The Crystal and Molecular Structure of (2Z)-2-[3-(4-Methoxybenzoyl)-4,4-dimethyl-1,2-oxazolidin-2-ylidene]-1-(4-methoxyphenyl)ethanone
Abstract
: The crystal and molecular structure of the title compound, viz., (2Z)-2-[3-(4-methoxybenzoyl)-4,4-dimethyl-1,2,-oxazolidin-2-ylidene]-1-(4-methoxyphenyl)ethanone (4), is reported. Compound 4 crystallises from toluene/hexanes mixtures in the P21/c space group with eight molecules in the unit cell. The unit cell parameters are: a = 20.9410(11) Å, b = 8.7523(5) Å, c = 21.2291(9) Å; β = 93.529(3)° and V = 3883.5(3) Å3. There are two structurally distinct molecules of 4 found in the solid-state which differ primarily in terms of the observed torsion angles and the overall intramolecular spacing between the aromatic groups. Bond lengths and angles of this tertiary amide are otherwise typical. This is the first crystallographically characterised example of this class of oxazoline precursors, which have previously found application in the syntheses of other heterocycles. Density functional theory (b3lyp 6-311++G** level of sophistication) has likewise been applied to estimate the gas-phase structure of the title compound.1. Introduction
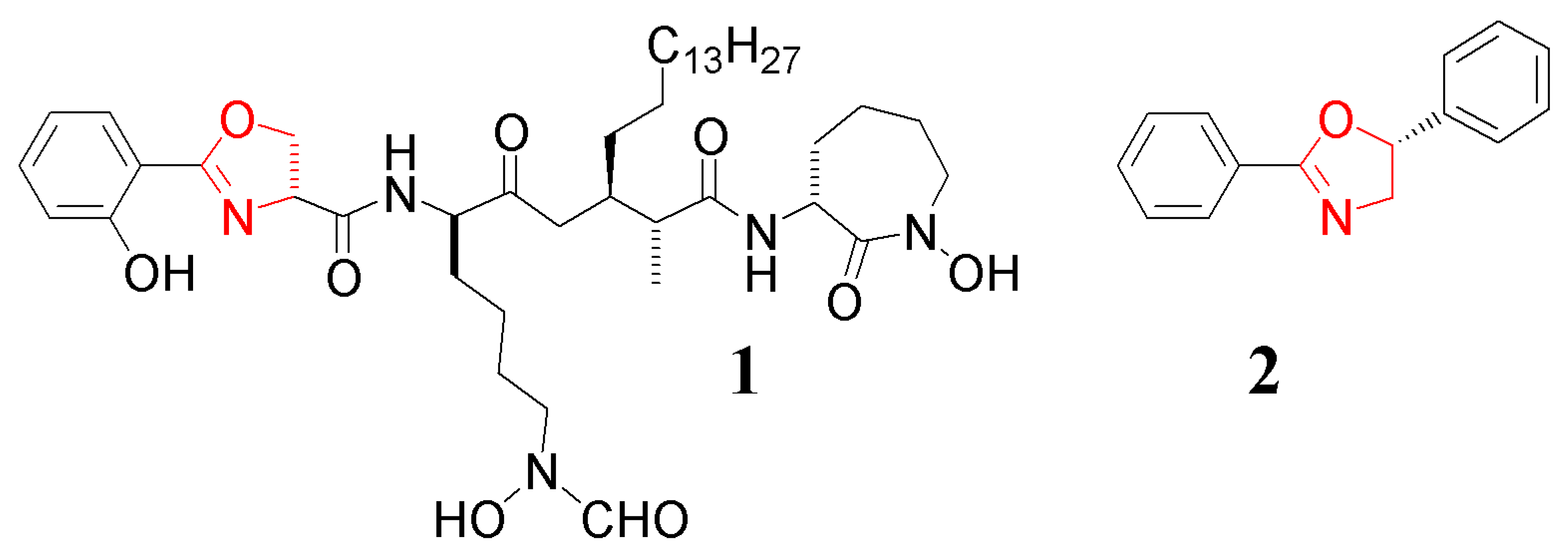
The 2-oxazolines, a sub-class of the azoles, consist of a 5-membered ring system consisting of one O- and one N-atom separated by a single carbon; these latter two atoms being formally sp2 hybridised [1,2]. The group itself represents an important heterocyclic functionality as these molecules are routinely used as ligands in coordination chemistry [3-6] and catalysis [7-10], as directing groups in chemical modification strategies [11-14] and as a protecting group for carboxylic acids [15-18]. The ring is also found in a number of natural products, many of which have been the subject of total synthesis. Examples of these include complex molecules such as Basilibactin A down to very simple small molecules such as Oxytriphine (1 and 2, respectively: Figure 1) [19-21].
