Monitoring the Invasion of Spartina alterniflora Using Multi-source High-resolution Imagery in the Zhangjiang Estuary, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Acquisition and Preprocessing
2.3. Classification Methods and Accuracy Assessment
2.4. Landscape Metrics and Dynamic Indices
3. Results
3.1. Classification Accuracy Assessment
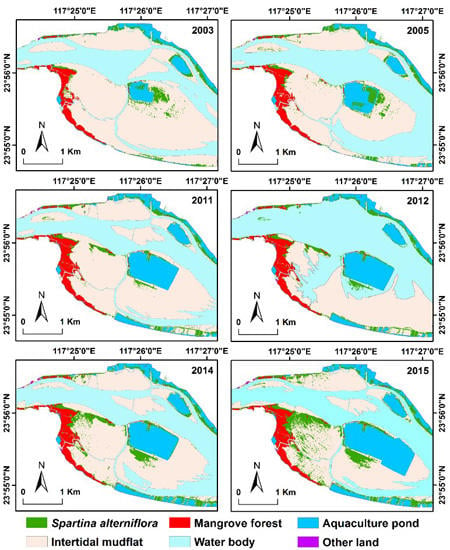
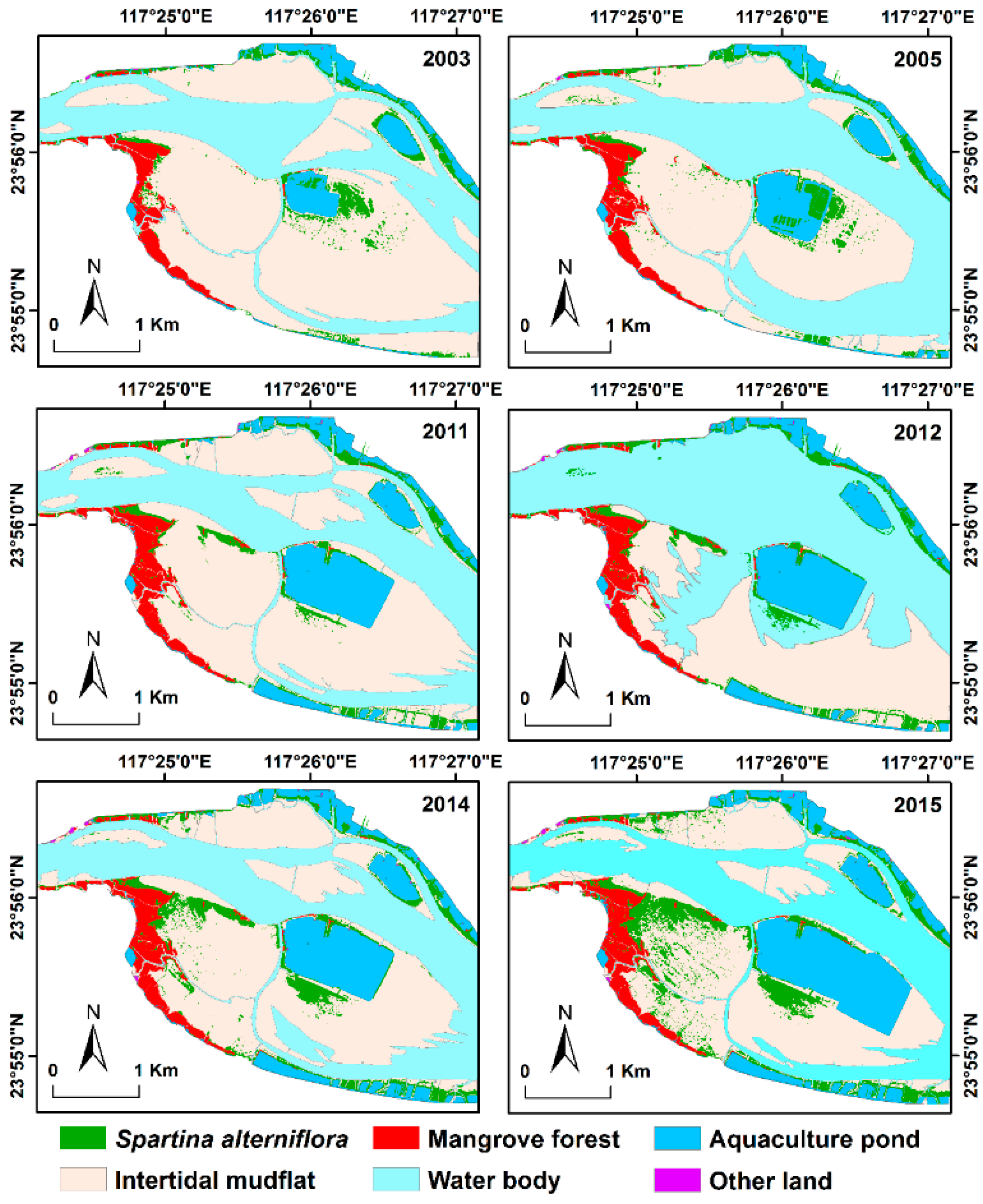
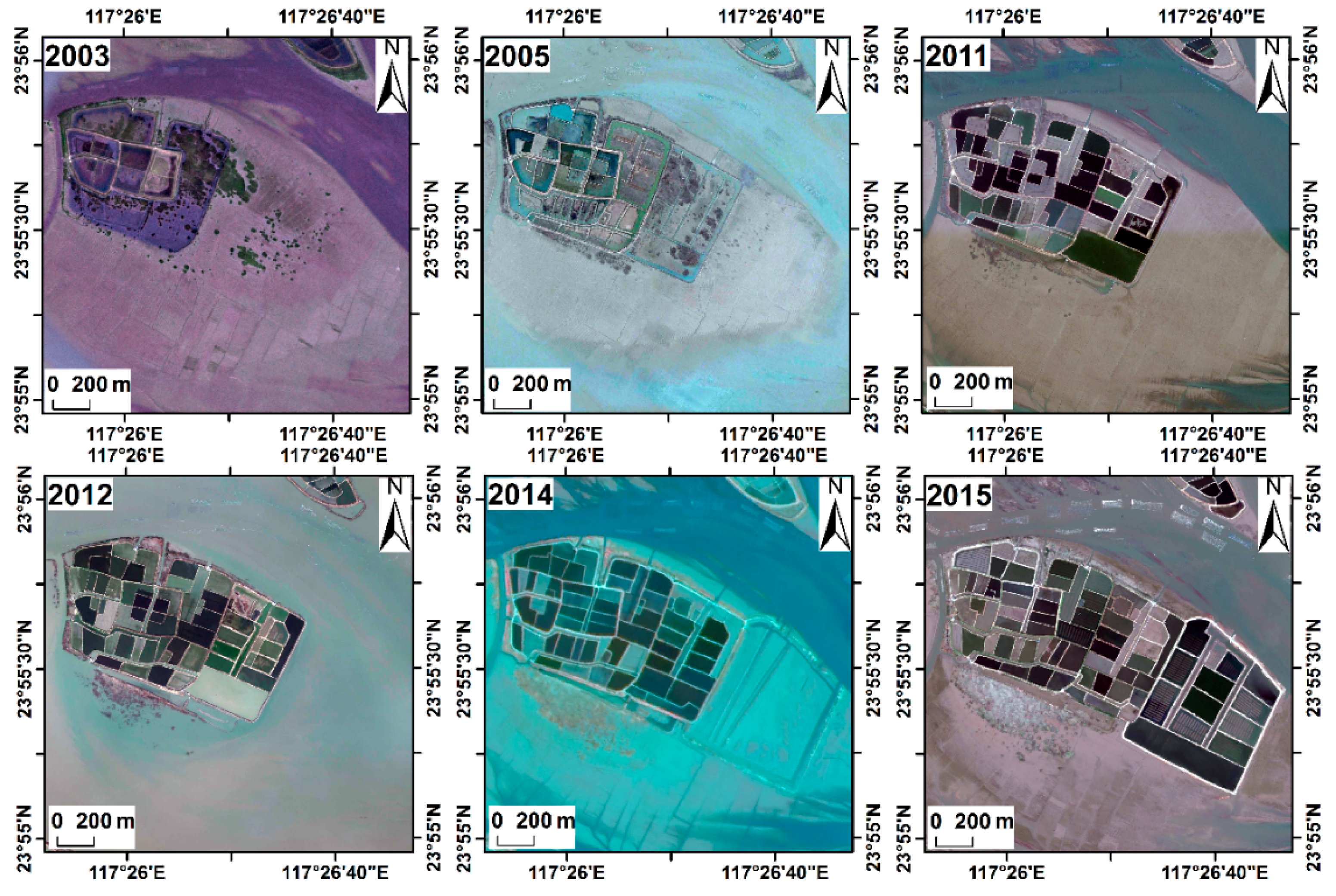
3.2. Spatial Distribution and Temporal Changes
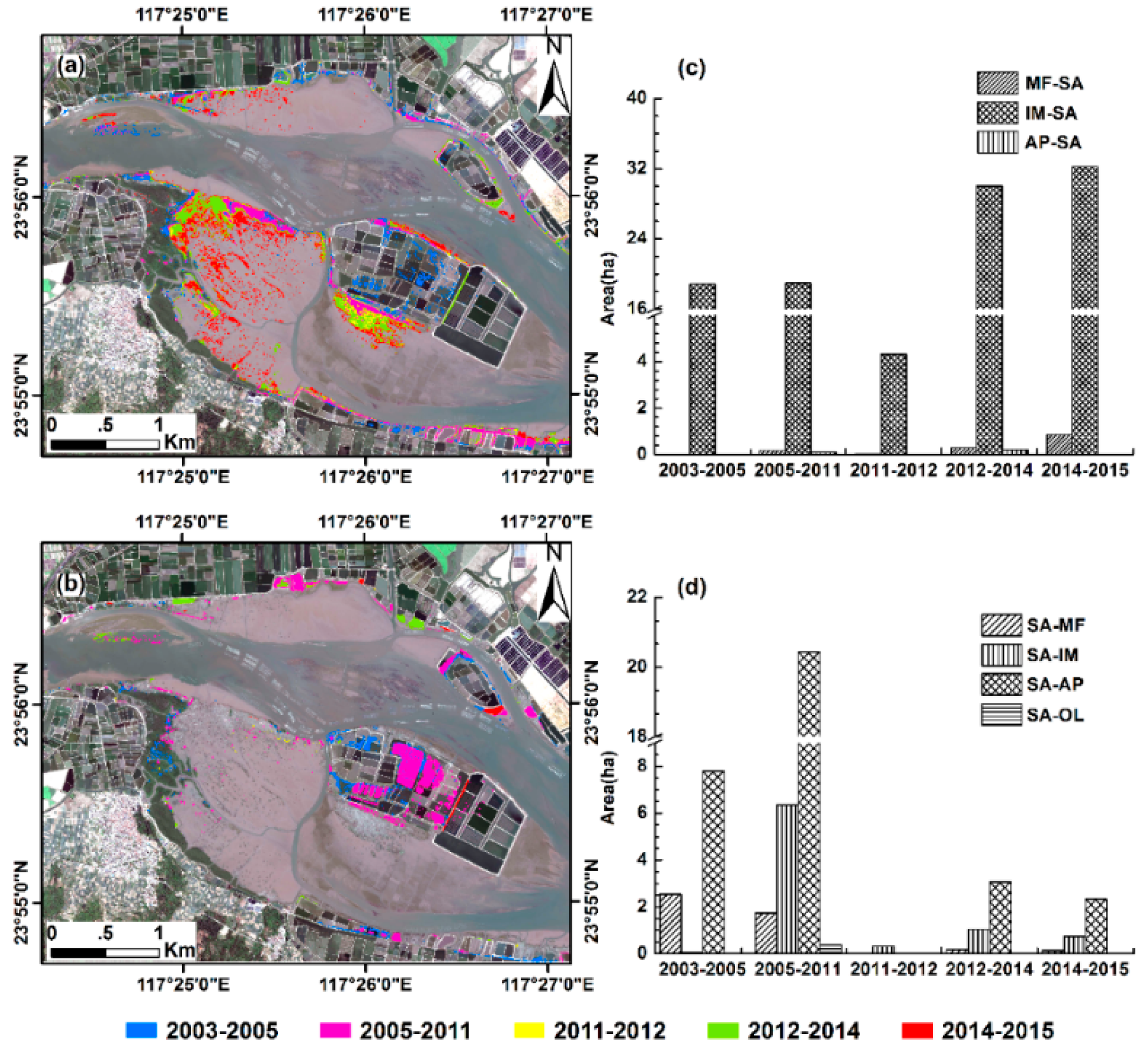
3.3. Conversions between S. alterniflora and Other Land Cover Types
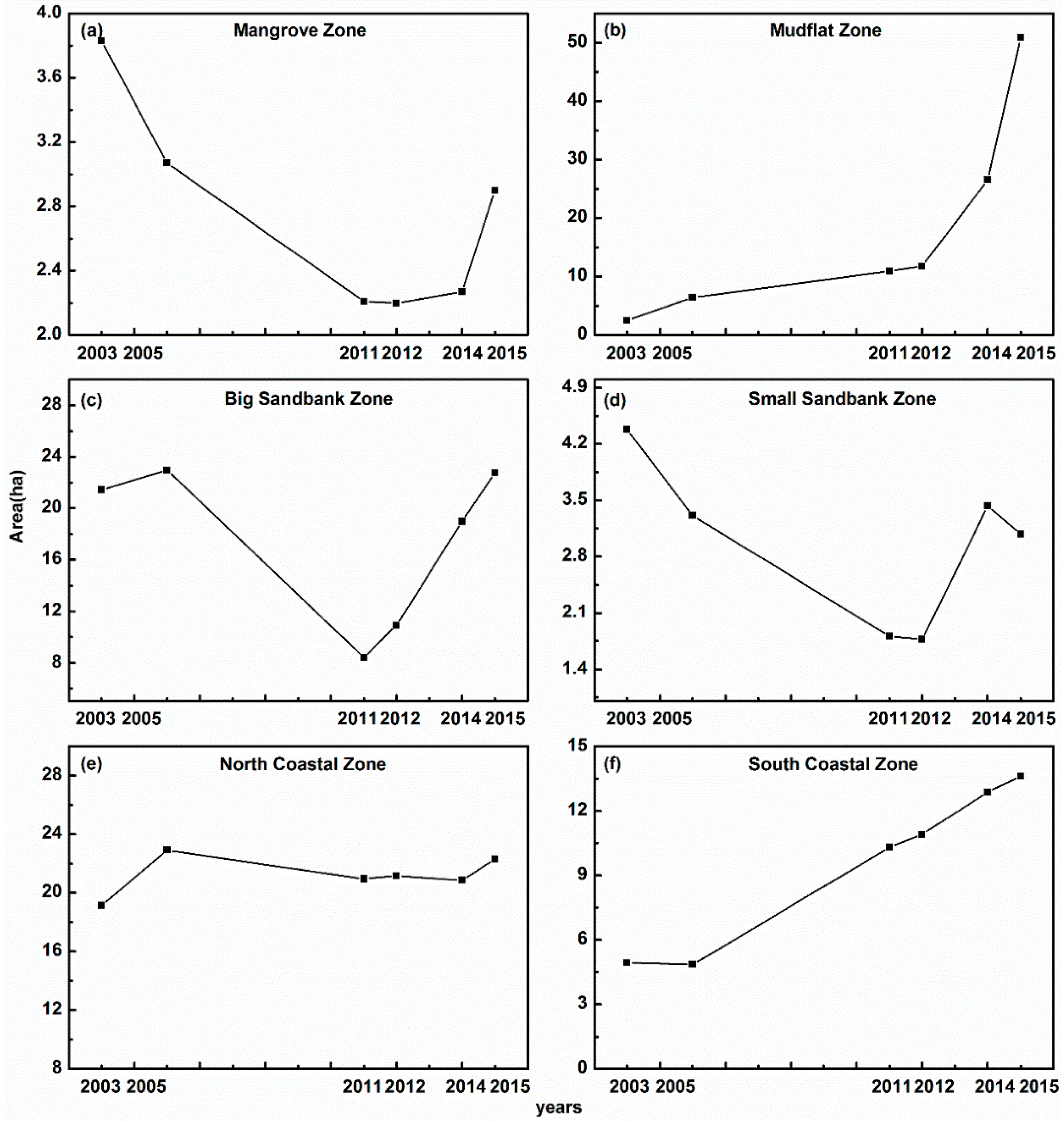
3.4. Expansion Patterns of S. alterniflora in the Six Subzones
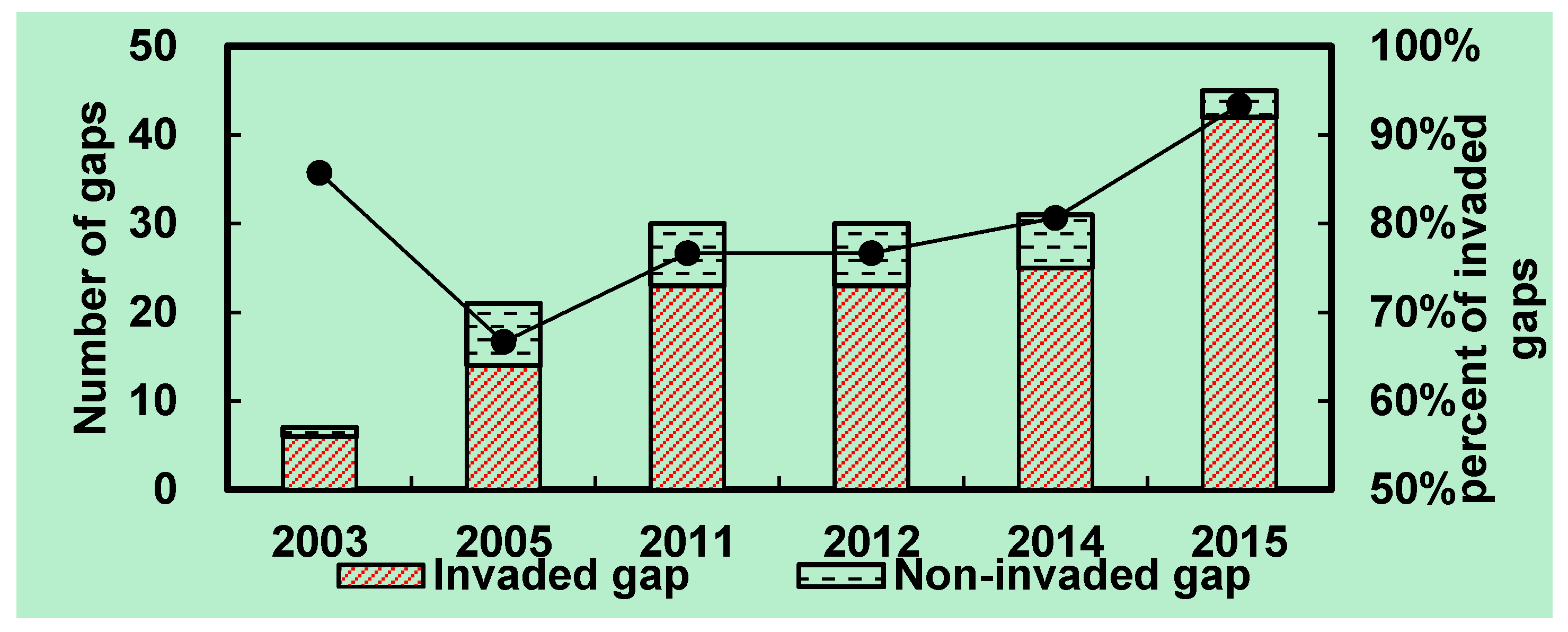
3.5. Invasion of S. alterniflora into Mangrove Forests
4. Discussion
4.1. Application of Multi-source High-resolution Imagery in Monitoring S. alterniflora Invasion
4.2. S. alterniflora Dynamics Driven by Human Activities
4.3. Possible Effects of S. alterniflora Invasion
