2.1. Textural Parameters for Activated Carbons
As adsorbents for sulfur compounds, a commercial activated carbon and two Fe-supported activated carbons were employed. Fe was supported on commercial activated carbon with the impregnation method. The impregnated Fe contents of these samples were 9 wt% and 17 wt%, respectively. The commercial activated carbon without modification is denoted by Fe0, and the two Fe-supported activated carbon samples with Fe contents of 9 wt% and 17 wt% are denoted by Fe9 and Fe17, respectively.
Table 1 shows the Brunauer–Emmett–Teller (BET) specific surface area, pore volume, and average pore diameter of the activated carbons.
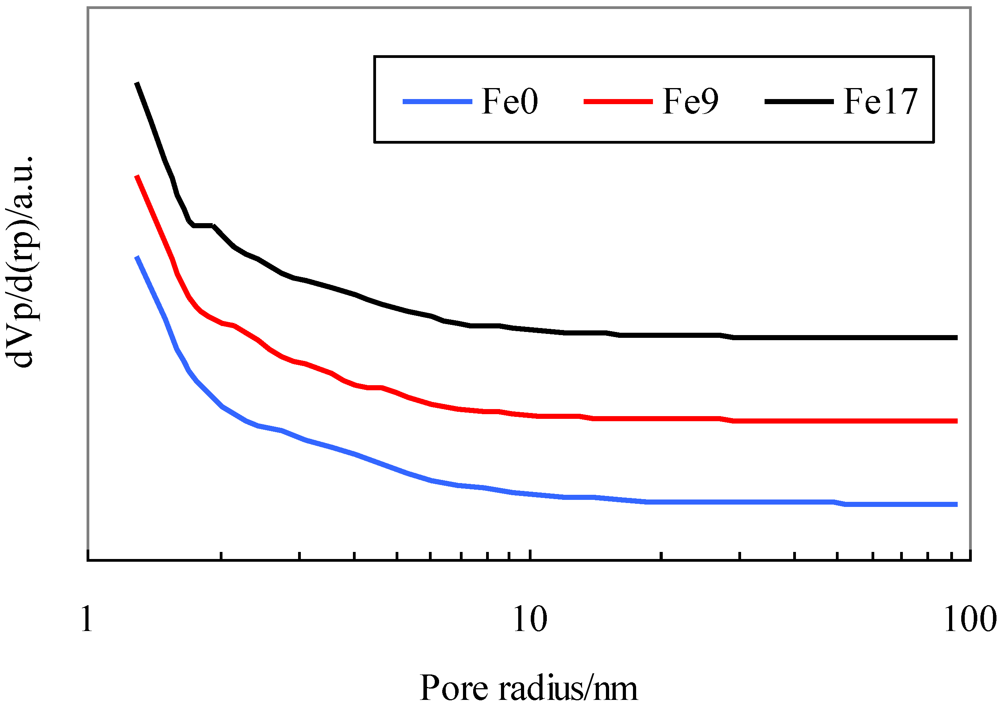
Figure 1 shows their pore distribution.
Table 1 indicates that the addition of Fe to the activated carbon decreases the BET surface area by approximately 6% and that the textural parameters of Fe9 are similar to those of Fe17.
Figure 1 indicates that all the activated carbons have not only micropores, but also mesopores with diameters up to 40 nm and that the addition of Fe has no effect on pore distribution.
Table 1.
Physical properties of activated carbons. BET = Brunauer–Emmett–Teller.
Table 1.
Physical properties of activated carbons. BET = Brunauer–Emmett–Teller.
| Sample | BET specific surface area | Pore volume | Average pore diameter |
|---|
| m2 g−1 | cm3(STP) g−1 | nm |
|---|
| Fe0 | 1206.3 | 277.2 | 2.0 |
| Fe9 | 1126.0 | 258.7 | 2.0 |
| Fe17 | 1128.5 | 259.3 | 2.0 |
Figure 1.
Pore distribution for activated carbons.
Figure 1.
Pore distribution for activated carbons.
