Häger, T.; Hofmeister, W. Micro-Raman spectroscopy of pigments contained in different calcium carbonate polymorphs from freshwater cultured pearls. J. Raman Spectrosc. 2008, 39, 525–536. [Google Scholar] [CrossRef]
- Koenig, M.-P. L’emploi du corail dans la parure hallstattienne d’alsace. Cah. Alsac. Arch. 1987, 30, 91–101. (In French) [Google Scholar]
- Müller, F. Überraschendes unter der Patina einer keltischen Fibel aus Münsingen. Arch. Schweiz 1993, 16, 60–64. (In German) [Google Scholar]
- Weiß, M.; von Zelewski, B. Untersuchungen zu einigen Ziereinlagen. In Waldalgesheim. Das Grab Einer Keltischen Fürstin; Joachim, H.-E., Ed.; Rheinland-Verlag GmbH: Bonn, Germany, 1995; pp. 148–158. (In German) [Google Scholar]
- Schüler, T. Röntgendiffraktometrische Untersuchungen an drei latènezeitlichen Schmuckeinlagen. Alt Thüringen 1997, 31, 57–63. (In German) [Google Scholar]
- Bente, K.; König, A.; Dehn, F.; Krüger, P.; Münster, T.; Berthold, C.; Wirth, R. Vergleichende computertomografische und elektronenmikroskopische Studien zu eisenzeitlicher Korallenzier. Metalla Sonderbd. 2015, 7, 59–61. (In German) [Google Scholar]
- Schrickel, M.; Bente, K.; Berthold, C.; Grill, W.; Sarge, C.; Hoppe, T. Vergleichende archäometrische Untersuchungen an Fibeln mit Korallenzier: Fragestellungen und methodischer Überblick. In Produktion—Distribution–Ökonomie: Siedlungs und Wirtschaftsmuster der Latènezeit Akten des Internationalen Kolloquiums in Otzenhausen, 28–30 Oktober 2011; Hornung, S., Ed.; Dr. Rudolf Habelt GmbH: Bonn, Germany, 2014. (In German) [Google Scholar]
- Schrickel, M.; Bente, K.; Franz, A.; Sarge, C. Vergleichende radiographische und 3D-röntgentomographische Untersuchungen an Fibeln mit Korallenzier. In Proceedings of the Handreichung zu Einem Poster im Rahmen des Internationalen Kolloquiums “Produktion–Distribution–Ökonomie”, Otzenhausen, Lkr. St. Wendel, Germany, 28–30 October 2011. (In German)
- Schrickel, M.; Bente, K.; Berthold, C.; Grill, W.; Tescher, U.; Scharf, O.; Hoppe, T. Rote Korallen? Archäometrische Studien an “Mitteldeutschen Korallenfibeln”. In Proceedings of the Poster Präsentation auf der internat. Tagung “Rot. Die Archäologie bekennt Farbe”, Halle, Germany, 4–5 October 2012. (In German)
- Schrickel, M.; Bente, K.; Fleischer, F.; Franz, A. Importation ou imitation du corail à la fin de l’âge du Fer? Première approche par analyses du matériau. In L’âge du Fer en Aquitaine et Sur Ses Marges. Mobilité des Hommes, Diffusion des Idées, Circulation des Biens dans L’espace Européen à L’âge du Fer, Actes du 35e Colloque International de l’AFEAF, Bordeaux 2011; Colin, A., Verdin, F., Eds.; Aquitania: Pessac, France, 2013; pp. 753–759. (In French) [Google Scholar]