4.4. Suggestions for Managing and Controlling the Invasion of S. alterniflora
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AI | Aggregation Index |
| AP | Aquaculture pond |
| CA | Class area |
| CLUMPY | Clumpiness index |
| GF-1 | Gaofen-1 |
| GCPs | Ground control points |
| GE | Google Earth |
| IM | Intertidal mudflat |
| LPI | Largest patch index |
| MF | Mangrove forest |
| MPS | Mean patch size |
| OL | Other land |
| RMSE | Root mean squared error |
| S. alterniflora | Spartina alterniflora |
| SA | S. alterniflora |
| UTM | Universal Transverse Mercator |
| WB | Water body |
| ZNMNR | Zhangjiangkou National Mangrove Nature Reserve |
References
- Simberloff, D.; Martin, J.L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; Garcia-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What‘s what and the way forward. Trends. Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Vitousek, P.M.; Antonio, C.M.; Loope, L.L.; Westbrooks, R. Biological invasions as global environmental change. Am. Sci. 1996, 84, 468–478. [Google Scholar]
- Ehrenfeld, J.G. Ecosystem consequences of biological invasions. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 59–80. [Google Scholar] [CrossRef]
- Brooks, M.L.; D’Antonio, C.M.; Richardson, D.M.; Grace, J.B.; Keeley, J.E.; DiTomaso, J.M.; Hobbs, R.J.; Pellant, M.; Pyke, D. Effects of invasive alien plants on fire regimes. Bioscience 2004, 54, 677–688. [Google Scholar] [CrossRef]
- D’Antonio, C.M.; Hobbie, S.E. Plant species effects on ecosystem processes: Insights from invasive species. In Species Invasions: Insights into Ecology, Evolution and Biogeography; Sax, D.F., Stachowicz, J.J., Gaines, S.D., Eds.; Sinauer Associates, Inc.: Sunderland, UK, 2005; pp. 65–84. [Google Scholar]
- Chung, C.H. Forty years of ecological engineering with Spartina plantations in China. Ecol. Eng. 2006, 27, 49–57. [Google Scholar] [CrossRef]
- Ge, Z.M.; Zhang, L.Q.; Yuan, L.; Zhang, C. Effects of salinity on temperature-dependent photosynthetic parameters of a native C3 and a non-native C4 marsh grass in the Yangtze Estuary, China. Photosynthetica 2014, 52, 484–492. [Google Scholar] [CrossRef]