2.2. Effect of Temperature on COS Capture
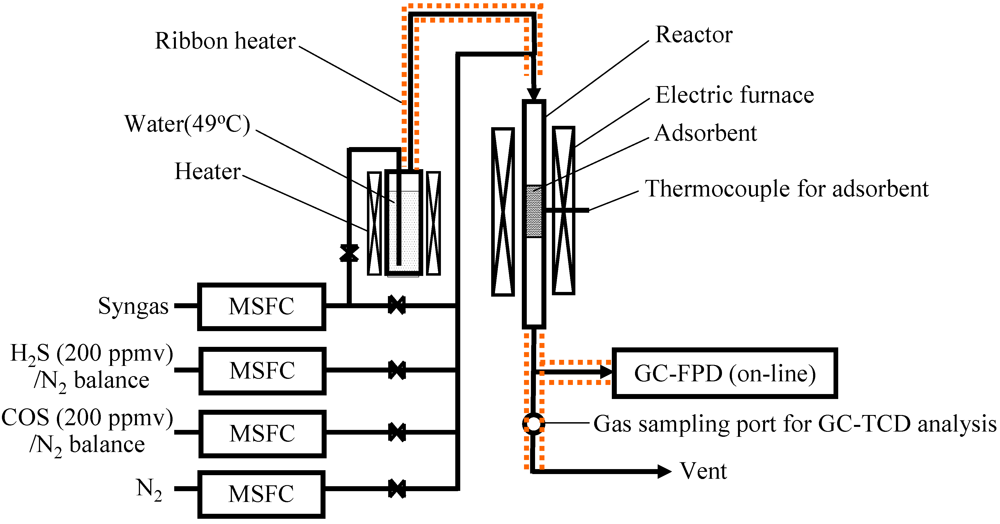
The effect of temperature on COS capture was investigated using Fe0 in order to understand removal mechanism for COS in the presence of product gas derived from biomass gasification. The product gas employed in the laboratory experiment was prepared throughout a bench-scale BTL plant operation with desulfurization and is denoted by syngas. The concentration of syngas was CO: 51.3 vol%, H2: 35.1 vol%, CH4: 5.2 vol%, CO2: 0.5 vol%, C2H4: 0.1 vol%, and N2: 7.7 vol%.
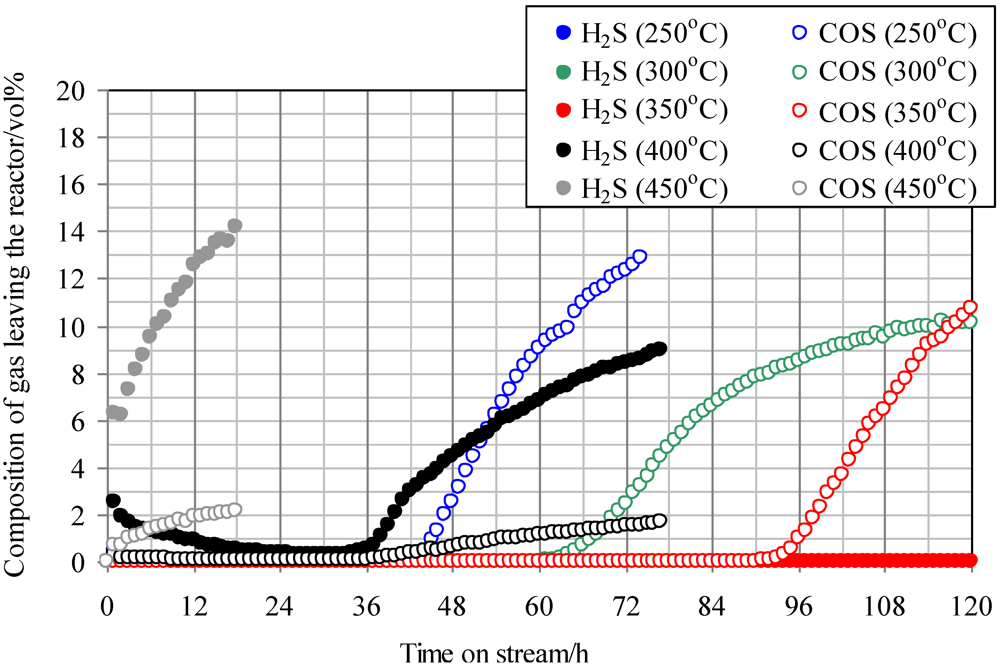
Figure 2 shows the temperature dependence of breakthrough curves using Fe0 when a mixture gas of syngas and COS was employed as the feed gas. The ratio of the volume flow rate of syngas and COS (200 ppmv in N
2) was 9:1; therefore, feed gas with a COS concentration of 20 ppmv was supplied into the adsorbent layers. With increasing reaction temperature, the breakthrough time increased, exhibiting the longest time at 350 °C (97 h). However, with an increase in temperature to more than 400 °C, the breakthrough time, which was regarded as the time when the concentration of sulfur compounds in the gas leaving the reactor reached 1 ppmv, decreased and the gas leaving the reactor contained both COS and H
2S. In the study by Sakanishi on COS removal using activated carbon, the gas leaving the reactor contained CO at 400 °C, while it contained no CO at 300 °C [