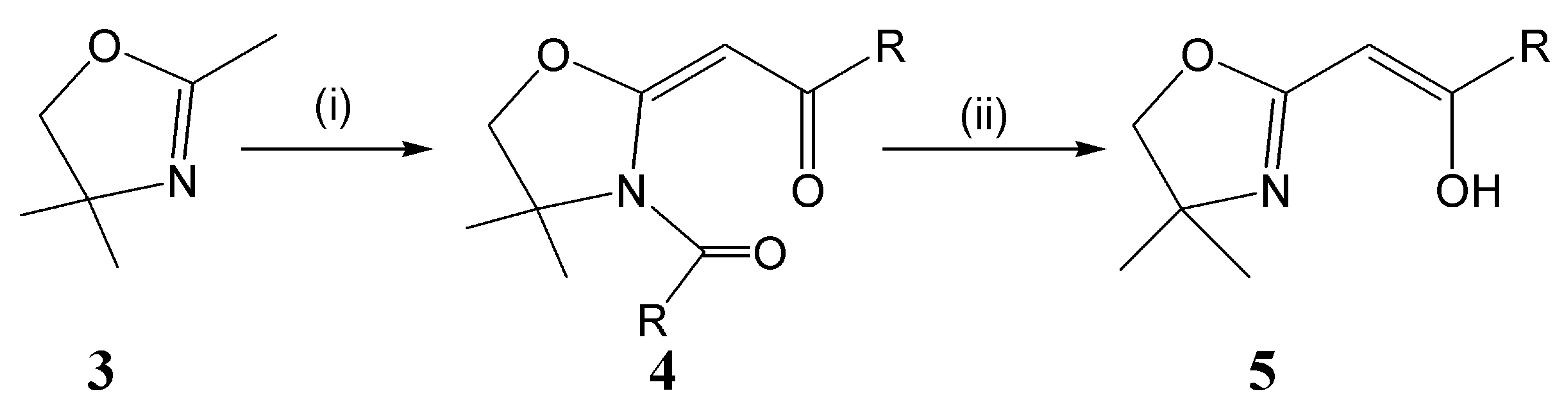
Sometime ago, Tohda and co-workers presented [22] a facile two-step strategy for the synthesis of enol-containing oxazolines initiating from 2,4,4-trimethyl-2-oxazoline (3). This protocol (Scheme 1) involves the (i) treatment of 3 with base (NEt3) in the presence of two equiv. of a benzoyl- or alkoyl-chloride to yield an intermediate ethanone. This latter compound is then hydrolysed with excess alcoholic base (ii) to yield the desired enol-oxazoline product (5: Scheme 1). Materials such as 4 have found application in the production of other heterocycles [23] and as general synthetic intermediates [24-27]. A compound of general formula 4 (Scheme 1) has not previously been the subject of study via single crystal X-ray diffraction. In this report, we detail the solid-state properties of an example of crystalline 4 in which the R group (Scheme 1) is −C6H4OCH3-p (i.e., 4: derived from 4-anisic acid).