- Daehler, C.C.; Strong, D.R. Status, prediction and prevention of introduced cordgrass Spartina spp. invasions in Pacific estuaries, USA. Biol. Conserv. 1996, 78, 51–58. [Google Scholar] [CrossRef]
- Callaway, J.C.; Josselyn, M.N. The introduction and spread of Smooth Cordgrass (Spartina alterniflora) in South San Francisco Bay. Estuaries Coasts 1992, 15, 218–226. [Google Scholar] [CrossRef]
- Wan, H.; Wang, Q.; Jiang, D.; Fu, J.; Yang, Y.; Liu, X. Monitoring the invasion of Spartina alterniflora using very high resolution unmanned aerial vehicle imagery in Beihai, Guangxi (China). Sci. World J. 2014, 2014, 638296:1–638296:7. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.J.; Hsu, C.B.; Liao, S.H.; Chen, C.P.; Hsieh, H.L. Effects of Spartina alterniflora invasion on the abundance and community of meiofauna in a subtropical wetland. Wetlands 2015, 35, 547–556. [Google Scholar] [CrossRef]
- Zuo, P.; Zhao, S.H.; Liu, C.A.; Wang, C.H.; Liang, Y.B. Distribution of Spartina spp. along China‘s coast. Ecol. Eng. 2012, 40, 160–166. [Google Scholar] [CrossRef]