15]. This result suggests that no COS decomposition reaction takes place at temperatures lower than 300 °C. However, COS is removed as a result of physical adsorption, and it decomposes well on the activated carbons at temperatures higher than 400 °C. In the present study, because H
2S was detected at more than 400 °C, reaction (1) would have proceeded on the Fe0 surface.
![Catalysts 02 00281 i001]()
Figure 2.
Breakthrough curves for COS using Fe0 at different temperatures. (Feed gas; CO: 46.1 vol%, H2: 31.6 vol%, CH4: 4.7 vol%, CO2: 0.5 vol%, C2H4: 0.1 vol%, N2: 17.0 vol%, COS: 20 ppmv).Commercial activated carbon without modification is denoted by Fe0.
Figure 2.
Breakthrough curves for COS using Fe0 at different temperatures. (Feed gas; CO: 46.1 vol%, H2: 31.6 vol%, CH4: 4.7 vol%, CO2: 0.5 vol%, C2H4: 0.1 vol%, N2: 17.0 vol%, COS: 20 ppmv).Commercial activated carbon without modification is denoted by Fe0.
It is considered that inorganic matter is contained in Fe0. At 350 °C, COS would be adsorbed by physical adsorption and react well with inorganic matter while reaction (1) was not dominant. As a result, the breakthrough capacity at 350 °C exhibited the maximum value. However, at more than 400 °C, reaction (1) became dominant and H2S was produced.
2.3. Effect of Addition of Fe to Activated Carbons
The effect of addition of Fe to activated carbon on desulfurization behavior was investigated in the presence of H
2S and COS.