2. Results and Discussion
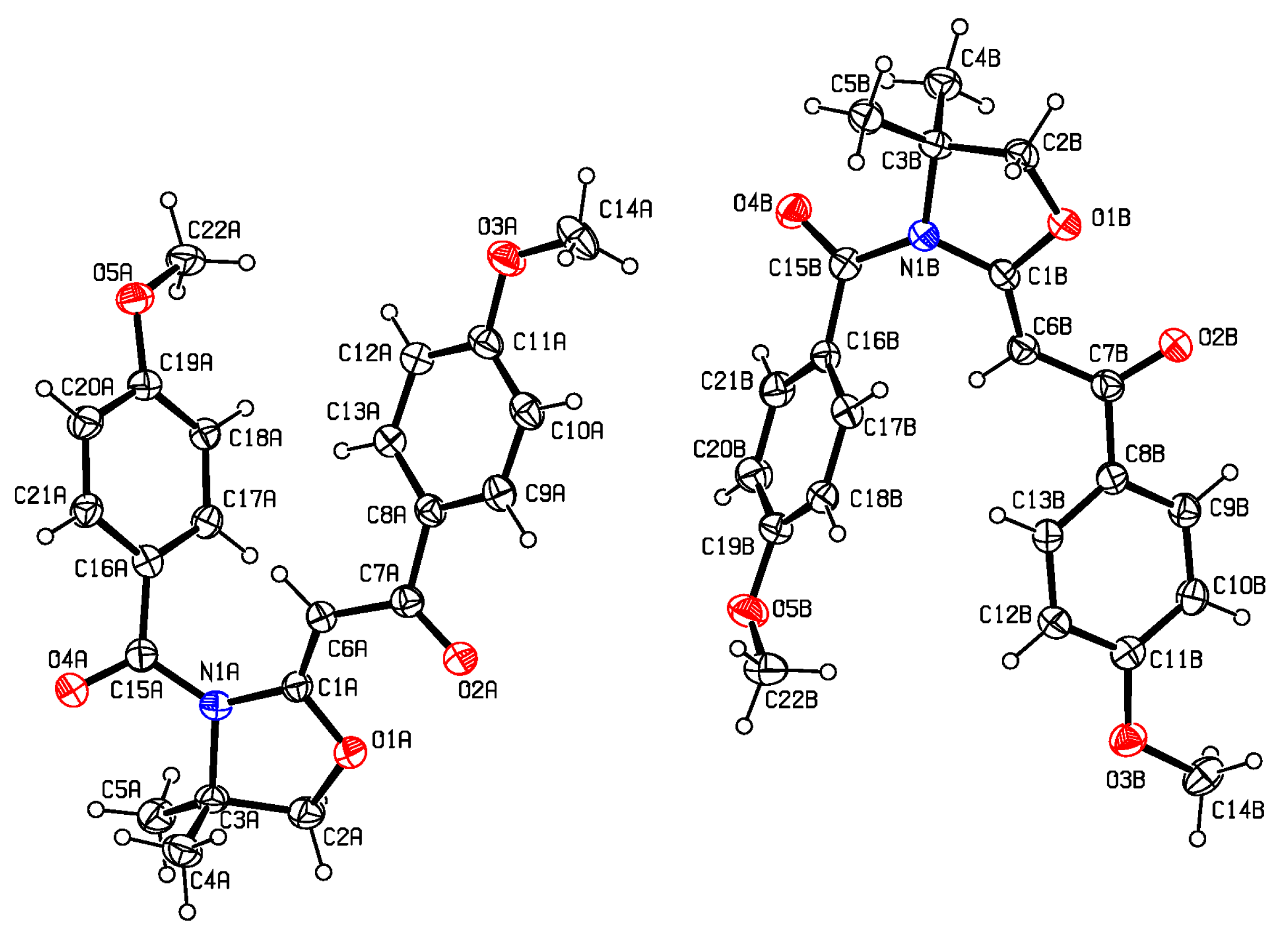
As noted above, the title compound is the first crystallographically characterised example of this class of tertiary amides; solvent free crystals of 4 were obtained from equal volume mixtures of C7H8 and hexanes. A list of selected bond lengths and angles appears in Table 1. A noteworthy feature of the crystal form is the presence of two structurally distinct molecules of 4 (Molecule A and B) which are found in the unit cell (Figure 2).