- Champion, S. Coral in Europe: Commerce and Celtic Ornament. In Celtic Art in Ancient Europe, Five Protohistoric Centuries L’Art Celtique en Europe Protohistorique: Débuts, Dévelopements, Styles, Techniques; Duval, P.-M., Hawkes, C., Eds.; Seminar Press: London, UK, 1976; pp. 29–40. [Google Scholar]
- Champion, S. The Use of Coral and Other Substances to Decorate Metal Work in Central and Western Europe in the Middle and Later Centuries of the First Millenium B.C. Ph.D. Thesis, Oxford University, Oxford, UK, 1977. [Google Scholar]
- Perrin, F. Technologie et économie du corail de Méditerranée Corallium rubrum L. en Gaule du VIe au Ier siècle avant J.-C. University Paris-Sorbonne, Paris, France, 1996. [Google Scholar]
- Morel, J.-P. Les conclusions d’un archéologue. In Corallo di ieri, corallo di oggi. Atti del convegno, Ravello, Villa Rufolo, 13–15 Dicembre 1996; Morel, J.-P., Rondi-Costanzo, C., Ugolini, D., Eds.; Edipuglia: Bari, Italy, 2000; pp. 301–303. (In French) [Google Scholar]
- Fürst, S. Die Südwestdeutschen Korallenfunde der Hallstatt- und Frühlatènezeit im Spiegel Raman-Spektroskopischer Analysen. Johannes Gutenberg University, Mainz, Germany, 2010. [Google Scholar]
- Fürst, S. Korallen am Übergang zur Frühlatènezeit: Zum wissenschaftlichen Potenzial eines problematischen Schmuckmaterials. In Produktion–Distribution–Ökonomie: Siedlungs- und Wirtschaftsmuster der Latènezeit Akten des Internationalen Kolloquiums in Otzenhausen, 28–30 Oktober 2011; Hornung, S., Ed.; Dr. Rudolf Habelt GmbH: Bonn, Germany, 2014; pp. 41–66. (In German) [Google Scholar]
- Debreuil, J.; Tambutté, S.; Zoccola, D.; Segonds, N.; Techer, N.; Marschal, C.; Allemand, D.; Kosuge, S.; Tambutté, É. Specific organic matrix characteristics in skeletons of Corallium species. Mar. Biol. 2011, 158, 2765–2774. [Google Scholar] [CrossRef]
- Tsounis, G. Demography, Reproductive Biology and Trophic Ecology of Red Coral (Corallium rubrum L.) at the Costa Brava (NW Mediterranean): Ecological Data as a Tool for Management. Available online: http://nbn-resolving.de/urn/resolver.pl?urn=urn:nbn:de:gbv:46-diss000012465 (accessed on 13 July 2009).
- Boavida, J.; Paulo, D.; Aurelle, D.; Arnaud-Haond, S.; Marschal, C.; Reed, J.; Gonçalves, J.M.S.; Serrão, E.A.; Pronzato, R. A well-kept treasure at depth: precious red coral rediscovered in atlantic deep coral gardens (SW Portugal) after 300 years. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Rondi-Costanzo, C.; Ugolini, D. Le corail dans le bassin nord-occidental de la Mediterranée entre le VIe et le IIe s. av. J.-C. In Corallo di ieri, Corallo di Oggi, Atti del Convegno, Ravello, Villa Rufolo, 13–15 Dicembre 1996; Morel, J.-P., Rondi-Costanzo, C., Ugolini, D., Eds.; Edipuglia: Bari, Italy, 2000; pp. 177–191. (In French) [Google Scholar]
- Pliny the Elder. The Natural History. XXXII. Available online: http://www.perseus.tufts.edu/hopper/text?doc=Plin.+Nat.+toc (accessed on 9 June 2016).