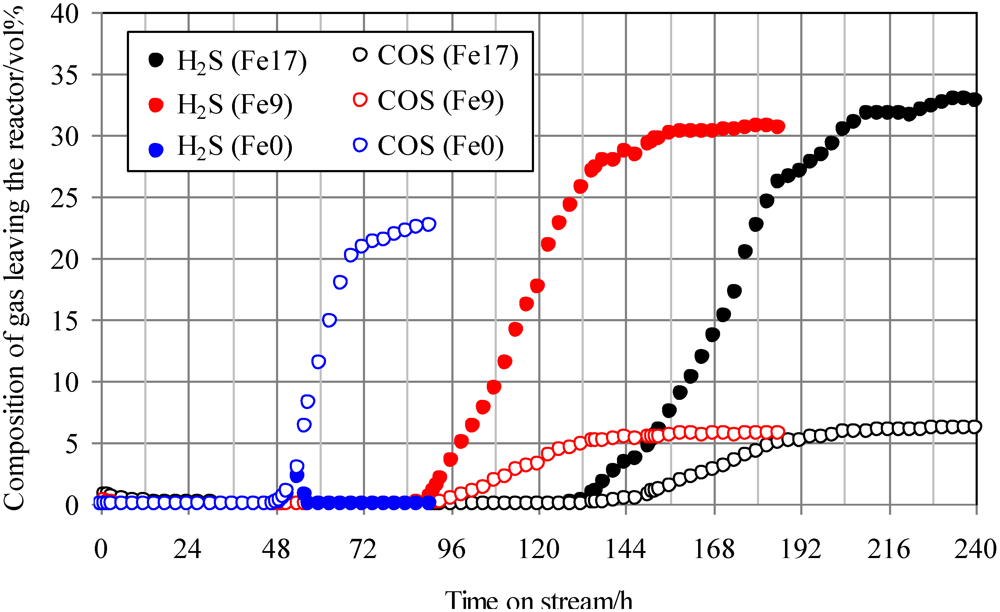
Figure 3 shows the breakthrough curves at 350 °C using Fe0, Fe9, and Fe17. The mixture gas of syngas, COS, and H
2S was employed as a feed gas. The ratio of the volume flow rate of syngas, COS (200 ppmv in N
2), and H
2S (200 ppmv in N
2) was 8:1:1; therefore, a feed gas with COS and H
2S concentrations of 20 ppmv was supplied to the adsorbent layers. With increasing Fe content, the breakthrough time increased from 48 to 136 h. Apparently, the addition of Fe to activated carbons contributed to the simultaneous removal of H
2S and COS. For Fe0, the COS concentration increased to 20 ppmv after the breakthrough, whereas no H
2S was detected after the H
2S breakthrough at 48 h. Comparing breakthrough behaviors using Fe0, shown in
Figure 2 and
Figure 3, the addition of H
2S to the syngas containing COS led to a decrease in the breakthrough time from 97 h (
Figure 2) to 51 h (
Figure 3). This comparison indicates that the interaction of H
2S and Fe0 is stronger than that of COS and Fe0, which is consistent with the result that H
2S was produced at temperatures greater than 400 °C (
Figure 2). Accordingly, H
2S and COS could be removed simultaneously at 350 °C using Fe0 with a gas mixture containing H
2S, COS, H
2, CO, CO
2, CH
4, and N
2, but it is relatively difficult for COS to be removed.
Figure 3.
Breakthrough curves at 350°C. (Feed gas; CO: 41.0 vol%, H2: 28.1 vol%, CH4: 4.2 vol%, CO2: 0.4 vol%, C2H4: 0.1 vol%, N2: 26.3 vol%, H2S: 20 ppmv, COS: 20 ppmv), SV(350°C) = 1592 h−1. The two Fe-supported activated carbon samples with Fe contents of 9 wt% and 17 wt% are denoted by Fe9 and Fe17.
Figure 3.
Breakthrough curves at 350°C. (Feed gas; CO: 41.0 vol%, H2: 28.1 vol%, CH4: 4.2 vol%, CO2: 0.4 vol%, C2H4: 0.1 vol%, N2: 26.3 vol%, H2S: 20 ppmv, COS: 20 ppmv), SV(350°C) = 1592 h−1. The two Fe-supported activated carbon samples with Fe contents of 9 wt% and 17 wt% are denoted by Fe9 and Fe17.
With increasing Fe content up to 17 wt%, the breakthrough time increased from 48 to 136 h. After the breakthroughs for Fe9 and Fe17, the concentrations of COS and H2S in the gas leaving the reactor were 5–6 ppmv and 30–33 ppmv, respectively. This result suggests that COS is converted to H2S on the Fe-supported activated carbons. Some portion of the COS supplied would react with inorganic matter contained in both Fe-supported activated carbon samples. The feed gas in the present study contains 28.1 vol% of H2. Therefore, reaction (1) is promoted on the surface of Fe9 and Fe17 to convert the residual portions of COS into H2S, followed by a sulfidation reaction of the Fe supported on the surface.