- An, S.Q.; Gu, B.H.; Zhou, C.F.; Wang, Z.S.; Deng, Z.F.; Zhi, Y.B.; Li, H.L.; Chen, L.; Yu, D.H.; Liu, Y.H. Spartina invasion in China: Implications for invasive species management and future research. Weed Res. 2007, 47, 183–191. [Google Scholar] [CrossRef]
- Gan, X.J.; Cai, Y.T.; Choi, C.Y.; Ma, Z.J.; Chen, J.K.; Li, B. Potential impacts of invasive Spartina alterniflora on spring bird communities at Chongming Dongtan, a Chinese wetland of international importance. Estuar. Coast. Shelf Sci. 2009, 83, 211–218. [Google Scholar] [CrossRef]
- Grosholz, E.D.; Levin, L.A.; Tyler, A.C.; Neira, C. Changes in community structure and ecosystem function following Spartina alterniflora invasion of Pacific estuaries. In Human Impacts on Salt Marshes; Silliman, B.R., Grosholz, E., Bertness, M.D., Eds.; University of California Press: Berkeley, CA, USA, 2009; pp. 23–40. [Google Scholar]
- List of the First Batch of Alien Invasive Species in China. Available online: http://www.biodiv.gov.cn/zyxw/gnxw/201601/t20160107_323373.html (accessed on 10 January 2003).