These differ primarily in the torsion angles observed and the relative spacing of the two intramolecular aromatic rings (Table 1). Specifically for Molecule A, the O2A-C7A-C8A-C9A, C6A-C7A-C8A-C13A and N1A-C15A-C16A-C17A torsions are all narrower (20.6(5)°, 22.0(6)° and 33.9(5)°, respectively) than their respective angles observed for Molecule B (24.2(6)°, 28.8(5)° and 38.2(5)°, respectively). In the case of Molecule A, the relative spacing and twisting of the two aromatic rings results in a longer C22A•••O3A distance (6.66 Å) than that observed for the same spacing in B (6.27 Å). The heterocyclic rings in both molecules display deviations from planarity of 9.7° (Molecule A) and 8.0° (Molecule B) [21] as measured by their respective N1-C1-O1-C3 torsion angles. The bond lengths observed (Table 1) for the various functional groups are well within the expected ranges for such bonds and hence are otherwise unsurprising [28]. A crystal packing diagram is found in Figure 3. This molecule was also subjected to examination of its gas-phase structure using Density Functional Theory (DFT) at the b3lyp 6-311++G** level of sophistication. The calculated bond lengths and angles also appear in Table 1. The DFT measurements do a reasonable job at mimicking the solid-state structure (Table 1); interestingly rotation of the C6-C7 bond to a situation in which the C7-O2 ketone functionality is rotated approximately 180° relative to that of Molecules A or B is found to be a more stable gas-phase configuration by approximately 3.0 kJ/mol. The gas-phase form is also predicted, perhaps not surprisingly, to have a longer C22•••O3 distance (dcalc = 9.00 Å). Of the three torsion angles mentioned above, the gas-phase form is closer to that of Molecule A with the respective values of 15.8°, 20.1° and 22.0°. The calculated deviation from planarity of the heterocycle (estimated similarly via the N1-C1-O1-C3 torsion, vide supra) is 12.5°.
3. Experimental Section
3.1. General
Compound 4 was prepared using the protocols described by Tohda and co-workers [22]. Crystals suitable for X-ray diffraction were obtained by recrystallisation of the said material from a sample dissolved in an equal volume mixture of warm toluene and hexanes which was allowed to cool and slowly evaporate at room temperature.
3.2. Data Collection and Refinement
The diffraction data of 4 were acquired and the data refined as described previously [29].
Crystal data (4). C22H23NO4, 381.41 g mol−1. Crystal size: 0.36 × 0.22 × 0.22 mm3. Monoclinic, P21/c (no. 14), a = 20.9410(11) Å, b = 8.7523(5) Å, c = 21.2291(9) Å, β = 93.529(3)°, V = 3883.5(3) Å3, Z = 8. Mo-Kα: λ = 0.71073 Å; T = 150(1) K; θ range: 2.65° to 25.14°; Index ranges: −24 ≤ h ≤ 24, −10 ≤ k ≤ 10, −25 ≤ l ≤ 25; Dcalc = 1.305 mg/m3; 17679 reflections measured of which 6659 were symmetrically independent; Rint = 0.087; F(000) = 1616; Abs. coeff.: 0.093 mm−1; Abs. corr.: semi-empirical from equivalents; Parameters/Restraints: 514/0. Max./min.: 0.990/0.701; completeness to θ at 25.00° = 96.4%. R values: R1/wR2 for 6659 reflections with [I > 2σ(I)]: 0.0727/0.1872; for all data: 0.1167/0.2117; gof on F2: 1.083, largest difference peak and hole: 0.235/−0.286 eÅ−3. CCDC number: 841810. Copies of the data can be obtained free of charge from the authors or on application to the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ U.K. (fax: +44 1223 336033; E-mail: [email protected]; website: http://www.ccdc.cam.uk/conts/retrieving.html).