2.4. Effect of Steam on Simultaneous Removal of COS and H2S
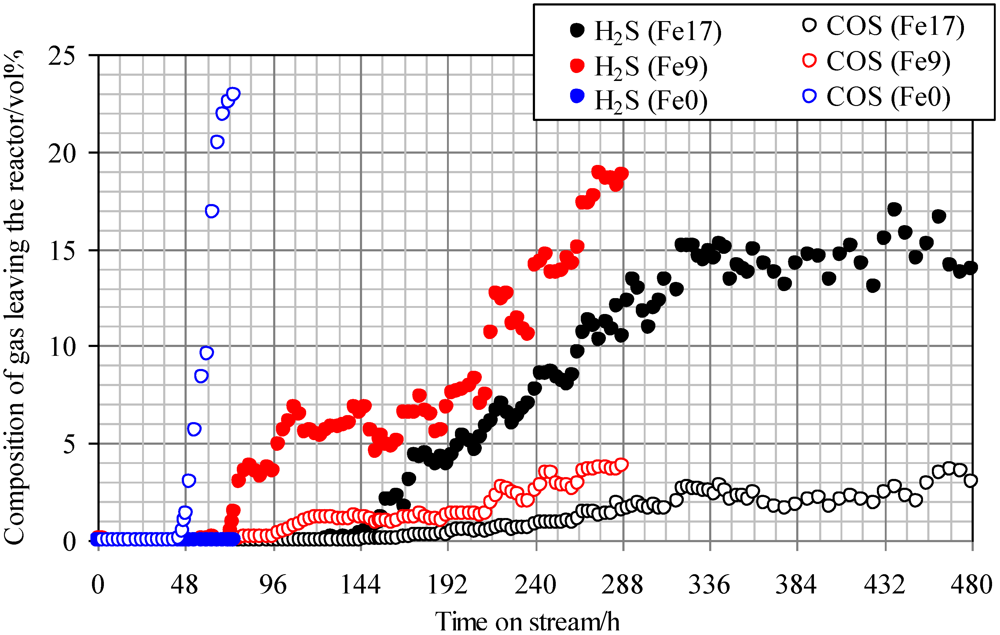
Figure 4 shows breakthrough curves at 350 °C using Fe0, Fe9, and Fe17. A mixture of syngas, COS, H
2S, and steam was employed as feed gas. The ratio of the volume flow rate of syngas, COS, H
2S, and steam was 8:1:1:1; therefore, a feed gas with COS and H
2S concentrations of 18 ppmv on a wet basis was supplied to the adsorbent layers.
Figure 4 indicates that with increasing Fe content, the breakthrough time increases. A comparison of the breakthrough behaviors with and without steam (
Figure 3 and
Figure 4) indicates that the presence of steam only slightly affected the breakthrough time. For Fe0, no H
2S was produced after the breakthrough of COS, while for Fe9 and Fe17, the concentrations of H
2S (15–20 ppmv) were higher than those of COS (3–4 ppmv) after the breakthrough. These results suggest that the presence of steam promotes reaction (2) on the Fe-supported activated carbons.
![Catalysts 02 00281 i002]()
Figure 4.
Breakthrough curves at 350°C using gas containing steam. (Feed gas; CO: 37.3 vol%, H2: 25.5 vol%, CH4: 3.8 vol%, CO2: 0.4 vol%, C2H4: 0.1 vol%, N2: 23.9 vol%, H2O: 9.1 vol%, H2S: 18 ppmv, COS: 18 ppmv), SV(350°C) = 1751 h−1.
Figure 4.
Breakthrough curves at 350°C using gas containing steam. (Feed gas; CO: 37.3 vol%, H2: 25.5 vol%, CH4: 3.8 vol%, CO2: 0.4 vol%, C2H4: 0.1 vol%, N2: 23.9 vol%, H2O: 9.1 vol%, H2S: 18 ppmv, COS: 18 ppmv), SV(350°C) = 1751 h−1.