- Bischoff, W.D.; Sharma, S.K.; Mackenzie, F.T. Carbonate ion disorder in synthetic and biogenic magnesian calcites: A Raman spectral study. Am. Mineral. 1985, 70, 581–589. [Google Scholar]
- Edwards Howell, G.M.; Villar Susana, E.J.; Jehlicka, J.; Munshi, T. FT-Raman spectroscopic study of calcium-rich and magnesium-rich carbonate minerals. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Hedegaard, C.; Bardeau, J.-F.; Chateigner, D. Molluscan shell pigments: An in situ resonance Raman study. J. Molluscan Stud. 2006, 72, 157. [Google Scholar] [CrossRef]
- Marschal, C.; Garrabou, J.; Harmelin, J.G.; Pichon, M. A new method for measuring growth and age in the precious red coral Corallium rubrum (L.). Coral Reefs 2004, 23, 423–432. [Google Scholar] [CrossRef]
- Fürst, S.; Müller, K.; Paris, C.; Bellot-Gurlet, L.; Pare, C.F.E.; Reiche, I. Neue Identifizierungsstrategie eisenzeitlicher Korallen anhand optischer und Raman-spektroskopischer Charakteristiken. Berl. Beitr. Archäom. Kunsttechnol. Konservierungswissenschaft 2014, 22, 25–35. (In German) [Google Scholar]
- Cuif, J.-P.; Dauphin, Y.; Sorauf, J.E. Biominerals and Fossils through Time; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- McGregor, H.V.; Gagan, M.K. Diagenesis and geochemistry of Porites corals from Papua New Guinea: Implications for paleoclimate reconstruction. Geochim. Cosmochim. Acta 2003, 67, 2147–2156. [Google Scholar] [CrossRef]
