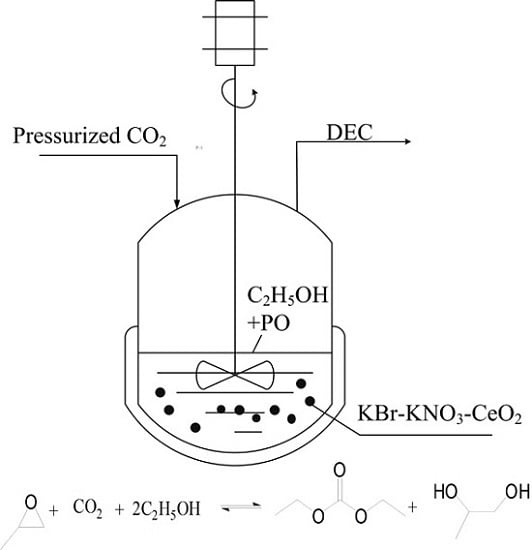
Synthesis of Diethyl Carbonate from Carbon Dioxide, Propylene Oxide and Ethanol over KNO3-CeO2 and KBr-KNO3-CeO2 Catalysts
Abstract
:1. Introduction
2. Results and Discussion
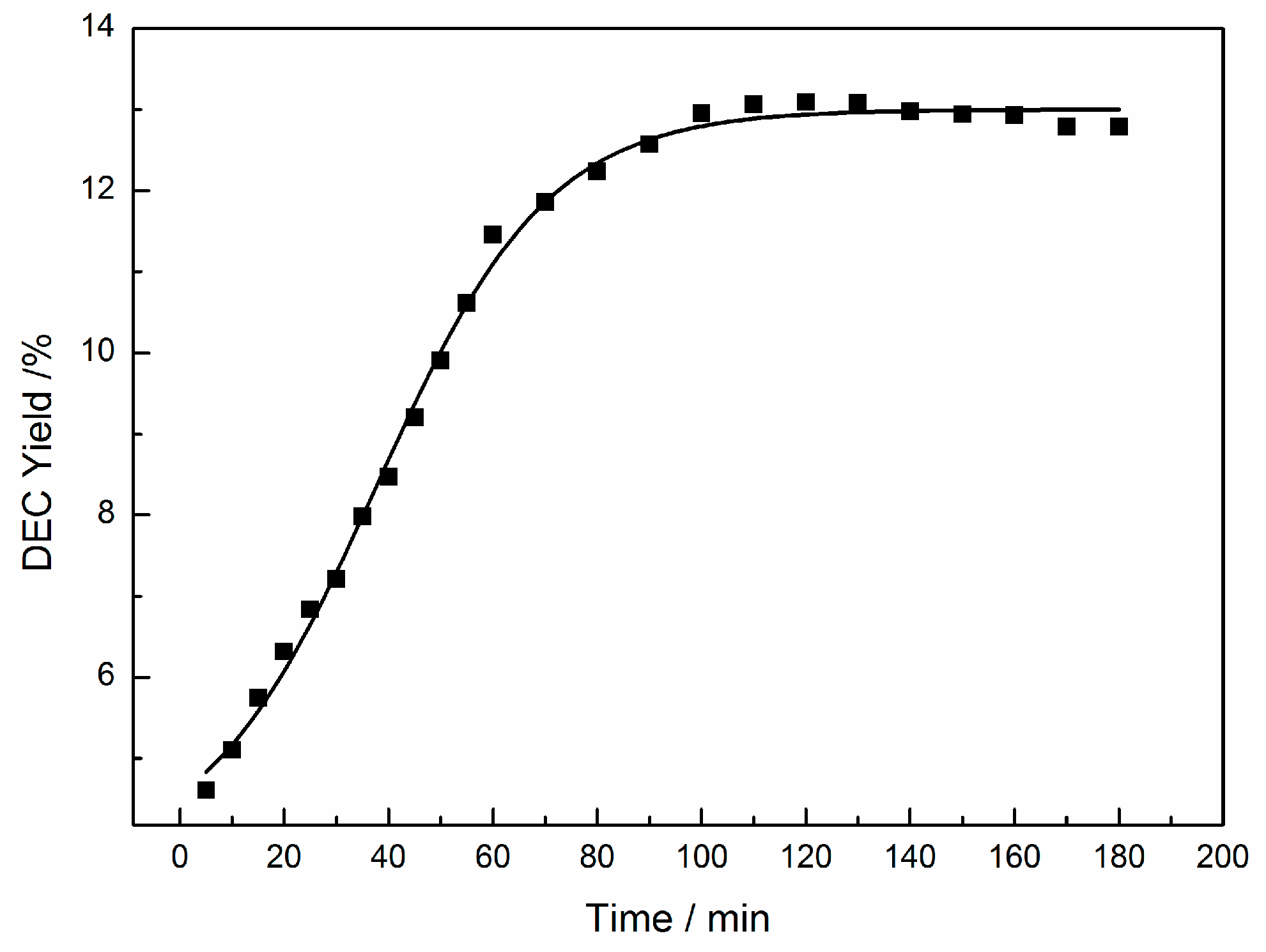
2.1. The One-Pot Synthesis of DEC over KNO3-CeO2 Catalyst
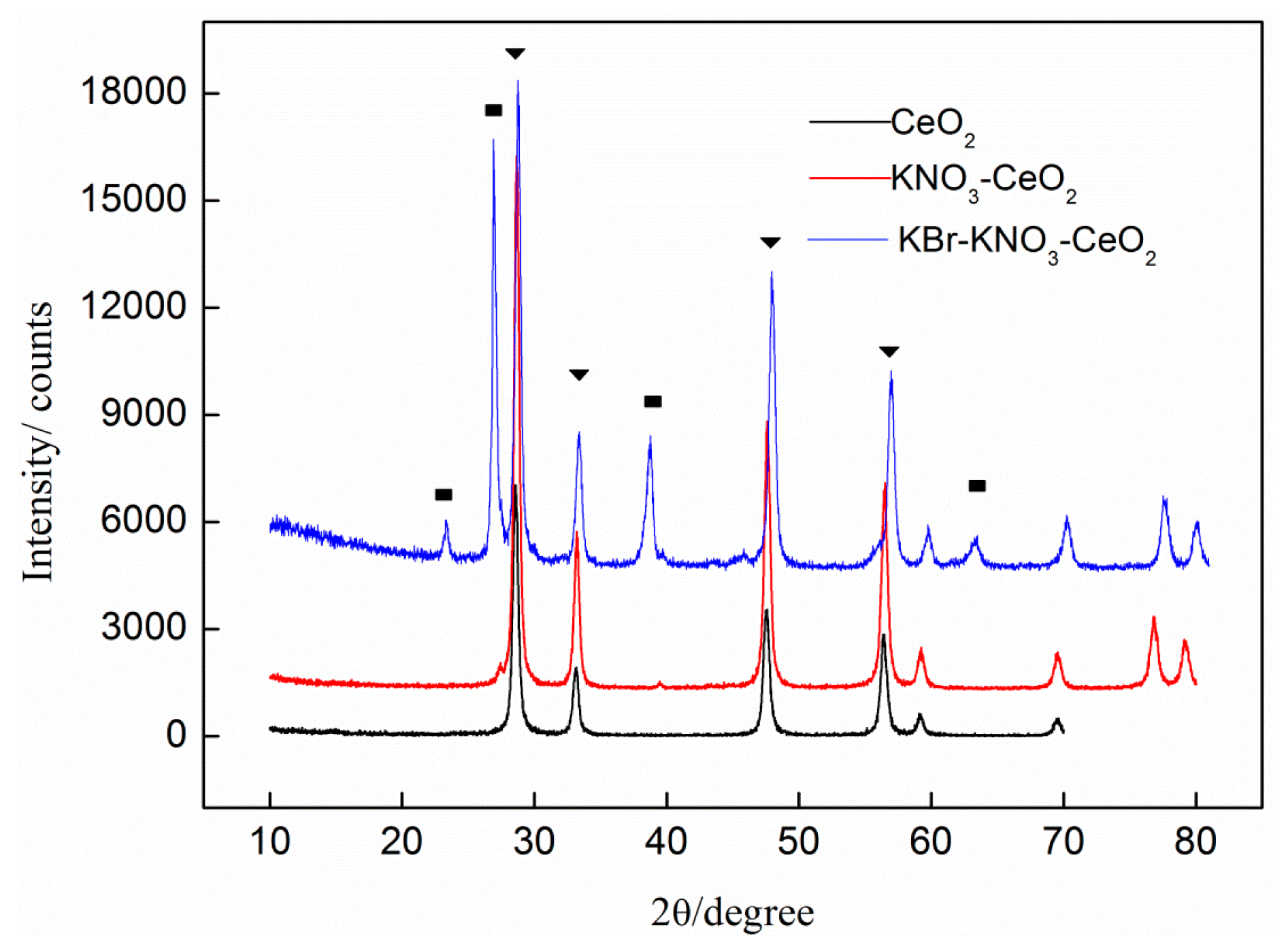
2.2. The One-Pot Synthesis of DEC over KBr-KNO3-CeO2 Catalyst
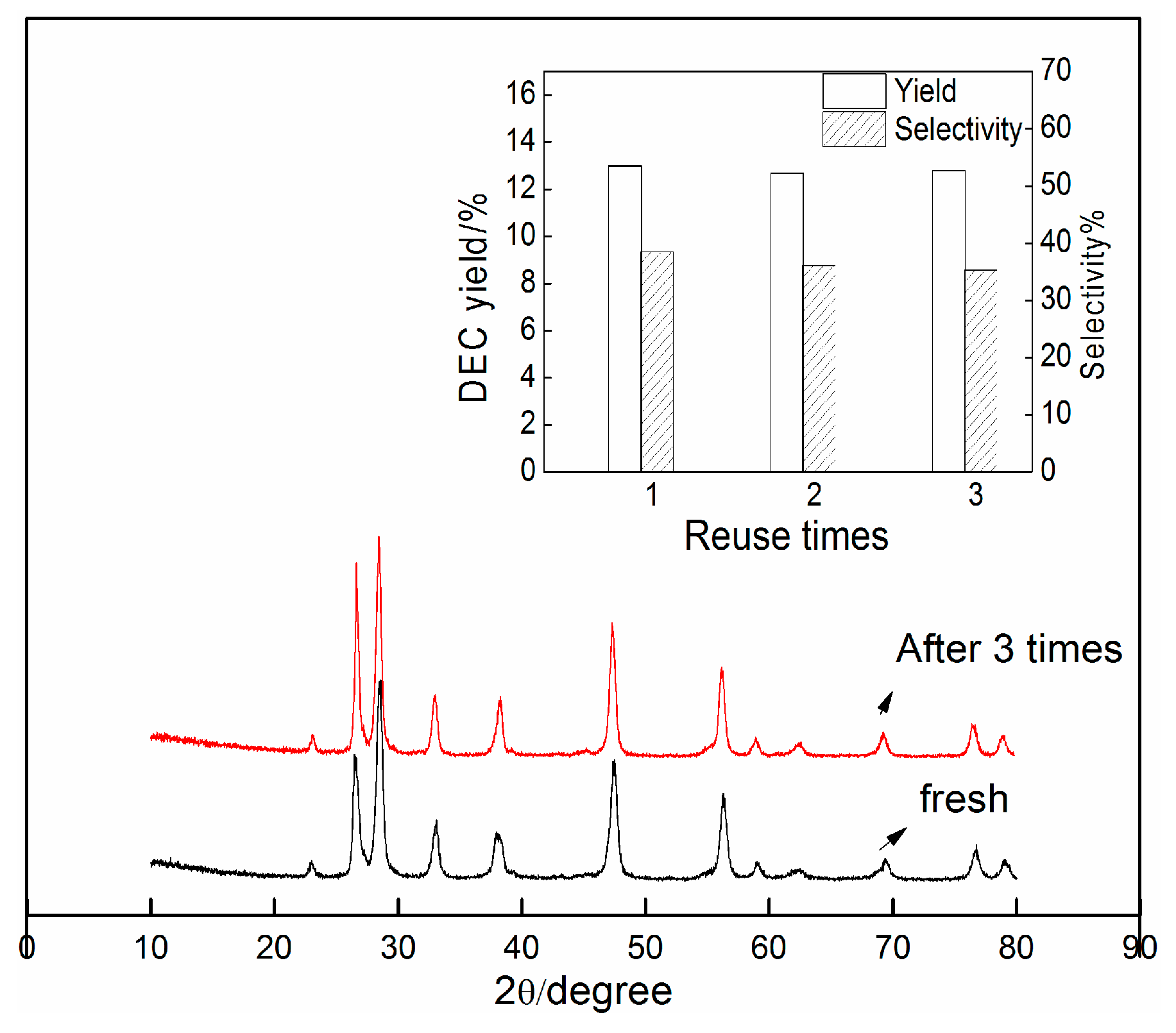
2.3. Recycling Experiments
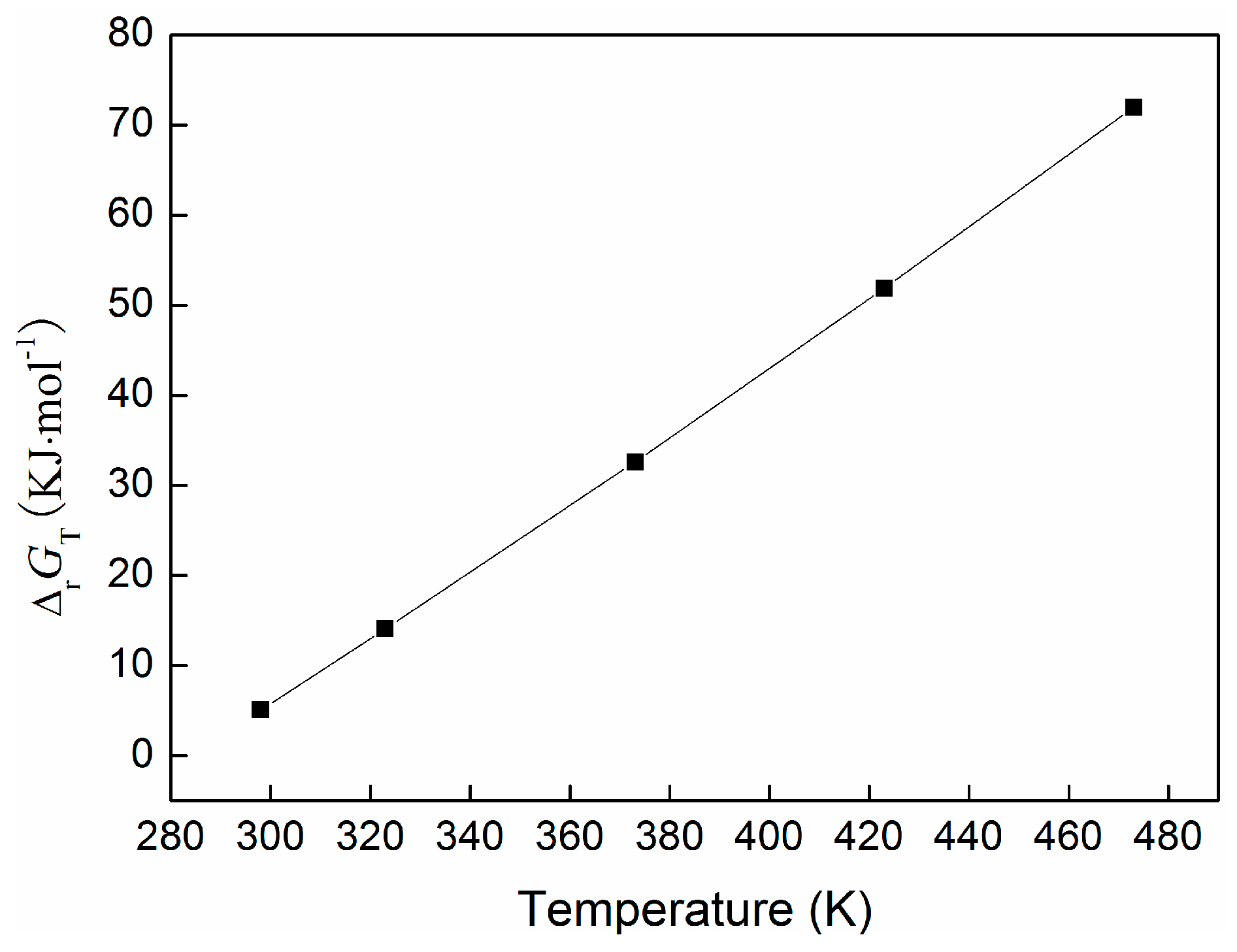
2.4. Thermodynamic Evaluation of the One-Pot Synthesis of DEC
3. Experimental Section
3.1. Material
3.2. Catalyst Preparation and Characterization
3.3. Catalytic Test
3.4. Analysis Method
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Subodh, K.; Suman, L.J. Polyethylene Glycol Enfolded KBr Assisted Base Catalyzed Synthesis of Dimethyl Carbonate from Methanol and Carbon Dioxide. Ind. Eng. Chem. Res. 2014, 53, 15798–15801. [Google Scholar]
- Wan Nor Roslam, W.I.; Zatil Amali, C.R.; Mohamed Wahab, M.H.; Mohd, A.Y. The formation of a series of carbonates from carbon dioxide: Capturing and utilization. Renew. Sustain. Energy Rev. 2015, 47, 93–106. [Google Scholar]
- Dibenedetto, A.; Angelini, A. Synthesis of Organic Carbonates. Adv. Inorg. Chem. 2014, 66, 25–81. [Google Scholar]