- Nagendra, H.; Lucas, R.; Honrado, J.P.; Jongman, R.H.G.; Tarantino, C.; Adamo, M.; Mairota, P. Remote sensing for conservation monitoring: Assessing protected areas, habitat extent, habitat condition, species diversity, and threats. Ecol. Indic. 2013, 33, 45–59. [Google Scholar] [CrossRef]
- Huang, C.Y.; Asner, G.P. Applications of remote sensing to alien invasive plant studies. Sensors 2009, 9, 4869–4889. [Google Scholar] [CrossRef] [PubMed]
- Müllerová, J.; Pyšek, P.; Jarošík, V.; Pergl, J. Aerial photographs as a tool for assessing the regional dynamics of the invasive plant species Heracleum mantegazzianum. J. Appl. Ecol. 2005, 42, 1042–1053. [Google Scholar] [CrossRef]
- Huang, H.M.; Zhang, L.Q. A study of the population dynamics of Spartina alterniflora at Jiuduansha shoals, Shanghai, China. Ecol. Eng. 2007, 29, 164–172. [Google Scholar] [CrossRef]
- Wang, A.Q.; Chen, J.D.; Jing, C.W.; Ye, G.Q.; Wu, J.P.; Huang, Z.X.; Zhou, C.S. Monitoring the Invasion of Spartina alterniflora from 1993 to 2014 with Landsat TM and SPOT 6 Satellite Data in Yueqing Bay, China. PLoS ONE 2015, 10, e0135538. [Google Scholar] [CrossRef] [PubMed]
- Madden, M. Remote sensing and geographic information system operations for vegetation mapping of invasive exotics. Weed Technol. 2004, 18, 1457–1463. [Google Scholar] [CrossRef]
- Bradley, B.A.; Mustard, J.F. Characterizing the landscape dynamics of an invasive plant and risk of invasion using remote sensing. Ecol. Appl. 2006, 16, 1132–1147. [Google Scholar] [CrossRef]
- Walsh, S.J.; McCleary, A.L.; Mena, C.F.; Shao, Y.; Tuttle, J.P.; Gonzalez, A.; Atkinson, R. QuickBird and Hyperion data analysis of an invasive plant species in the. Galapagos Islands of Ecuador: Implications for control and land use management. Remote Sens. Environ. 2008, 112, 1927–1941. [Google Scholar] [CrossRef]
- Fuller, D.O. Remote detection of invasive Melaleuca trees (Melaleuca quinquenervia) in South Florida with multispectral IKONOS imagery. Int. J. Remote Sens. 2005, 26, 1057–1063. [Google Scholar] [CrossRef]
- Visser, V.; Langdon, B.; Pauchard, A.; Richardson, D.M. Unlocking the potential of Google Earth as a tool in invasion science. Biol. Invasions 2014, 16, 513–534. [Google Scholar] [CrossRef]
- Hu, Q.; Wu, W.B.; Xia, T.; Yu, Q.Y.; Yang, P.; Li, Z.G.; Song, Q. Exploring the use of Google Earth imagery and object-based methods in land use/cover mapping. Remote Sens. 2013, 5, 6026–6042. [Google Scholar] [CrossRef]
- Li, Z.J.; Wang, W.Q.; Zhang, Y.H. Recruitment and herbivory affect spread of invasive Spartina alterniflorain China. Ecology 2014, 95, 1972–1980. [Google Scholar] [CrossRef] [PubMed]
- Information Sheet on Ramsar Wetlands (RIS) Version 2006–2008. Available online: https://rsis.ramsar.org/ris/1726 (accessed on 2 February 2008).