A comparison of H
2S concentrations after the breakthrough, shown in
Figure 3 and
Figure 4, indicates that the concentration with steam (
Figure 4) is lower than that without steam (
Figure 3). Because the feed gas contained steam and H
2, it is considered that Fe9 and Fe17 would promote reactions (1) and (2). Taking the H
2S concentration after the breakthrough into account, the presence of steam would possibly lead to an increase in the number of adsorption sites for H
2S. In a study by Adib
et al. [
20], steam had an affinity with the functional group on the surface of activated carbon and was therefore likely to be condensed. The removal of H
2S was promoted by the presence of steam in the feed gas because of the interaction of condensed water and H
2S [
20]. In a study by Xie
et al., the simultaneous removal of H
2S and COS at temperatures greater than 420 °C was favored by using Fe‑supported CeO
2 [
21]. Lower temperatures are favorable for the promotion of reaction (2). In the present study, Fe-supported activated carbon would promote reactions (1) and (2) even at temperatures less than 420 °C.
The presence of steam in the feed gas decreased the breakthrough capacity of adsorbents for sulfur compounds [
22]. In this study by Wakker
et al., the breakthrough capacity for the simultaneous removal of H
2S and COS using FeO/γ-Al
2O
3 decreased by 70% with increasing steam concentration up to 14 vol% [
22]. The addition of steam to the feed gas led to the promotion of a reversible reaction (3).
In the present study, because Fe is supported on the activated carbon, it would be difficult to carry out a sulfidation reaction such as reaction (3) in the presence of steam. Reaction (2) is promoted; however, the amount of H2O consumed as a result of the reaction would be negligible compared to the steam concentration (9 vol%) in the feed gas. It is therefore considered that for Fe9 and Fe17, steam is mainly consumed by a reaction different from reaction (2).
For Fe17 after the breakthrough mentioned in
Figure 4, the effect of temperature on gas composition was studied by changing the temperature of the Fe-supported activated carbon bed.
Table 2 shows the temperature dependence of the composition on a dry basis of gas leaving the reactor. With increasing temperature up to 450 °C, the CO content decreased from 41.0 to 30.8 vol%, while the CO
2 and H
2 content increased from 0.4 to 7.5 vol% and from 28.1 to 30.5 vol%, respectively. This result indicates that the water-gas shift reaction (CO + H
2O → CO
2 + H
2) proceeds with an increase in the temperature. In a study by Encinar
et al., similar results were reported using Zn-supported carbonaceous materials [