- Li, J.; Qi, X.; Wang, L.; He, Y.; Deng, Y. New attempt for CO2 utilization: One-pot catalytic syntheses of methyl, ethyl and n-butyl carbamates. Catal. Commun. 2014, 12, 1224–1227. [Google Scholar] [CrossRef]
- Sanguineti, P.B.; Baltanas, M.A.; Bonivardi, A.L. Copper-gallia interaction in Cu-Ga2O3-ZrO2 catalysts for methanol production from carbon oxide(s) hydrogenation. Appl. Catal. A 2014, 504, 476–481. [Google Scholar] [CrossRef]
- Ma, J.; Sun, N.; Zhang, X.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y. A short review of catalysis for CO2 conversion. Catal. Today 2009, 148, 221–231. [Google Scholar] [CrossRef]
- Dibenedetto, A.; Angelini, A.; Stufano, P. Use of carbon dioxide as feedstock for chemicals and fuels: Homogeneous and heterogeneous catalysis. J. Chem. Technol. Biotechnol. 2014, 89, 334–353. [Google Scholar] [CrossRef]
- Leino, E.; Maki-Arvela, P.; Eranen, K.; Tenho, M.; Murzin, D.Y.; Salmi, T.; Mikkola, J.P. Enhanced yields of diethyl carbonate via one-pot synthesis from ethanol, carbon dioxide and butylene oxide over cerium (IV) oxide. Chem. Eng. J. 2011, 176–177, 124–133. [Google Scholar] [CrossRef]