- Müller, K.; Chadefaux, C.; Thomas, N.; Reiche, I. Microbial attack of archaeological bones versus high concentrations of heavy metals in the burial environment. A case study of animal bones from a mediaeval copper workshop in Paris. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 310, 39–51. [Google Scholar] [CrossRef]
- Kimmig, W. Les tertres funéraires préhistoriques dans la forêt de Haguenau: Rück- und Ausblick. Prähist. Zeitschr. 1979, 54, 47–176. (In French) [Google Scholar] [CrossRef]
- Wigg, A. Koralle und Email als Einlage bei Metallarbeiten. In Hundert Meisterwerke Keltischer Kunst: Schmuck und Kunsthandwerk Zwischen Rhein und Mosel; Cordie-Hackenberg, R., Born, H., Eds.; Rheinisches Landesmuseum: Trier, Germany, 1992; pp. 207–209. (In German) [Google Scholar]
- Koenig, M.-P. Haguenau: trois ensembles avec or et corail. In Trésors Celtes et Gaulois: Le Rhin Supérieur Entre 800 et 50 Avant J.-C. Exposition Présentée au Musée d’Unterlinden du 16 Mars au 2 Juin 1996; Plouin, S., Ed.; Direction Régionale des Affaires Culturelles d’Alsace: Colmar, France, 1996; pp. 88–93. (In French) [Google Scholar]
- Stöllner, T. “Verborgene Güter”-Rohstoffe und Spezereien als Fernhandelsgut in der Späthallstatt- und Frühlatènezeit. In Die Hydria von Grächwil, Zur Funktion und Rezeption Mediterraner Importe in Mitteleuropa im 6. und 5. Jahrhundert v. Chr. Internationales Kolloquium, 12–13 Oktober 2001; Guggisberg, M.A., Ed.; Bernisches Historisches Museum: Bern, Switzerland, 2004. (In German) [Google Scholar]