23]. Not only is steam consumed on the surface of Fe-supported activated carbons as a result of the water-gas shift reaction, but also reaction (2) and the sulfidation reaction of Fe are promoted at 350 °C. Accordingly the breakthrough times for Fe9 and Fe17 are much longer than that for Fe0, even in the presence of steam.
Table 2.
Effect of temperature on composition of gas leaving the reactor.
Table 2.
Effect of temperature on composition of gas leaving the reactor.
| Temperature | Gas composition/vol% on a dry basis | H2/CO |
|---|
| °C | H2 | CO | CO2 | CH4 | C2H4 | N2 | - |
| 20 a | 28.1 | 41.0 | 0.4 | 4.2 | 0.1 | 26.3 | 0.69 |
| 250 | 27.1 | 39.8 | 0.6 | 4.0 | 0.1 | 28.3 | 0.68 |
| 300 | 27.3 | 39.2 | 1.2 | 4.0 | 0.1 | 28.2 | 0.70 |
| 350 | 27.4 | 38.7 | 1.7 | 4.0 | 0.1 | 28.1 | 0.71 |
| 400 | 29.2 | 34.8 | 4.4 | 3.9 | 0.1 | 27.6 | 0.84 |
| 450 | 30.5 | 30.8 | 7.5 | 3.9 | 0.1 | 27.2 | 0.99 |
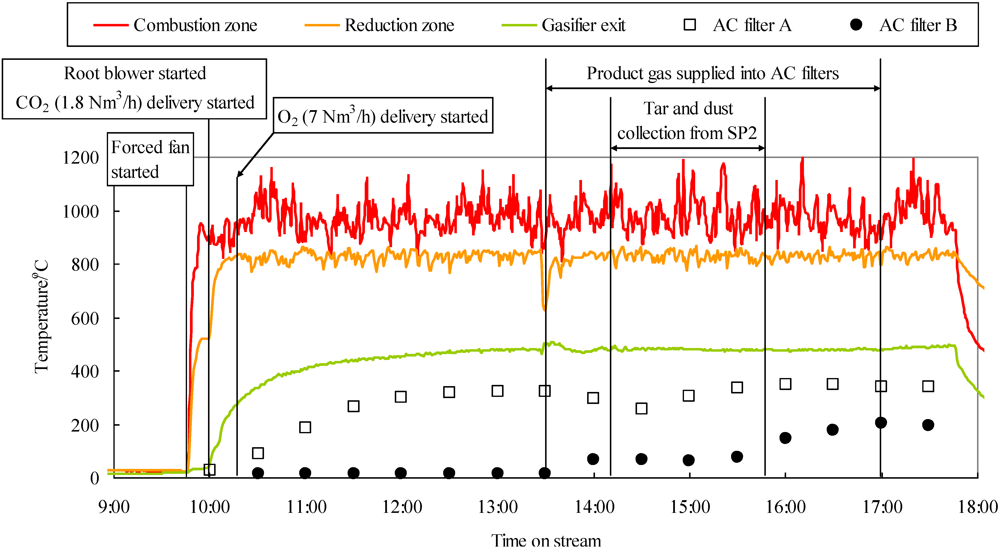
2.6. Improvement of Gas-Cleaning Process Using Fe-Supported Activated Carbons for BTL System
When the cleaned gas is compressed using a compressor before the FT synthesis reaction in the BTL total system, the typical tolerance levels for tar and particles are 50–500 mg Nm
−3 and 0.02 mg Nm
−3, respectively [
31]. If the tarry compound consists only of benzene, the tolerance level is 2500 ppmv at 4 MPa, 20 °C [
26]. In a study by Garcia
et al., the phenanthrene adsorption capacity decreased with increasing CO and CO
2-type groups on the surface of activated carbons using H
2O
2 and HNO
3 [
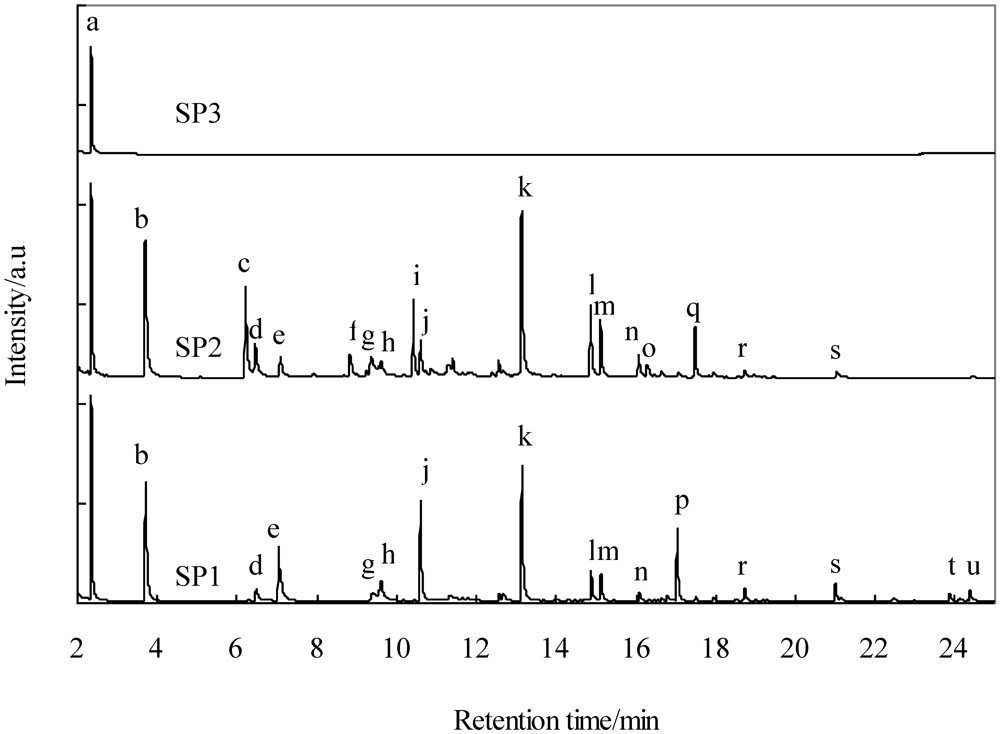
32]. This suggests that aromatic compounds are removed as a result of the interaction between the π-electron-rich regions of the graphene layers and the aromatic rings of phenanthrene. It is possible that benzene molecules are removed simultaneously by modifying the surface of Fe-supported activated carbons. As a result, tar, except that composed of benzene, was removed and its concentration decreased below the tolerance level, while the removal of particles was not accomplished. Since particles are solid at temperatures less than in the gasifier, they should be physically removed. At present, we have no regeneration system for blockage of activated carbon filters due to particles and need to exchange new filters after a prolonged time on stream. In order to protect the activated deactivation due to particles, we suggest the process in which particles are removed at more than 350 °C between the cyclone and activated filters. For example the cleaned gas without small particles could be supplied to the activated carbon filter through a process such as electrostatic precipitator.