- Dunn, B.C.; Guenneau, C.; Hilton, S.A.; Pahnke, J.; Eyring, E.M.; Dworzanski, J.; Meuzelaar, H.L.C.; Hu, J.Z.; Solum, M.S.; Pugmire, P.J. Production of diethyl carbonate from ethanol and carbon monoxide over a heterogeneous catalyst. Energy Fuel 2002, 16, 177–181. [Google Scholar] [CrossRef]
- Ma, X.; Shi, H.; Zhang, Z.; Wang, B.; Wang, S.; Xu, G. Catalytic synthesis of diethyl carbonate from carbon monoxide and ethyl nitrite on supported Pd catalysts. Prepr. Pap. Am. Chem. Soc. Div. Fuel Chem. 2003, 48, 906–907. [Google Scholar]
- Ma, X.B.; Fan, M.M.; Zhang, P.B. Study on the catalytic synthesis of diethyl carbonate from CO and ethyl nitrite over supported Pd catalysts. Catal. Commun. 2004, 5, 765–770. [Google Scholar] [CrossRef]
- Muskat, I.E.; Strain, F. Preparation of carbonic acid esters. US Patent 2,379,250, June 1941. [Google Scholar]
- Wang, D.; Yang, B.; Zhai, X.; Zhou, L. Synthesis of diethyl carbonate by catalytic alcoholisis of urea. Fuel Process. Technol. 2007, 88, 807–812. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, D.D.; Zhang, J.; Sun, Y.Y. Direct Synthesis of Diethyl Carbonate from CO2 and Ethanol Catalyzed by ZrO2/Molecular Sieve. Catal. Lett. 2014, 144, 2144–2150. [Google Scholar] [CrossRef]
- Choi, J.C.; Kohno, K.; Ohshima, Y.; Yasuda, H.; Sakakura, T. Tin- or titanium-catalyzed dimethyl carbonate synthesis from carbon dioxide and methanol: Large promotion by a small amount of triflate salts. Catal. Commun. 2008, 9, 1630–1633. [Google Scholar] [CrossRef]