- Bersani, D.; Lottici, P.P. Applications of Raman spectroscopy to gemology. Anal. Bioanal. Chem. 2010, 397, 2631–2646. [Google Scholar] [CrossRef] [PubMed]
- Reiche, I.; Pages-Camagna, S.; Lambacher, L. In situ Raman spectroscopic investigations of the adorning gemstones on the reliquary Heinrich’s Cross from the treasury of Basel Cathedral. J Raman Spectrosc. Spec. Issue Raman Spectrosc. Art Archaeol. 2004, 35, 719–725. [Google Scholar]
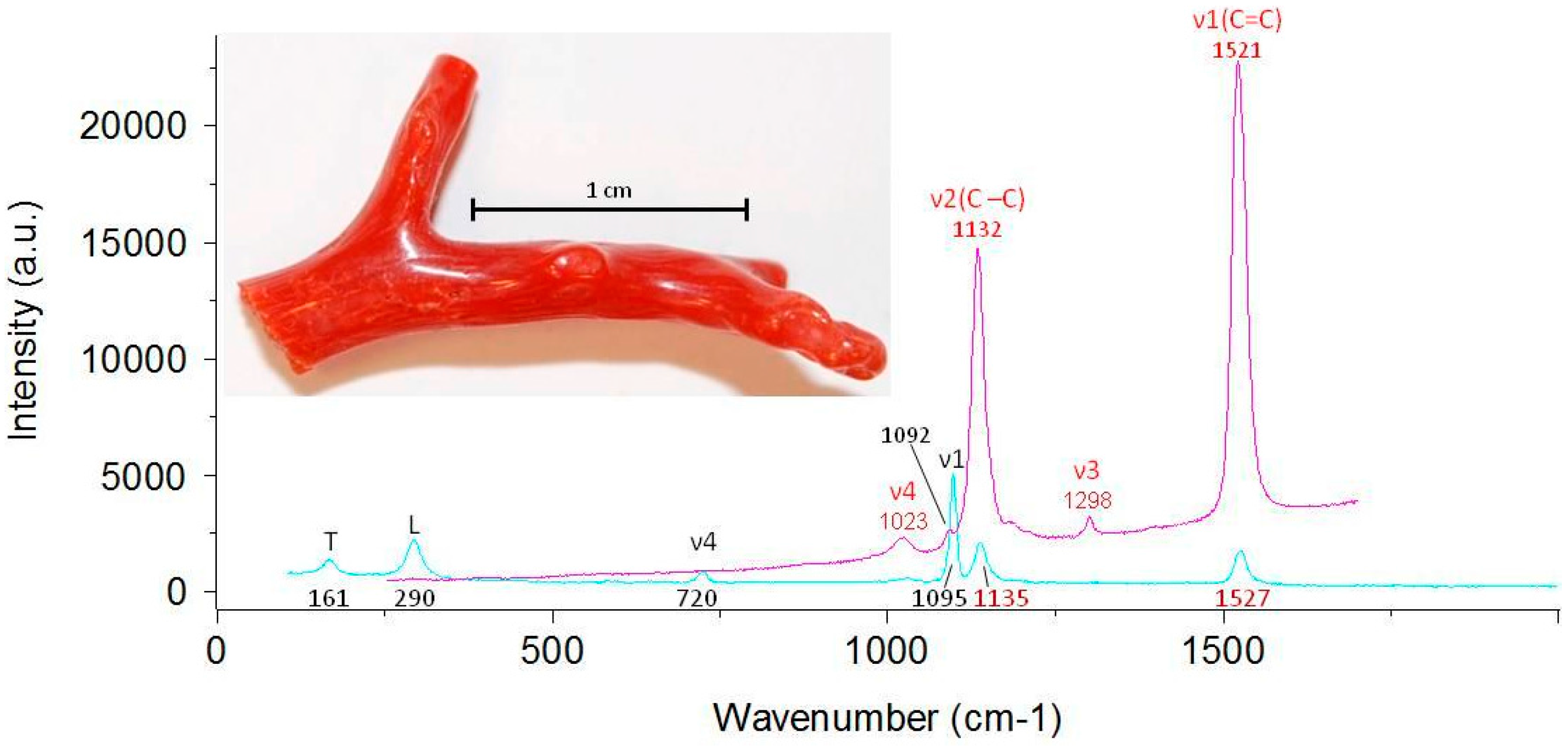





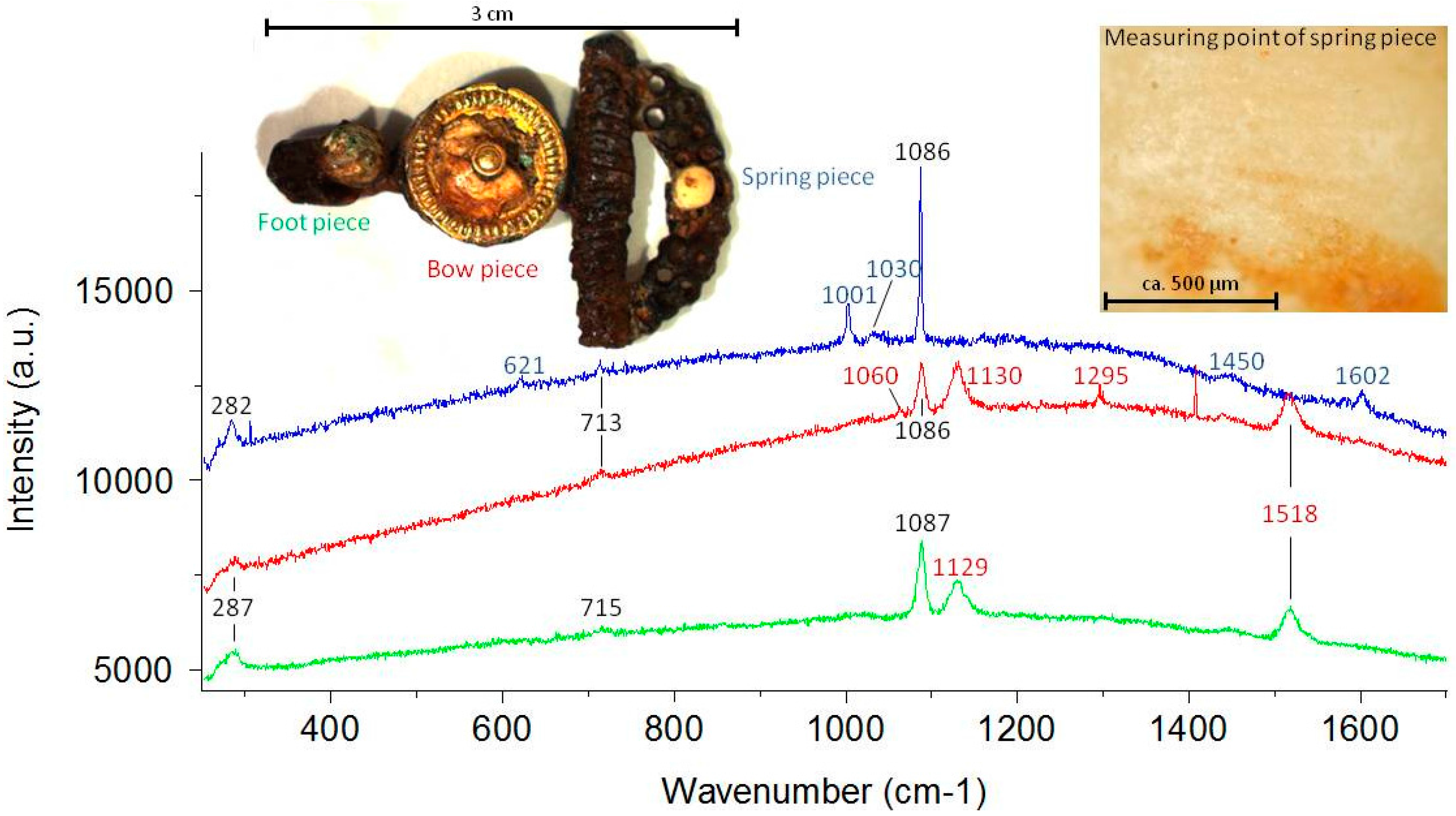


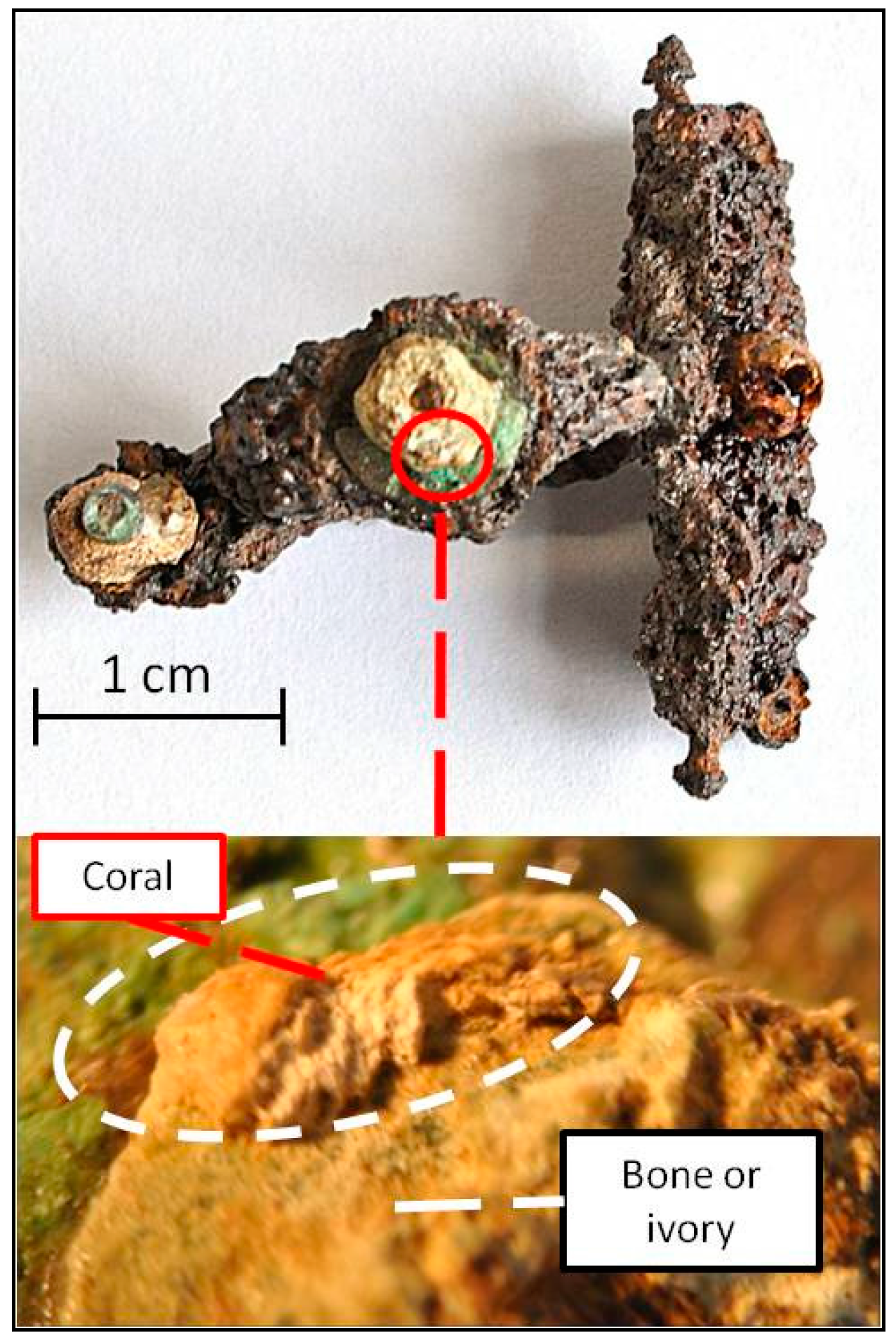


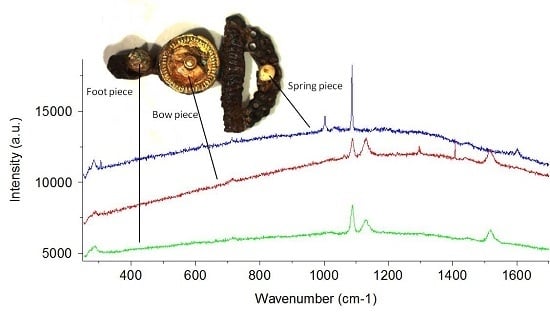
| Species * | Origin * | Analyzed Objects |
|---|---|---|
| Corals (Anthozoa) | ||
| Corallium rubrum (red precious coral) | French coast (Marseille), Greece, Italy (Sardinia, Sicily, Tyrrhenian Sea), Malta, Spain (La Escala), Portugal (Algarve) | 19 |
| Corallium rubrum (Sciacca coral) | Italy (Sicily, Sciacca) | 1 |
| Corallium sp., deep sea | Portugal (Azores, 1557 m depth) | 1 |
| Corallium sp./konojoi(?) (white coral) | Japan | 1 |
| Corallium sp. (white coral) | Unknown | 1 |
| Corallium sp., Pleistocene | Italy (Sicily, Augusta) | 1 |
| Corallium sp., Miocene, Tortonian 11.6–7.2 million years | Spain (Mazarrón) | 1 |
| Corallium secundum (red Momo coral) | Taiwan | 1 |
| Corallium elatius (porous Mushi coral) | Philippines, Nepal | 2 |