In the present study, both the simultaneous removal of impurities and the promotion of a water-gas shift reaction were demonstrated using Fe-supported activated carbon packed into two filters on a bench scale. Sulfur compounds, three- and four-ring PAHs, and particles were removed and a water-gas shift reaction was promoted through the first filter. Except that of benzene, one- and two-ring PAHs were subsequently removed through the second filter. Apparently the addition of Fe to activated carbon produced the active sites for desulfurization and water gas shift reaction. Probably the heavy tar would be adsorbed on not only catalytically active Fe, but also on the surface of activated carbon. Therefore the textual parameter of activated carbons would affect the lifetime for the removal of impurities. We believe that not only the lifetime, but also the absorption capacity of heavy tar would increase with an increase in the surface area and pore size. The lifetime of Fe-supported activated carbon employed in the first filter and the choice of adsorbent used in the second filter are important considerations for optimizing the gas-cleaning process. In our proposed process, it is necessary to renew the Fe-supported activated carbon after an extended time on stream, which makes the economics less favorable. In the present paper, unfortunately we did not study the lifetime of the activated carbon filter. Therefore in the future it will be investigated together with the effect of adsorption capacity for sulfur compounds and the tar, and pressure drop through the bench plant operation.
A product gas with a H
2/CO ratio of 1.2 was obtained by the promotion of the water-gas shift reaction. Because a feed gas with a H
2/CO ratio of one is desirable for dimethyl ether synthesis from syngas [
33], the water-gas shift process can be omitted if the proposed gas-cleaning process is optimized. However, because a feed gas with H
2/CO of 2.0–2.2 is favorable for hydrocarbon and alcohol synthesis from syngas [
34,
35,
36], it is necessary to develop a more effective reaction.
It is also important to study inexpensive adsorbents and their regeneration. In order to make heavy tarry compound molecules diffuse easily into the pores, even at higher temperatures, for the water-gas shift reaction, Fe-supported inexpensive carbonaceous materials with mesopores up to 40 nm could be used. For example, unreacted char obtained in the gasification process can be employed as an adsorbent in the first filter. A saturated adsorbent with sulfur compounds and heavy tar could be used as a heating fuel. In this study, the one- and two-ring PAHs, except that of benzene and sulfur compounds, were adsorbed on the surface of activated carbons used in the second filter at temperatures less than 200 °C. It is possible that such compounds can be collected under an inert gas stream at temperatures greater than 200 °C; these collected compounds could then be useful as raw materials for chemicals and the adsorbent reused. In the near future, we will report on the lifetime and regeneration of adsorbents based on the results of a laboratory test.