- Sakakura, T.; Saito, Y.; Okano, M.; Choi, J.C.; Sako, T. Selective Conversion of Carbon Dioxide to Dimethyl Carbonate by Molecular Catalysis. J. Org. Chem. 1998, 63, 7095–7096. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Kuno, S.; Begum, N.; Fujimoto, K.; Suzuki, K.; Nakagawa, Y.; Tomishige, K. Catalytic synthesis of dialkyl carbonate from low pressure CO2 and alcohols combined with acetonitrile hydration catalyzed by CeO2. Appl. Catal. A 2010, 384, 165–170. [Google Scholar] [CrossRef]
- Honda, M.; Sonehara, S.; Yasuda, H.; Nakagawaa, Y.; Tomishige, K. Heterogeneous CeO2 catalyst for the one-pot synthesis of organic carbamates from amines, CO2 and alcohols. Green Chem. 2011, 13, 3406–3413. [Google Scholar] [CrossRef]
- Leino, E.; Mäki-Arvela, P.; Eta, V.; Kumar, N.; Demoisson, F.; Samikannu, A.; Leino, A.R.; Shchukarev, A.; Murzin, D.Y.; Mikkola, J.P. The influence of various synthesis methods on the catalytic activity of cerium oxide in one-pot synthesis of diethyl carbonate starting from CO2, ethanol and butylene oxide. Catal. Today 2013, 210, 47–54. [Google Scholar] [CrossRef]