3.3. Density Functional Treatment of 4
The calculated parameters (Table 1) for a hypothetical gas phase molecule of 4 were derived using DFT at the b3lyp 6-311++G** level of theory using software (Spartan 10.0®) and parameters as previously described [29]. Zero point energy calculations were also carried out and no imaginary frequencies or vibrations were noted. Data files (.mol) are available from the authors on request.
4. Conclusions
The crystal and molecular structure of the title compound, viz., (2Z)-2-[3-(4-methoxybenzoyl)-4,4-dimethyl-1,2,-oxazolidin-2-ylidene]-1-(4-methoxyphenyl)ethanone (4), has been reported. These data represent the first crystallographically characterised example of this class of tertiary amides. The gas phase structure of 4 has also been estimated by DFT and the calculated parameters are in good agreement with those observed in the solid-state form.

| Molecule A | Calculated | Molecule B | ||
|---|---|---|---|---|
| Designation | Parameter | Parameter | Parameter | Designation |
| O1A-C1A | 1.351(4) | 1.361 | 1.351(4) | O1B-C1B |
| O1A-C2A | 1.456(5) | 1.440 | 1.459(5) | O1B-C2B |
| O2A-C7A | 1.231(4) | 1.234 | 1.248(4) | O2B-C7B |
| O4A-C15A | 1.222(4) | 1.214 | 1.222(4) | O4B-C15B |
| N1A-C15A | 1.401(5) | 1.427 | 1.405(5) | N1B-C15B |
| N1A-C1A | 1.405(5) | 1.377 | 1.407(5) | N1B-C1B |
| N1A-C3A | 1.496(5) | 1.500 | 1.496(5) | N1B-C3B |
| C1A-C6A | 1.344(5) | 1.363 | 1.350(5) | C1B-C6B |
| C6A-C7A | 1.456(5) | 1.451 | 1.438(5) | C6B-C7B |
| C7A-C8A | 1.489(5) | 1.501 | 1.491(5) | C7B-C8B |
| C1A-C6A-C7A | 125.5(4) | −125.74 * | 126.0(4) | C1B-C6B-C7B |
| O2A-C7A-C8A | 119.9(3) | 119.41 | 119.0(4) | O2B-C7B-C8B |
| O4A-C15A-N1A | 119.4(3) | 118.85 | 118.9(3) | O4B-C15B-N1B |
*see text.
Acknowledgments
The authors are indebted to the support of NSERC Canada, Acadia University and Ryerson University.
References
- Wiley, R.H.; Bennett, L.L., Jr. The chemistry of the oxazolines. Chem. Rev. 1948, 44, 447–476. [Google Scholar]
- Frump, J.A. Oxazolines. Their preparation, reactions, and applications. Chem. Rev. 1971, 71, 483–505. [Google Scholar]
- Gossage, R.A.; Yadav, P.N.; MacInnis, T.D.; Quail, J.W.; Decken, A. Zinc halide oxazoline complexes – the quest for structural diversity. Can. J. Chem. 2009, 87, 368–379. [Google Scholar]
- Gossage, R.A. Pincer oxazolines: emerging tools in coordination chemistry and catalysis – where to next? Dalton Trans. 2011, 40, 8755–8759. [Google Scholar]
- Gómez, M.; Muller, G.; Rocamora, M. Coordination chemistry of oxazoline ligands. Coord. Chem. Rev. 1999, 193-195, 769–835. [Google Scholar]
- Baerlocher, F.J.; Bucur, R.; Decken, A.; Eisnor, C.R.; Gossage, R.A.; Jackson, S.M.; Jolly, L.; Wheaton, S.L.; Wylie, R.S. Oxazoles XXII. The cobalt(II) coordination chemistry of 2-(ortho-anilinyl)-4,4-dimethyl-2-oxazoline: syntheses, properties, and solid-state structural characterization. Aust. J. Chem. 2010, 63, 47–55. [Google Scholar]