| Tubipora musica (organ pipe coral) | Unknown, Red Sea or Indo-Pacific | 1 |
| Scleractinia | Unknown | 2 |
| Shells (Bivalvia) | ||
| Spondylus gaederopus (thorny/spiny oysters) | Greece (Morea), Italy (Trieste, Sardinia); Egypt (Red Sea, Hurghada) 1 | 5 |
| Spondylus barbatus (thorny/spiny oysters) | Indo-Pacific | 1 |
| Spondylus sp. (thorny/spiny oysters) | United Arab Emirates | 1 |
| Sea Snails (Gastropoda) | ||
| Cypraecassis testiculus (reticulated cowrie helmet) | Dominican Republic, Cape Verde (Sao Vicente) 1 | 2 |
| Semicassis saburon (helmet/bonnet snail) | Italy (Naples) | 1 |
| Number of Pieces 1 | Previous Material | Status | New Material |
|---|---|---|---|
| 169 | Red coral | approved | Red coral |
| Proportion of approved materials: 64.3% | |||
| 5 | Previously unspecified | clarification | Red coral |
| 1 | Amber or coral | clarification | Red coral |
| 1 | Not specified | clarification | Enamel |
| 4 | Amber or coral | clarification | Not coral, possibly amber |
| Proportion of clarifications: 4.1% | |||
| 5 | Red coral | partial specification of substance | I.g. some kind of carbonate |
| Proportion of partial specifications 2: 1.9% | |||
| 1 | “White paste” | different material | Red coral |
| 2 | Bone | different material | Red coral |
| 2 | Bronze | different material | Red coral |
| 7 | Red coral | different material | White coral or shell |