- Honda, M.; Tamura, M.; Nakao, K.; Suzuki, K.; Nakagawa, Y.; Tomishige, K. Direct Cyclic Carbonate Synthesis from CO2 and Diol over Carboxylation/Hydration Cascade Catalyst of CeO2 with 2-Cyanopyridine. ACS Catal. 2014, 4, 1893–1896. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; Xin, S.; He, P.; Cao, Y.; Li, F.; Hou, X. Highly efficient synthesis of diethyl carbonate via one-pot reactionfrom carbon dioxide, epoxides and ethanol over KI-based binarycatalyst system. Appl. Catal. A 2014, 471, 19–27. [Google Scholar] [CrossRef]
- Qiu, P.; Wang, L.; Jiang, X.; Yang, B. Synthesis of Diethyl Carbonate by the Combined Process of Transesterification with Distillation. Energy Fuel 2012, 26, 1254–1258. [Google Scholar] [CrossRef]
- Chang, Y.; Jiang, T.; Han, B.; Liu, Z.; Wu, W.; Gao, L.; Li, J.; Gao, H.; Zhao, G.; Huang, J. One-pot synthesis of dimethyl carbonate and glycols from supercritical CO2, ethylene oxide or propylene oxide, and methanol. Appl. Catal. A 2004, 263, 179–186. [Google Scholar] [CrossRef]
- Eta, V.; Maki-Arvela, P.; Leino, A.R.; Kordas, K.; Salmi, T.; Murzin, D.Y.; Mikkola, J.P. Synthesis of Dimethyl Carbonate from Methanol and Carbon Dioxide: Circumventing Thermodynamic Limitations. Ind. Eng. Chem. Res. 2010, 49, 9609–9617. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, W.Y.; Wu, Z.; Chun, Y.; Zhu, J.H. Superbase derived from zirconia-supported potassium nitrate. Mater. Lett. 2000, 46, 198–204. [Google Scholar] [CrossRef]