- Jacobson, A.; Dhanota, J.; Godfrey, J.; Jacobson, H.; Rossman, Z.; Stanish, A.; Walker, H.; Riggio, J. A novel approach to mapping land conversion using Google Earth with an application to East Africa. Environ. Model. Softw. 2015, 72, 1–9. [Google Scholar] [CrossRef]
- Potere, D. Horizontal Positional Accuracy of Google Earth’s High-Resolution Imagery Archive. Sensors 2008, 8, 7973–7981. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.L.; Shao, X.W.; Xu, Y.W.; Miyazaki, H.; Ohira, W.; Shibasaki, R. Identification of Village Building via Google Earth Images and Supervised Machine Learning Methods. Remote Sens. 2016, 8, 271. [Google Scholar] [CrossRef]
- Aiazzi, B.; Alparone, L.; Baronti, S.; Selva, M. MS + Pan Image Fusion by an Enhanced Gram-Schmidt Spectral Sharpening. In Proceedings of the 26th Annual Symposium of the European Association of Remote Sensing Laboratories, Warsaw, Poland, 29 May–2 June 2006. [Google Scholar]
- Yuhendra; Sumantyo, J.; Kuze, H. Performance Analyzing of High Resolution Pan-sharpening Techniques: Increasing Image Quality for Classification using Supervised Kernel Support Vector Machine. Res. J. Inf. Technol. 2011, 3, 12–23. [Google Scholar]
- Zhang, Y.; Mishra, R.K. A Review and Comparison of Commercially Available Pan-Sharpening Techniques for High Resolution Satellite Image Fusion. In 2012 IEEE International Geoscience and Remote Sensing Symposium; IEEE: Munich, Gremany, 2012; pp. 182–185. [Google Scholar]
- Baatz, M.; Schäpe, A. Multiresolution Segmentation: An Optimization Approach for High Quality Multi-scale Image Segmentation. In Proceedings of the 12th Angewandte Geographische Informationsverarbeitung; Wichmann-Verlag: Heidelberg, Germany, 2000. [Google Scholar]
- Blaschke, T. Object based image analysis for remote sensing. ISPRS. J. Photogramm. Remote Sens. 2010, 65, 2–16. [Google Scholar] [CrossRef]
- Benz, U.C.; Hofmann, P.; Willhauck, G.; Lingenfelder, I.; Heynen, M. Multi-resolution, object-oriented fuzzy analysis of remote sensing data for GIS-ready information. ISPRS. J. Photogramm. Remote Sens. 2004, 58, 239–258. [Google Scholar] [CrossRef]
- Mathieu, R.; Aryal, J.; Chong, A.K. Object-based classification of ikonos imagery for mapping large-scale vegetation communities in urban areas. Sensors 2007, 7, 2860–2880. [Google Scholar] [CrossRef]
- Jia, M.M.; Liu, M.Y.; Wang, Z.M.; Mao, D.H.; Ren, C.Y.; Cui, H.S. Evaluating the effectiveness of conservation on mangroves: A remote sensing-based comparison for two adjacent protected areas in Shenzhen and Hong Kong, China. Remote Sens. 2016, 8, 627. [Google Scholar] [CrossRef]
- Definiens Imaging. In eCognition Developer Software: 8.7.1. Reference Book; Trimble GmbH: Raunheim, Germany, 2012.
- Congalton, R.; Green, K. Assessing the Accuracy of Remotely Sensed Data: Principles and Practices; Mapping Science Series; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Wang, Z.M.; Song, K.S.; Zhang, B.; Liu, D.W.; Ren, C.Y.; Luo, L.; Yang, T.; Huang, N.; Hu, L.; Yang, H.J.; et al. Shrinkage and fragmentation of grasslands in the West Songnen Plain, China. Agric. Ecosyst. Environ. 2009, 129, 315–324. [Google Scholar] [CrossRef]
- Fragstats Help Version 4.2. Available online: http://www.umass.edu/landeco/research/fragstats/documents/fragstats.help.4.2.pdf (accessed on 21 April 2015).
- He, H.S.; DeZonia, B.E.; Mladenoff, D.J. An aggregation index (AI) to quantify spatial patterns of landscapes. Landsc. Ecol. 2000, 15, 591–601. [Google Scholar] [CrossRef]
- Müllerová, J.; Pergl, J.; Pyšek, P. Remote sensing as a tool for monitoring plant invasions: Testing the effects of data resolution and image classification approach on the detection of a model plant species Heracleum mantegazzianum (giant hogweed). Int. J. Appl. Earth Obs. Geoinf. 2013, 25, 55–65. [Google Scholar] [CrossRef]
- Ecological Effectiveness of Mangrove Forests in the Zhangjiang Estuary. Available online: http://www.wetwonder.org/P_show.asp?ID=4541 (accessed on 27 May 2017).