- McManus, H.A.; Guiry, P.J. Recent developments in the application of oxazoline-containing ligands in asymmetric catalysis. Chem. Rev. 2004, 104, 4151–4202. [Google Scholar]
- Hargaden, G.C.; Guiry, P.J. Recent applications of oxazoline-containing ligands in asymmetric catalysis. Chem. Rev. 2009, 109, 2505–2550. [Google Scholar]
- Eisnor, C.R.; Gossage, R.A.; Yadav, P.N. Oxazoline chemistry. Part 11: synthesis of natural and synthetic isoflavones, stilbenes and related species via C-C bond formation promoted by a Pd-oxazoline complex. Tetrahedron 2006, 62, 3395–3401. [Google Scholar]
- Gossage, R.A.; Jenkins, H.A.; Yadav, P.N. Application of an air stable Pd oxazoline complex for Heck, Suzuki, Sonogashira and related C-C bond-forming reactions. Tetrahedron Lett. 2004, 45, 7689–7691, (Corrigendum: 2005, 46, 5243). [Google Scholar]
- Snieckus, V.; Beaulieu, F.; Mohri, K.; Han, W.; Murphy, C.K.; Davis, F.A. Directed ortho-metalation-mediated F+ introduction. Regiospecific synthesis of fluorinated aromatics. Tetrahedron Lett. 1994, 35, 3465–3468. [Google Scholar]
- Green, L.; Chauder, B.; Snieckus, V. The directed ortho metalation – cross-coupling symbiosis in heteroaromatic synthesis. J. Heterocycl. Chem. 1999, 36, 1453–1468. [Google Scholar]
- Meyers, A.I. Chiral Oxazolines – their legacy as key players in the renaissance of asymmetric synthesis. J. Heterocycl. Chem. 1998, 35, 991–1002. [Google Scholar]
- Meyers, A.I. Chiral Oxazolines and their legacy in asymmetric carbon-carbon bond-forming reactions. J. Org. Chem. 2005, 70, 6137–6151. [Google Scholar]
- Jung, M.Y.; Jung, M.O. Identification of conjugated linoleic acids in hydrogenated soybean oil by silver ion-impregnated HPLC and gas chromatography-ion impacted mass spectrometry of their 4,4-dimethyloxazoline derivatives. J. Agric. Food Chem. 2002, 50, 6188–6193. [Google Scholar]
- Dobson, G.; Christie, W.W. Structural analysis of fatty acids by mass spectrometry of picolinyl esters and dimethyloxazoline derivatives. Trends Anal. Chem. 1996, 15, 130–137. [Google Scholar]
- Yu, Q.T.; Liu, B.N.; Zhang, J.Y.; Hunag, Z.H. Location of double bonds in fatty acids of fish oil and rat testis lipids. Gas chromatography-mass spectrometry of the oxazoline derivatives. Lipids 1989, 24, 79–83. [Google Scholar]
- Christie, W.W. Gas chromatography-mass spectrometry methods for structural analysis of fatty acids. Lipids 1999, 33, 343–353. [Google Scholar]
- Ying, Y.; Hong, J. Synthesis of Brasilibactin A and confirmation of absolute configuration of β–hydroxy acid fragment. Tetrahedron Lett. 2007, 48, 8104–8107. [Google Scholar]
- Akhmedzhanova, V.I.; Batsurén, D.; Shakirov, R.Sh. Oxytropis alkaloids II. Structure of Oxytriphine. Chem. Nat. Cmpd. (Engl. Transl.) 1993, 29, 778–780. [Google Scholar]Khim. Prir. Soedin. 1993, 873–876.