- Ma, P.S. Chemical Engineering Thermodynamics; Chemical Industry Press: Beijing, China, 2005. [Google Scholar]
- Ma, P.S. Chemical Engineering Data; China Petrochemical Press: Beijing, China, 2003. [Google Scholar]





| Run | Catalyst | Specific surface area/m2/g | Ethanol conversion/% | DEC yield/% | Selectivity/% | ||
|---|---|---|---|---|---|---|---|
| DEC | PG | EP | |||||
| 1 | CeO2 | - | 1.5 | 1.3 | 84.0 | - | - |
| 2 | NaNO3-CeO2 | - | 12.6 | 3.6 | 28.3 | 24.1 | 19.3 |
| 3 | Ba(NO3)2-CeO2 | - | 8.1 | 0.9 | 12.6 | 10.2 | 16.6 |
| 4 | Mg(NO3)2-CeO2 | - | 15.9 | 2.2 | 13.0 | 15.9 | 17.4 |
| 5 | Ca(NO3)2-CeO2 | - | 6.6 | 1.3 | 22.3 | 16.7 | 19.8 |
| 6 | KOH-CeO2 | - | 13.4 | 3.6 | 28.0 | 20.1 | 33.6 |
| 7 | K2CO3-CeO2 | - | 9.1 | 1.8 | 19.0 | 17.6 | 25.1 |
| 8 | KNO3-CeO2 | 30.5 | 34.0 | 11.2 | 33.3 | 23.7 | 23.5 |
| 9 | KNO3-γ-Al2O3 | 102 | 46.8 | 11.5 | 24.5 | 23.1 | 16.5 |
| 10 | KNO3-ZrO2 | 22.9 | 23.7 | 6.3 | 27 | 24.4 | 22.9 |
| 11 | KNO3-SiO2 | 78.1 | 12.3 | 3.1 | 25.3 | 26.1 | 18.8 |
| 12 | KNO3-La2O3 | 31.8 | 0.6 | 0.4 | 69.1 | 20.7 | 10.9 |
| 13 | KI-CeO2 A | - | 18.0 | 0.9 | 6.3 | 6.4 | 10.3 |
| 14 | KI-CeO2 B | - | 9.0 | 1.3 | 14.4 | 17.4 | 12.8 |
| 15 | KI-KNO3-CeO2 A | - | 17.1 | 0.9 | 5.8 | 4.9 | 7.7 |
| 16 | KI-KNO3-CeO2 B | - | 9.6 | 0.4 | 7.5 | 7.9 | 7.2 |