- Zhang, Y.H.; Huang, G.M.; Wang, W.Q.; Chen, L.Z.; Lin, G.H. Interactions between mangroves and exotic Spartina in an anthropogenically disturbed estuary in southern China. Ecology 2012, 93, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.C.; Bin, Z.X.; Qun, L.C. Function assessment and ecological controls on alien species cordgrass. Chin. J. Ecol. 2004, 23, 93–98. (In Chinese) [Google Scholar]
- Lin, W.P.; Chen, G.S.; Guo, P.P.; Zhu, W.Q.; Zhang, D.H. Remote-sensed monitoring of dominant plant species distribution and dynamics at Jiuduansha Wetland in Shanghai, China. Remote Sens. 2015, 7, 10227–10241. [Google Scholar] [CrossRef]



| Year | Source | Acquisition Date | Band | Resolution (m) | Tidal Level | Remark |
|---|---|---|---|---|---|---|
| 2003 | SPOT-5 | 2003.01.04 | 3 | 2.5 | Low | Classification |
| 2005 | Google Earth 1 | 2005.02.09 | 3 | 1 | Low | Classification |
| 2011 | Google Earth 1 | 2011.04.24 | 3 | 1 | Low | Classification |
| 2012 | Google Earth 1 | 2011.12.24 | 3 | 1 | Middle | Classification |
| 2014 | Google Earth 2 | 2014.01.31 | 3 | 1 | Low | Classification |
| GF-1 | 2014.01.18 | 4 | 2 | Low | Ancillary data | |
| GF-1 | 2014.08.23 | 4 | 2 | High | Ancillary data | |
| 2015 | Google Earth1 | 2015.10.17 | 3 | 1 | Low | Classification |
| Year Source | 2003 | 2005 | 2011 | 2012 | 2014 | 2015 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SPOT-5 | GE | GE | GE | GE&GF-1 | GE | ||||||||
| Land Cover Type | Pro | Use | Pro | Use | Pro | Use | Pro | Use | Pro | Use | Pro | Use | |
| S. alterniflora | 0.82 | 0.90 | 0.82 | 0.93 | 0.89 | 0.94 | 0.90 | 0.90 | 0.84 | 0.88 | 0.91 | 0.89 | |
| Mangrove Forest | 0.90 | 0.90 | 0.91 | 0.91 | 0.96 | 0.92 | 0.93 | 0.90 | 0.89 | 0.89 | 0.91 | 0.91 | |
| Water Body | 0.86 | 0.86 | 0.91 | 0.87 | 0.95 | 0.90 | 0.86 | 0.82 | 0.92 | 0.88 | 0.88 | 0.83 | |
| Aquaculture Pond | 0.78 | 0.78 | 0.82 | 0.90 | 0.92 | 0.85 | 1.00 | 0.93 | 1.00 | 0.88 | 0.82 | 0.90 | |
| Intertidal Mudflat | 0.91 | 0.86 | 0.88 | 0.78 | 0.82 | 0.90 | 0.63 | 0.71 | 0.77 | 0.85 | 0.80 | 0.86 | |
| Other Land | 0.80 | 1.00 | 0.75 | 1.00 | 0.80 | 0.80 | 0.83 | 1.00 | 1.00 | 0.75 | 0.83 | 0.83 | |
| Overall accuracy | 0.87 | 0.87 | 0.90 | 0.86 | 0.87 | 0.88 | |||||||
| Kappa coefficient | 0.83 | 0.84 | 0.88 | 0.82 | 0.84 | 0.85 | |||||||
| Year | Area (ha) | Stage | Change of Area (ha) | Annual Change Rate (%) |
|---|---|---|---|---|
| 2003 | 57.94 | 2003–2005 | 8.82 | 7.61 |
| 2005 | 66.76 | 2005–2011 | −9.41 | −2.35 |
| 2011 | 57.34 | 2011–2012 | 4.02 | 7.01 |
| 2012 | 61.36 | 2012–2014 | 25.95 | 21.14 |
| 2014 | 87.31 | 2014–2015 | 28.80 | 32.98 |
| 2015 | 116.11 | 2003–2015 | 58.17 | 8.37 |
| Year | CA | LPI | MPS | CLUMPY | AI |
|---|---|---|---|---|---|
| 2003 | 12.13 | 0.11 | 0.12 | 0.91 | 91.15 |
| 2005 | 18.27 | 0.17 | 0.14 | 0.92 | 91.63 |
| 2011 | 27.42 | 0.24 | 0.21 | 0.93 | 93.31 |
| 2012 | 29.51 | 0.24 | 0.22 | 0.93 | 93.49 |
| 2014 | 45.11 | 0.71 | 0.36 | 0.94 | 94.14 |
| 2015 | 49.76 | 1.32 | 0.37 | 0.95 | 94.65 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Li, H.; Li, L.; Man, W.; Jia, M.; Wang, Z.; Lu, C. Monitoring the Invasion of Spartina alterniflora Using Multi-source High-resolution Imagery in the Zhangjiang Estuary, China. Remote Sens. 2017, 9, 539. https://doi.org/10.3390/rs9060539
Liu M, Li H, Li L, Man W, Jia M, Wang Z, Lu C. Monitoring the Invasion of Spartina alterniflora Using Multi-source High-resolution Imagery in the Zhangjiang Estuary, China. Remote Sensing. 2017; 9(6):539. https://doi.org/10.3390/rs9060539
Chicago/Turabian StyleLiu, Mingyue, Huiying Li, Lin Li, Weidong Man, Mingming Jia, Zongming Wang, and Chunyan Lu. 2017. "Monitoring the Invasion of Spartina alterniflora Using Multi-source High-resolution Imagery in the Zhangjiang Estuary, China" Remote Sensing 9, no. 6: 539. https://doi.org/10.3390/rs9060539