- Deshpande, A.A.; Gossage, R.A.; Jackson, S.M.; Quail, J.W.; Sadowy, A.L.; Yadav, P.N. An exploration of the metal oxide-assisted decomposition and rearrangement of N-acyl-1,3-oxazolidin-2-ones leading to 2-aryl-2-oxazolines. Z. Naturforsch. 2009, 64b, 1046–1052. [Google Scholar]
- Tohda, Y.; Kawashima, T.; Ariga, M.; Akiyama, R.; Shudoh, H.; Mori, Y. A convenient synthesis of 2-acylmethyl-4,4-dimethyl-2-oxazolines. Useful reagents for β–keto ester synthesis. Bull. Chem. Soc. Jpn. 1984, 57, 2329–2330. [Google Scholar]
- Tohda, Y.; Yanagidani, T.; Hiramatsu, S.; Nishiwaki, N.; Tani, K.; Imagawa, K.; Ariga, M. Synthesis via 2-acylmethyl-2-oxazolines. I. A novel synthesis of 3-acyl-2-pyridones by Michael Addition of 2-acylmethyl-2-oxazoline to α,β–acetylenic ketones. Bull. Chem. Soc. Jpn. 1997, 70, 2781–2790. [Google Scholar]
- Castan, F.; Denonne, F.; Bigg, D.C.H. Preparation of 2-(β-oxo)-2-oxazolines and thiazolines by reaction of enamines with 2-chloroethyl iso(thio)cyanates. Synthesis 1993, 1081–1083. [Google Scholar]
- Chatterjee, S.; Ye, G.; Song, Y.; Barker, B.L.; Pittman, C.U., Jr. Reactions of substituted oxazoles and thiazoles with acid chlorides: carbon-carbon bond formation through cyclic ketene acetals. Synthesis 2010, 3384–3394. [Google Scholar]
- Zhou, A.; Pittman, C.U., Jr. Cyclizations of 2-alkylthiazolines and 2-alkyloxazolines with α,α-disubstituted diacid chlorides or N-(chlorocarbonyl) isocyanate. Tetrahedron Lett. 2005, 46, 2045–2048. [Google Scholar]
- Song, Y.; De Silva, H.I.; Henry, W.P.; Ye, G.; Chatterjee, S.; Pittman, C.U., Jr. Regiochemistry of an ambident cyclic keten-N,O-acetal nucleophile and its anion toward electrophiles. Tetrahedron Lett. 2011, 52, 4507–4511. [Google Scholar]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. 1987, II, S1–S19. [Google Scholar]
- Chojnacka, M.W.; Lough, A.J.; Wylie, R.S.; Gossage, R.A. Piperonylic anhydride: isolation and conformational analysis by X-ray crystallography and density functional theory calculations. J. Mol. Struct. 2011, 991, 158–161. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Petrov, A.; Jones, R.C.; Vaughan, D.G.; Lough, A.J.; Gossage, R.A. The Crystal and Molecular Structure of (2Z)-2-[3-(4-Methoxybenzoyl)-4,4-dimethyl-1,2-oxazolidin-2-ylidene]-1-(4-methoxyphenyl)ethanone. Crystals 2011, 1, 229-235. https://doi.org/10.3390/cryst1040229
Petrov A, Jones RC, Vaughan DG, Lough AJ, Gossage RA. The Crystal and Molecular Structure of (2Z)-2-[3-(4-Methoxybenzoyl)-4,4-dimethyl-1,2-oxazolidin-2-ylidene]-1-(4-methoxyphenyl)ethanone. Crystals. 2011; 1(4):229-235. https://doi.org/10.3390/cryst1040229
Chicago/Turabian StylePetrov, Anna, Roderick C. Jones, Douglas G. Vaughan, Alan J. Lough, and Robert A. Gossage. 2011. "The Crystal and Molecular Structure of (2Z)-2-[3-(4-Methoxybenzoyl)-4,4-dimethyl-1,2-oxazolidin-2-ylidene]-1-(4-methoxyphenyl)ethanone" Crystals 1, no. 4: 229-235. https://doi.org/10.3390/cryst1040229