| 17 | KCl-KNO3-CeO2 A | - | 14.4 | 2.7 | 17.4 | 14.4 | 5.7 |
| 18 | KCl-KNO3-CeO2 B | - | 39.9 | 10.3 | 25.9 | 22.3 | 5.9 |
| 19 | KBr-KNO3-CeO2 A | - | 23.1 | 9.4 | 40.8 | 30.5 | 7.6 |
| 20 | KBr-KNO3-CeO2 B | - | 33.9 | 13.0 | 38.5 | 27.7 | 7.5 |
| Substance | /kJ·mol−1 | /J·mol−1·K−1 | /J·mol−1·K−1 |
|---|---|---|---|
| C2H5OH | −235.10 | 282.70 | 112.6 |
| CO2 | −393.509 | 213.74 | 37.13 |
| DEC | −637.9 | 412.94 a | 211 |
| H2O | −241.82 | 188.82 | 33.58 |
| PO | −94.68 | 281.15 | 72.55 |
| PG | −421.29 | 288 a | 189.9 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Jia, D.; Zhu, Z.; Sun, Y. Synthesis of Diethyl Carbonate from Carbon Dioxide, Propylene Oxide and Ethanol over KNO3-CeO2 and KBr-KNO3-CeO2 Catalysts. Catalysts 2016, 6, 52. https://doi.org/10.3390/catal6040052
Wang Y, Jia D, Zhu Z, Sun Y. Synthesis of Diethyl Carbonate from Carbon Dioxide, Propylene Oxide and Ethanol over KNO3-CeO2 and KBr-KNO3-CeO2 Catalysts. Catalysts. 2016; 6(4):52. https://doi.org/10.3390/catal6040052
Chicago/Turabian StyleWang, Yanlou, Dongdong Jia, Zhen Zhu, and Yongyue Sun. 2016. "Synthesis of Diethyl Carbonate from Carbon Dioxide, Propylene Oxide and Ethanol over KNO3-CeO2 and KBr-KNO3-CeO2 Catalysts" Catalysts 6, no. 4: 52. https://doi.org/10.3390/catal6040052