| 1 | Amber | different material | White coral or shell |
| 3 | Red coral | different material | Ivory (or bone) |
| 4 | Red coral | different material | Enamel |
| 7 | Red coral | different material | Unidentified material |
| Proportion of changed material interpretations: 10.3% | |||
| 45 | Red coral | No results due to patina | Unknown |
| Proportion of pieces without result due to patina 3: 17.1% | |||
| 6 | Red coral, not specified, bone | No signal, only fluorescence | Unknown |
| Proportion of pieces without result due to fluorescence: 2.3% | |||
| Total: 263 | |||
| Proportion of red coral after investigations: 68.4% | |||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fürst, S.; Müller, K.; Gianni, L.; Paris, C.; Bellot-Gurlet, L.; Pare, C.F.E.; Reiche, I. Raman Investigations to Identify Corallium rubrum in Iron Age Jewelry and Ornaments. Minerals 2016, 6, 56. https://doi.org/10.3390/min6020056
Fürst S, Müller K, Gianni L, Paris C, Bellot-Gurlet L, Pare CFE, Reiche I. Raman Investigations to Identify Corallium rubrum in Iron Age Jewelry and Ornaments. Minerals. 2016; 6(2):56. https://doi.org/10.3390/min6020056
Chicago/Turabian StyleFürst, Sebastian, Katharina Müller, Liliana Gianni, Céline Paris, Ludovic Bellot-Gurlet, Christopher F.E. Pare, and Ina Reiche. 2016. "Raman Investigations to Identify Corallium rubrum in Iron Age Jewelry and Ornaments" Minerals 6, no. 2: 56. https://doi.org/10.3390/min6020056